Abstract
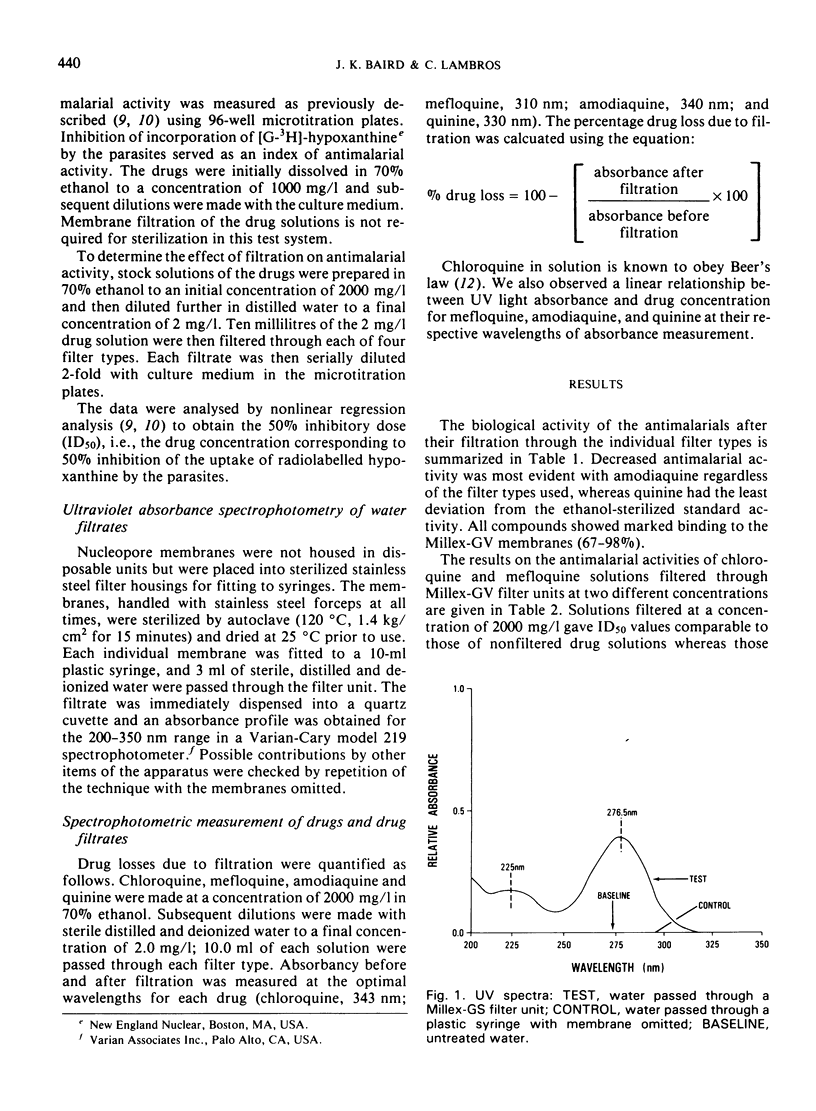
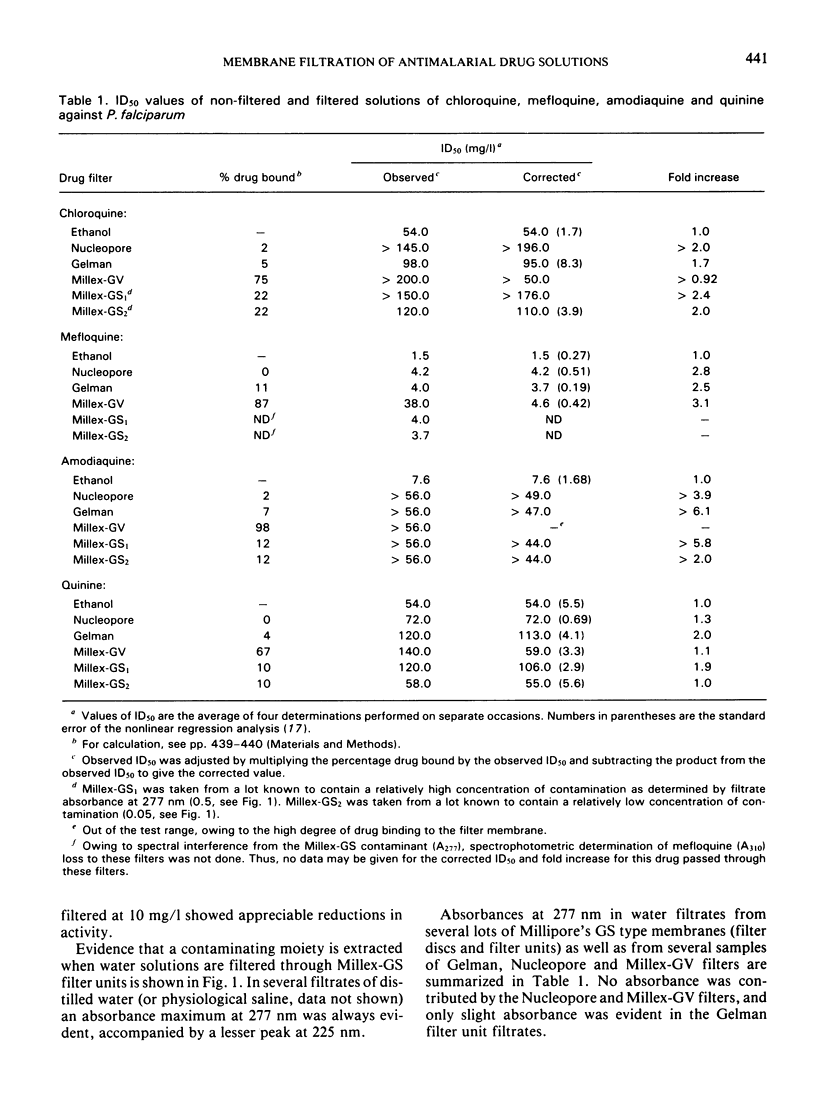
Antimalarial activities of chloroquine, mefloquine, amodiaquine, and quinine in vitro against Plasmodium falciparum were diminished as a consequence of membrane filtration. Filtered drug solutions gave ID50 values up to 25-fold greater than those of non-filtered (ethanol-sterilized) drug solutions. Loss of activity by filtration was overcome by increasing the drug concentration prior to filtration. Water solutions filtered through Millex-GS filter units consistently showed an absorbance maximum at 277 nm, accompanied by a lesser peak at 225 nm. Water filtrates from Nucleopore and Millex-GV filters showed no absorbance at 277 nm and only slight absorbance was evident for the Gelman filter unit. Activity losses were attributed to extractable contaminating moieties in the membrane filters and/or drug binding to the membrane filters.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN F., CARTWRIGHT B., NEWMAN J. F. INHIBITION OF VIRUS GROWTH BY A TOXIC FACTOR FROM ASBESTOS PAD AND CELLULOSE ACETATE MEMBRANE FILTERS. Nature. 1965 Jan 16;205:310–311. doi: 10.1038/205310b0. [DOI] [PubMed] [Google Scholar]
- COHEN S. N., YIELDING K. L. SPECTROPHOTOMETRIC STUDIES OF THE INTERACTION OF CHLOROQUINE WITH DEOXYRIBONUCLEIC ACID. J Biol Chem. 1965 Jul;240:3123–3131. [PubMed] [Google Scholar]
- Cahn R. D. Detergents in membrane filters. Science. 1967 Jan 13;155(3759):195–196. doi: 10.1126/science.155.3759.195. [DOI] [PubMed] [Google Scholar]
- Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicchio V. S., Murphy M. J., Jr Effect of membrane dialysis and filtration-sterilization on erythropoietin activity. Yale J Biol Med. 1981 Jul-Aug;54(4):249–254. [PMC free article] [PubMed] [Google Scholar]
- Lambros C., Childs G. E., Notsch J. D., Scovill J. P., Klayman D. L., Davidson D. E., Jr In vitro assessment of 2-acetylpyridine thiosemicarbazones against chloroquine-resistant Plasmodium falciparum. Antimicrob Agents Chemother. 1982 Dec;22(6):981–984. doi: 10.1128/aac.22.6.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R., Niles A. C. Effect of filtration on antimicrobial solutions. Antimicrob Agents Chemother. 1981 Nov;20(5):686–687. doi: 10.1128/aac.20.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth S. A., Cripps G. W. Inhibitory effects of detergents in membrane filters. J Pharm Sci. 1969 Apr;58(4):440–442. doi: 10.1002/jps.2600580410. [DOI] [PubMed] [Google Scholar]
- Simpson L. Toxic impurities in nalgene filter units. Science. 1966 Jul 29;153(3735):548–548. doi: 10.1126/science.153.3735.548. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Wakeland J. R., Crie J. S., Wildenthal K. Toxicity to organ cultured hearts of media prepared with disposable filter units. In Vitro. 1982 Aug;18(8):715–718. doi: 10.1007/BF02796427. [DOI] [PubMed] [Google Scholar]


