Abstract
Dipyridamole (Persantine) is a clinically used vasodilator with equilibrative nucleoside transporters 1, and 2 (ENT1 and ENT2) inhibitory activity albeit less potent than the prototype ENT1 inhibitor nitrobenzylmercaptopurine riboside (NBMPR). Dipyridamole is a good candidate for further exploration because it is a non-nucleoside and has a proven record of safe use in humans. A series of dipyridamole analogs were synthesized with systematic modification, and evaluated as ENT1 inhibitors by flow cytometry. Compounds with much higher potency were identified, the best being 2,6-bis(diethanolamino)-4,8-diheptamethyleneimino-pyrimido[5,4-d]pyrimidine (13), with a Ki of 0.49 nM, compared to a Ki of 308 nM for dipyridamole. Compound 13 is similar in potency to the prototype potent ENT1 inhibitor NBMPR (0.43 nM). For the first time, a dipyridamole analog has been identified that is equipotent with NBMPR. The SAR indicated that diethanolamine substituted analogs were more active than monoethanolamine compounds. Also, free hydroxyl groups are not essential for activity.
Keywords: Equilibrative nucleoside transporter ENT1 Inhibitors, Dipyridamole Analogs, NBMPR
Introduction
Nucleoside transporters are specialized integral membrane glycoproteins known to mediate the cellular influx or efflux of physiological nucleosides or nucleobases, as well as many synthetic analogs.1-2 Currently, nucleoside transporters have been classified into two families: (i) the equilibrative nucleoside transporter family (ENTs), and (ii) the concentrative nucleoside transporter family (CNTs).3-4 The equilibrative family facilitates the transport of nucleosides or nucleobases down their concentration gradients; in contrast, the concentrative family transports nucleosides against their concentration gradients by coupling with a sodium ion gradient. Nucleoside transporter inhibitors have potential therapeutic applications in ischemic heart disease and stroke5-10, in inflammatory disease,11 and as biological response modifiers in antimetabolite chemotherapy.12 A comprehensive summary of nucleoside transport inhibitors as potential therapeutic agents has been published.13
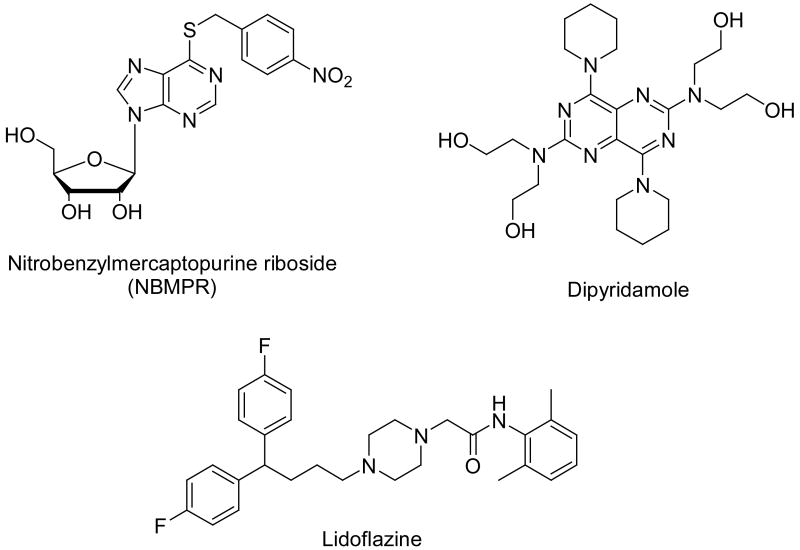
Equilibrative nucleoside transporters were the first to be identified because of their broad tissue distribution. They were initially subdivided into es (equilibrative sensitive) or ENT1, and ei (equilibrative insensitive) or ENT2 according to their sensitivities to inhibition by nanomolar concentrations of 4-nitrobenzylmercaptopurine ribonucleoside (NBMPR). Four subtypes of ENTs (ENT1, ENT2, ENT3 and ENT4) have now been identified and cloned.3 The ENT1 transporter is the most widely distributed nucleoside transporter with the highest abundance in most tissues studied.14-15 This makes it the most relevant NT target for therapeutic exploration. Several chemical classes have been shown to inhibit ENT1.13 Among them, three classes are most significant (Figure 1). These are purine nucleoside analogs of which NBMPR is the prototype, pyrimidopyrimidine analogs such as the antithrombotic and vasodilating agent dipyridamole, and flazine calcium channel blockers represented by lidoflazine.
Figure 1.
Representatives of the three main ENT1 inhibitory chemical classes
NBMPR is a more potent ENT1 inhibitor (e.g. Ki of 0.7 nM)16 than dipyridamole (e.g. Ki of 8.8)17. Draflazine, a lidoflazine analog, also exhibits high ENT1 inhibitory activity (IC50 = 0.28-10 nM).18 However, NBMPR and the flazine compounds like draflazine are poor candidates for further exploration. NBMPR has immunosuppressive and mutagenic activities deriving from its 6-mercaptopurine metabolite.19-21 The flazines are nosnspecific, having calcium channel antagonist activity that is thought to contribute significantly to their cardioprotective effects.22-24 As a potent ENT1 inhibitor, dipyridamole has broad pharmacological effects. It is an effective coronary vasodilator (used as an antianginal drug) through the increasing of extracellular adenosine concentration stemming from its ENT inhibitory activity.5,25,26 Dipyridamole also has antiplatelet effects attributed to phosphodiesterase inhibition.5 Co-administration of ENT1 inhibitors such as dipyridamole and antimetabolites such as 5-fluorouracil, has been shown to result in synergism and might improve the therapeutic index of antimetabolites, where target cells have a higher ENT1 expression than normal cells.27,28 Synergism results not only from inhibition of nucleoside salvage, but also from increasing the intracellular concentration of 5-fluorodeoxyuridine caused by blockade of its efflux by dipyridamole. Thus, the intracellular level of the active product, 5-fluorodeoxyuridine monophosphate, increases, resulting in higher therapeutic efficacy.29-30
Besides mammalian tissues, nucleoside transporters are also found in parasites such as Plasmodium falciparum, the malarial parasite.31,32 Parasites rely on salvage pathways to meet their purine and purine nucleoside needs since they do not have de novo purine biosynthetic pathways.33 Nucleoside transporters of parasites have limited homologies with the human ENT1, and have been shown to be inhibited by dipyridamole but not NBMPR or lidoflazine.34 Some parasites like Toxoplasma gondii can even transport NBMPR.35 A study of the antimalarial activity of dipyridamole showed that it was effective against all of the erythrocytic stages such as rings, trophozoites and schizonts; it had an IC50 of 30 nM by itself, and lowered the IC50 of chloroquine from 97.0 nM to 13.7 nM at a concentration of 0.1 nM.36
In light of these positive attributes of dipyridamole, we selected it as a candidate for further structure-activity relationship (SAR) exploration for ENT1 transporter inhibitory activity. Many dipyridamole analogs have been reported, and evaluated for their effects as antiplatelet and cardioprotective agents.37-41 Some dipyridamole analogs have also been synthesized and evaluated for their inhibitory activities against cyclin dependent kinases (CDKs), with negative results.42 A more recent publication disclosed the synthesis and biological evaluation of a series of dipyprdamole analogs for their ENT1 inhibitory activities, and some of them showed only slightly higher activities than dipyridamole.43 In this paper, a series of dipyridamole analogues were synthesized for a more systematic and comprehensive evaluation of ENT1 SAR. Some of the compounds showed comparative activity to NBMPR, which is a much more potent ENT1 inhibitor than dipyridamole.
Chemistry
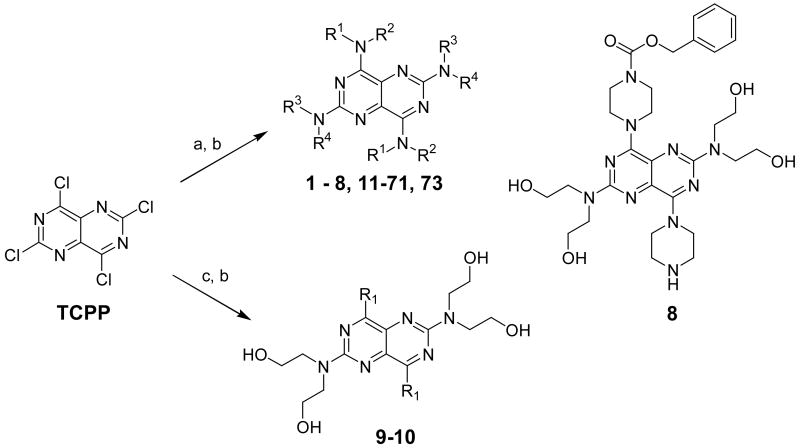
For the synthesis of these dipyridamole analogs, commercially available starting materials, 2,4,6,8-tetrachloropyrimido[5,4-d]pyrimidine (TCPP) and dipyridamole, were used based on the structures of individual final products. For the preparation of the major dipyridamole analogs (compounds 1-8, 11-71, and 73) (Scheme 1), an excess of the appropriate amine (about 4-fold excess) was reacted with TCPP in anhydrous THF. The resulting 2,6-dichloro intermediates were individually reacted with diethanolamine, ethanolamine or morpholine at 150 °C in DMSO as solvent to obtain the target products. For the preparation of compounds 9 and 10 (Scheme 1), the appropriate Grignard reagents were used for the first step, followed by reaction with diethanolamine in the second step.
Scheme 1a.
aReagents and conditiond: (a) NHR1R2, Anhydrous THF, 0 - 5 °C; (b) NHR3R4, DMSO, 150 °C; (c) R1MgCl, Anhydrous THF, 0 - 5 °C.
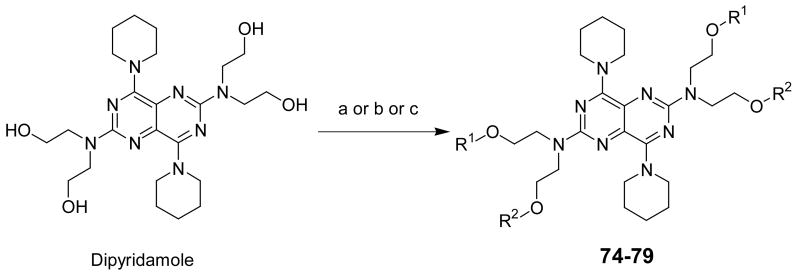
For the preparation of compounds 74-79 (Scheme 2), dipyridamole was used as starting material. Dipyridamole was acylated or alkylated44 to afford the desired products. Compound 78 was a dialkylated product, instead of the intended tetra-alkylated product. It appears that the introduction of the first isopropyl group at each side of dipyridamole prevented the introduction of a second isopropyl group on the remaining hydroxyl groups under the reaction conditions. This could be possibly due to steric hindrance. In total, 79 dipyridamole analogs with diverse substituents were synthesized in this study. The core pyrimido[5,4-d]pyrimidine system and the symmetrical feature in dipyridamole was maintained, with the exception of compound 8, which had two different substituents at the at the 4- and 8-positions. Compound 8 was planned to be symmetrical, but the conditions in the second reaction step caused a loss of one Cbz group to produce the unsymmetrical compound.
Scheme 2a.
aReagents and conditions: (a) HCOOH, 100 °C (compound 74); (b) CH3COCl, DMAP, anhydrous THF, 0-5 °C (compound 75); (c) NaH, R1I, anhydrous DMF (R2 = R1 for 76-78; R2 = H for 79).
Biological Studies
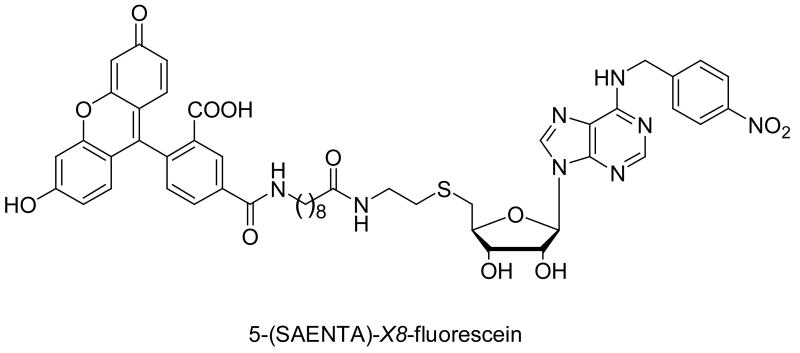
The compounds and positive controls, dipyridamole, NBMPR and lidoflazine were subjected to a flow cytometric assay with SAENTA-fluorescein (Figure 2) as the fluorescent probe.44 Flow cytometry has several advantages over the conventional radioligand binding assays, in that it eliminates radiation hazards and disposal problems and allows the use of much less amount of cells, as few as 5000 cells compared to 2 million cells per sample for comparable radioligand assays. SAENTA-fluorescein is a NBMPR analog, and it was used successfully used in several studies to determine the ENT1 inhibitory activities of NBMPR analogs.16,17 Studies with radiolabeled ligands have shown that NBMPR, dipyridamole and lidoflazine displace each other at the binding sites on the ENT1 transporter.45-47 Thus, we expected the new compounds would similarly displace SAENTA-fluorescein from the NBMPR binding site on the ENT1 transporter.
Figure 2.
Structure of SAENTA-fluorescein
Dipyridamole itself is a fluorescent molecule (Exλmax = 280 nm, Em λmax = 490 nm),48 but at the experimental wavelengths sets for SAENTA-fluorescein (Ex λ = 488 nm, Em λ = 533 nm), dipyridamole and its analogs, with the exception of compounds 9 and 10, had insignificant absorbance and emission, which did not interfere with the detection of bound SAENTA-fluorescein. Human erythroleukemia K562 cells were used as the ENT1 transporter source for the binding experiments. This cell line expresses high levels of ENT1 protein, with very limited fraction of other nucleoside transporters,49 and has been used widely for assessing ENT1 binding affinity of compounds.50-53 Compounds were first screened at 10 μM, and those compounds that showed good inhibitory activities (% Inhibition > 40 %) were further tested at 10 concentration levels to generate dose-dependent curves from which the IC50 values were derived and used to calculate the corresponding Ki values. The inhibitory activities of the highly fluorescent dipyridamole analogs like 9 and 10 could not be determined by this method.
Structure–Activity Relationships
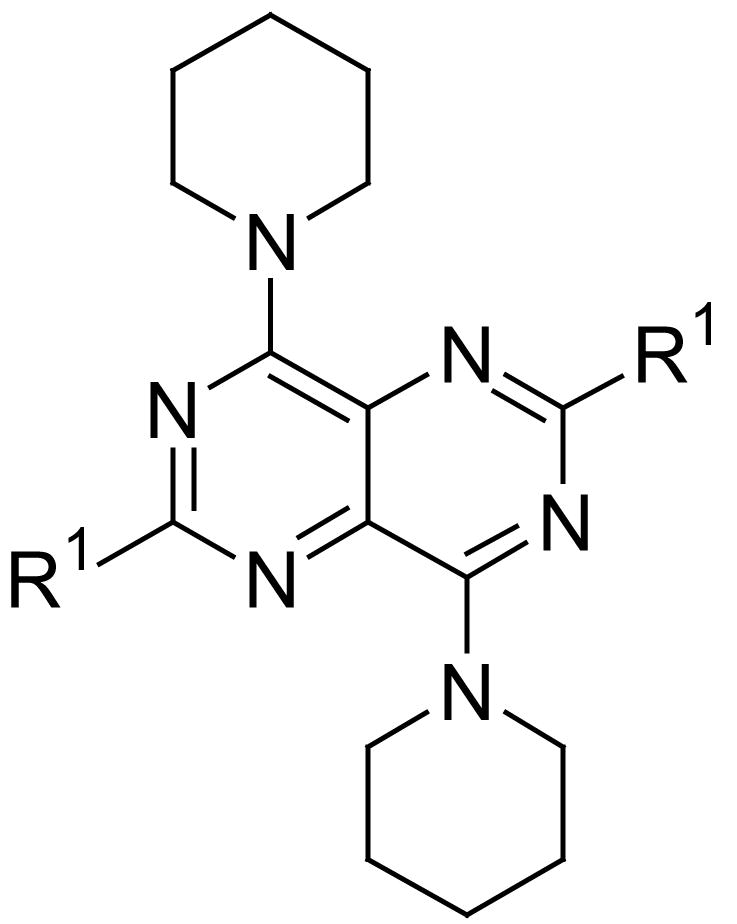
All dipyridamole analogs had the core structure of 2,4,6,8-tetra-substituted-pyrimido[5,4-d]pyrimidine. They maintained the symmetric feature as in the case of dipyridamole, with the exception of compound 8, which had two different substituents at the 4-, and 8-positions of the core pyrimidopyrimidine structure. The ENT1 inhibitory activities are summarized in Tables 1-4. In all tables, the activities of one negative control (DMSO) and three positive controls, NBMPR, lidoflazine and dipyridamole, are listed for comparison.
Table 2.
Inhibitory activities of compounds with different ring systems at the 4- and 8-positions
 | |||||
|---|---|---|---|---|---|
| Comp. | Type | R1 | ENT1 inhibitory activity in K562 cells determined by flow cytometry | ||
| %Inhibition at 10μM | IC50 (nM) | Ki (nM) | |||
| DMSO | - | - | 0.0 ± 0.7 | N. D.a | N. D. |
| NBMPR | - | - | 97.1 ± 0.4 | 7.6 | 0.43 |
| Lidoflazine | - | - | 90.0 ± 0.2 | 4954 | 279.9 |
| Dipyridamole | A |
|
86.7 ± 0.1 | 144.8 | 8.18 |
| 1 | B | 53.5 ± 0.1 | 12,229 | 690.9 | |
| 2 | A |
|
85.7 ± 0.3 | 1,764 | 99.7 |
| 3 | B | 19.3 ± 1.1 | ND | ND | |
| 4 | A |
|
70.4 ± 0.4 | 6,956 | 393 |
| 5 | B | 13.5 ± 0.3 | ND | ND | |
| 6 | A |
|
0.9 ± 0.7 | ND | ND |
| 7 | A |
|
44.6 ± 2.1 | ND | ND |
| 8b | A | - | 68.8 ± 0.0 | 7,947 | 449 |
| 9 | A |
|
N. D. | ND | ND |
| 10 | A |
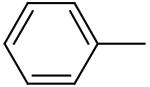
|
N. D. | ND | ND |
| 11 | A |
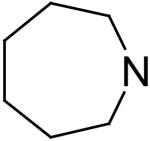
|
94.4 ± 0.2 | 15.2 | 0.86 |
| 12 | B | 28.7 ± 0.1 | ND | ND | |
| 13 | A |
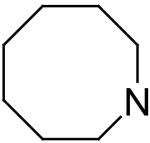
|
93.1 ± 0.3 | 8.67 | 0.49 |
| 14 | B | 78.6 ± 0.3 | 375 | 21.2 | |
| 15 | A |
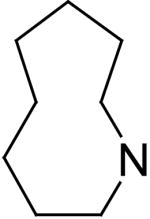
|
78.0 ± 0.4 | 13.6 | 0.77 |
| 16 | B | 69.3 ± 0.3 | 672 | 38 | |
| 17 | A |
|
85.0 ± 0.1 | 3,416 | 193 |
| 18 | B | 14.9 ± 1.7 | ND | ND | |
| 19 | A |
|
24.7 ± 0.2 | ND | ND |
| 20 | B | 16.2 ± 1.9 | ND | ND | |
ND = Not Determined.
For structure of compound 8, see Scheme 1.
Table 4.
Inhibitory activities of compounds with modification at the hydroxyl groups of dipyridamole
 | ||||
|---|---|---|---|---|
| Comp. | R1 | ENT1 inhibitory activity in K562 cells determined by flow cytometry | ||
| % Inhibition at 10μM | IC50 (nM) | Ki (nM) | ||
| DMSO | - | 0.0 ± 0.7 | NDa | ND |
| NBMPR | - | 97.1 ± 0.4 | 7.6 | 0.43 |
| Lidoflazine | - | 90.0 ± 0.2 | 4954 | 279.9 |
| DP | N(CH2CH2OH)2 | 86.7 ± 0.1 | 144.8 | 8.18 |
| 72b | OCH2CH2OH | 60.7 ± 0.7 | 5,746 | 325.2 |
| 73 |
|
13.0 ± 0.4 | ND | ND |
| 74 | N(CH2CH2OOCH3)2 | 91.3 ± 0.2 | 145 | 8.2 |
| 75 | N(CH2CH2OOCCH3)2 | 90.4 ± 0.3 | 302 | 17.1 |
| 76 | N(CH2CH2OCH3)2 | 66.2 ± 1.1 | 1,621 | 91.6 |
| 77 | N(CH2CH2OCH2CH3)2 | 8.4 ± 0.1 | ND | ND |
| 78 | N(CH2CH2OCH2CH2CH3)2 | 2.3 ± 0.4 | ND | ND |
| 79 |
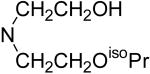
|
73.8 ± 1.1 | 76 | 4.3 |
ND = Not Determined.
Prepared by a literature procedure.40
Compounds listed in Table 1 are dipyridamole analogs with ring structures at the 4- and 8-positions of the pyrimidopyrimidine template; compounds listed in Table 2 are analogs with open-chain tertiary amines at the pyrimidopyrimidine 4- and 8-positions. Compounds listed in Table 3 have primary or secondary amine substituents at the 4- and 8-positions of the core structure. Compounds listed in Table 4 are derivatives of dipyridamole. In this study, NBMPR had a Ki of 0.43 nM, dipyridamole a Ki of 8.18 nM and lidoflazine a Ki of 279.9 nM, which are in agreement with the literature.
Table 2.
Inhibitory activities of compounds with open chain 4- and 8-position substituents
 | |||||
|---|---|---|---|---|---|
| Comp. | Type | R1 | ENT1 inhibitory activity in K562 cells determined by flow cytometry | ||
| %Inhibition at 10μM | IC50 (μM) | Ki (nM) | |||
| DMSO | - | - | 0.0 ± 0.7 | NDa | ND |
| NBMPR | - | - | 97.1 ± 0.4 | 7.6 | 0.43 |
| Lidoflazine | - | - | 90.0 ± 0.2 | 4,954 | 279.9 |
| DP | A |
|
86.7 ± 0.1 | 144.8 | 8.18 |
| 21 | A | Me2N | 49.7 ± 0.1 | 3,828 | 216.7 |
| 22 | B | 9.9 ± 0.4 | ND | ND | |
| 23 | A | Et2N | 57.0 ± 0.7 | 3,831 | 216.4 |
| 24 | B | 30.4 ± 0.1 | ND | ND | |
| 25 | A | (n-Propyl)2N | 41.6 ± 1.4 | ND | ND |
| 26 | B | 13.0 ± 0.1 | ND | ND | |
| 27 | A | (n-Butyl)2N | 53.6 ± 2.9 | ND | ND |
| 28 | B | 3.0 ± 4.3 | ND | ND | |
| 29 | A | (iso-Butyl)2N | 19.3 ± 1.8 | ND | ND |
| 30 | B | 6.6 ± 0.1 | ND | ND | |
| 31 | A | (n-Pentyl)2N | 7.2 ± 1.3 | ND | ND |
| 32 | B | -4.6 ± 1.9 | ND | ND | |
| 33 | A | (iso-Pentyl)2N | 8.2 ± 0.8 | ND | ND |
| 34 | B | 2.7 ± 1.2 | ND | ND | |
| 35 | A |
|
38.4 ± 0.4 | ND | ND |
| 36 | B | 9.9 ± 0.4 | ND | ND | |
| 37 | A |
|
-65.6 ± 1.8 | ND | ND |
| 38 | B | -8.0 ± 1.6 | ND | ND | |
ND = Not Determined.
Table 3.
Inhibitory activities of compounds with free hydrogen on the nitrogen of 4- and 8-position substituents
 | |||||
|---|---|---|---|---|---|
| Comp. | Type | R1 | ENT1 inhibitory activity in K562 cells determined by flow cytometry | ||
| %Inhibition at 10μM | IC50 (nM) | Ki (nM) | |||
| DMSO | - | - | 0.0 ± 0.7 | ND a | ND |
| NBMPR | - | - | 97.1 ± 0.4 | 7.6 | 0.43 |
| Lidoflazine | - | - | 90.0 ± 0.2 | 4954 | 279.9 |
| DP | A |
|
86.7 ± 0.1 | 144.8 | 8.18 |
| 39 | A | NH2 | 6.5 ± 0.1 | ND | ND |
| 40 | A | MeNH | 4.1 ± 0.9 | ND | ND |
| 41 | B | -1.0 ± 0.1 | ND | ND | |
| 42 | A | EtNH | 24.0 ± 1.0 | ND | ND |
| 43 | B | 0.7 ± 0.5 | ND | ND | |
| 44 | A | n-PropylNH | 79.5 ± 1.0 | 5,310 | 300 |
| 45 | B | 5.7 ± 0.3 | ND | ND | |
| 46 | A | iso-PropylNH | 81.7 ± 1.4 | 3,381 | 191 |
| 47 | B | 14.1 ± 1.2 | ND | ND | |
| 48 | A | n-ButylNH | 87.3 ± 0.1 | 2,407 | 136 |
| 49 | B | 61.6 ± 0.1 | 8,655 | 489 | |
| 50 | A | iso-ButylNH | 92.0 ± 0.2 | 673 | 38 |
| 51 | B | 24.9 ± 0.1 | ND | ND | |
| 52 | A | tert-ButylNH | 81.2 ± 0.1 | 297 | 16.8 |
| 53 | B | 28.0 ± 0.1 | ND | ND | |
| 54 | A | n-PentylNH | 56.4 ± 0.4 | 2,476 | 139.9 |
| 55 | B | 12.5 ± 1.4 | ND | ND | |
| 56 | A | iso-PentylNH | 86.8 ± 0.6 | 2,136 | 120.7 |
| 57 | B | 7.56 ± 0.1 | ND | ND | |
| 58 | A | tert-PentylNH | 94.4 ± 0.2 | 260 | 14.7 |
| 59 | B | 16.1 ± 0.3 | ND | ND | |
| 60 | A |
|
48.7± 0.9 | 7,554 | 427.6 |
| 61 | B | 6.2 ± 1.3 | ND | ND | |
| 62 | A |
|
84.1 ± 0.3 | 1,838 | 104.1 |
| 63 | B | 23.0 ± 2.6 | ND | ND | |
| 64 | A |
|
90.8 ± 0.2 | 279.7 | 15.8 |
| 65 | B | 4.9 ± 0.2 | ND | ND | |
| 66 | A |
|
80.9 ± 0.1 | 940 | 53.1 |
| 67 | B | 1.4 ± 0.3 | ND | ND | |
| 68 | A |
|
5.8 ± 0.5 | ND | ND |
| 69 | B | 6.6 ± 3.9 | ND | N. D. | |
| 70 | A |
|
11.9 ± 1.8 | ND | ND |
| 71 | B | 3.8 ± 0.2 | ND | ND | |
ND = Not Determined.
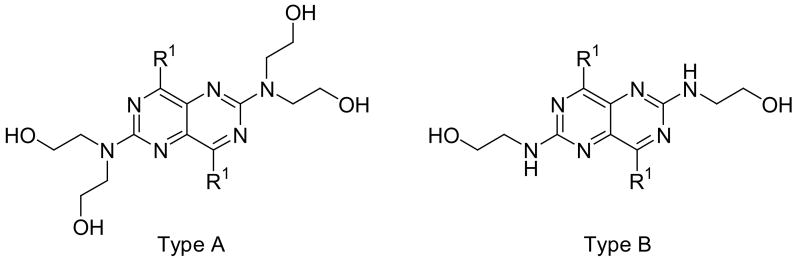
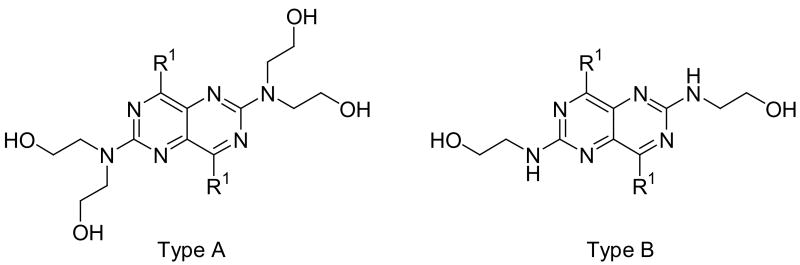
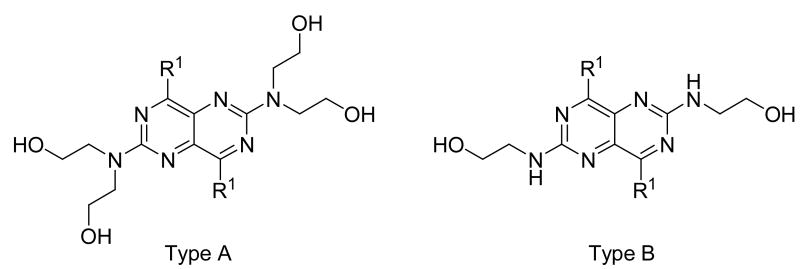
For substituents at the 4- and 8-positions of the pyrimido[5,4-d]pyrimidine, nitrogen-containing monocyclic ring structures usually gave analogs with good inhibitory activities, as in the case of compounds 2, 4, 11, 13 and 15. Increasing ring size from 5 (compound 2) to 8 (compound 13), increased inhibitory activity accordingly, with Ki values going from 99.7 nM to 0.49 nM, about 200-fold increase in inhibitory activity. Compound 13 was the most active analog in the series with comparable activity to one of best ENT1 nucleoside analog inhibitors, NBMPR (Ki of 0.43 nM). Compared to dipyridamole (Ki = 8.18 nM), compound 13 is 16 times more potent. A ring size of eight was optimal since a further increase in ring size to nine, decreased activity as can be seen with compound 15, which had a Ki of 0.77 nM. The effect of ring size could be due to an increased hydrophobic effect since the piperidine ring in dipyridamole (Ki = 8.18 nM) provided higher inhibitory activity than the morpholino or piperazine rings in compounds 5 (Ki = 6,956 nM) and 6 (practically inactive), respectively. The binding pocket at the 4- and 8-positions also has limits on the ring size it can accommodate. Further, not only does the ring size matter, but also the ring flexibility is important, with flexible rings affording higher activity than rigid ring systems. This is evident in comparing the activities of compound 15 (Ki = 13.6 nM) and compound 17 (Ki = 3,416 nM). Compounds with N-(bis-hydroxyethyl) substituents at the 2- and 6-positions (Type A in Tables 1-4) were much more potent than the corresponding N-(monohydroxyethyl) substituted analogs (Type B in Tables 1-4).
The open chain analogs (compound 21-38) were less active than the cyclic counterparts. Compounds with carbon chain length from 1 to 4 (compounds 21, 23, 25 and 27) exhibited low inhibitory activities. Increasing the chain length (compound 31), or branching it (compounds 29 and 33) led to a decrease in activity. Compound 35 has polar oxygen atoms in the side chain, which also resulted in low activity. Compound 37 has dibenzylamino groups at the 4- and 8-positions and was inactive. In this set also, the N-(monohydroxyethyl) substituted analogs (compounds 22, 24, 26, 28, 30, 32, 34, 36, and 38) were less active than the N-(bis-hydroxyethyl) counterparts.
The analogues which contained a primary or secondary amine (compounds 39-71) at the 4- and 8-positions had lower inhibitory activities relative to dipyridamole. The most active compounds in the group, 52, 58 and 64 were only about half as active as dipyridamole. These are analogs with tert-butylamino, iso-pentylamino and cyclopentylamnio groups at the 4- and 8-positions. Again, analogs with N-(monohydroxyethyl) substitution were less active than the N-(bis-hydroxyethyl) counterparts.
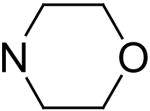
Compounds 72-79 are 2- and 6-substituted dipyridamole analogs. The presence of a 2′-hydroxyethoxy group at the 2- and 6-positions (compound 72) resulted in a steep drop in activity, compared to dipyridamole. However, compound 72 exhibited higher activity than compound 1, the N-(monohydroxyethyl) counterpart of dipyridamole. This indicates that a hydrogen atom on the 2- and 6-position nitrogen is unfavorable for potent activity. Compound 73 has the diethanolamino groups at the 2- and 6-postions locked into morpholino rings; and this modification caused a loss of activity. Esterification of dipyridamole (compound 74 and 75) maintained relatively good activity compared to dipyridamole, which indicates that free hydroxyl groups are not necessary for activity. Esterification introduces additional oxygen atoms, which might participate in additional hydrogen-bonding that probably compensates for the loss of activity caused by an increase in lipophilicity. In contrast, ether type lipophilic modification at same positions caused a decrease in activity as in the case compounds 76 to 78. Interestingly, compound 79, which has one free hydroxyl group at the 2- and 6-positions, exhibited a higher potency than dipyridamole. The reasons for the higher potency of 79 relative to dipyridamole are not apparent. Some compounds, namely 7, 25, and 27, had a % inhibition above 40 %, but no IC50s, could be determined due to low solubility.
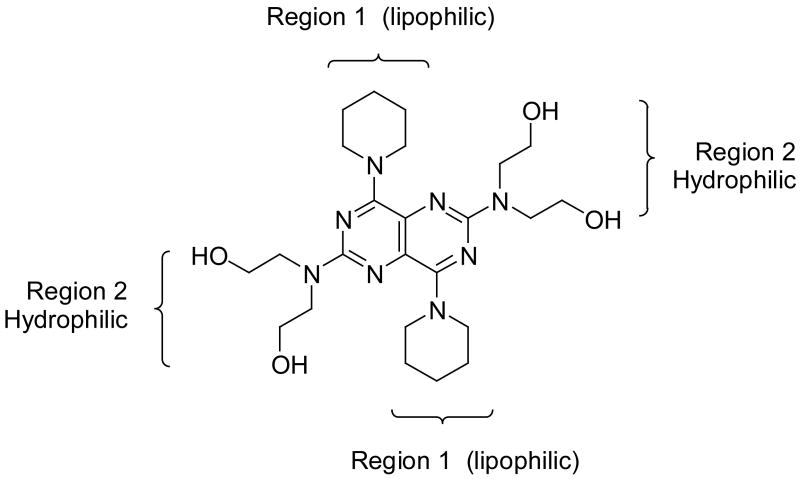
These dipyridamole analogs had modifications at two important regions with regard to ENT1 inhibitory activity (see Figure 3). Region 1 should be lipophilic to obtain the highest ENT1 inhibitory activities, with single nitrogen-containing flexible rings being preferred to carbocyclic, morpholine, piperazine or rigid multicyclic ring systems. For the nitrogen-containing flexible rings, an 8-membered ring is optimal. Region 2 should be hydrophilic region with diethanolamino group providing optimal activity, although it is not essential; small lipophilic modifications over the hydroxyl groups are well tolerated.
Figure 3.
Representative regions for dipyridamole analogs
Conclusion
In this study, a substantial number of dipyridamole analogs were synthesized and explored for their inhibitory activity against ENT1 transporter using a flow cytometric method. Compounds with much higher activity than dipyridamole were identified for the first time, with the best, compound 13, being 16 times more active than dipyridamole, and having comparative activity to the potent ENT1 standard inhibitor NBMPR. The study has also revealed important structural determinants for ENT1 inhibitory activity in this series, among which are the requirements for a lipophilic medium to large size nitrogen containing lipophilic rings at the 4- and 8-positions, and hydrophilic, hydrogen-bond acceptor substituents at the 2- and 6-positions. The newly identified higher potency dipyridamole analog, compound 13, may facilitate the therapeutic exploitation of the ENT1 inhibitory activity of dipyridamole and related compounds.
Experimental Section
Chemistry
Thin-layer chromatography (TLC) was conducted on silica gel plates (Analtech). Compounds were visualized by UV light (254 and 365 nm). 1D NMR spectra were recorded on a Varian Inova 500 MHz NMR instruments by using CDCl3 or (CD3)2SO as solvents and tetramethylsilane (TMS) as an internal standard. Flash column chromatography was performed on Fisher silica gel (170-400 mesh). Melting points were determined using a Fisher-Johns Melting Point Apparatus and were reported uncorrected. Mass spectra were obtained on a Bruker-HP ESQUIRE Ion Trap LC/MS(n) system. Elemental analyses were performed by Atlantic Microlab Inc., Norcross, GA. All solvents and reagents were purchased from Aldrich or other major chemical companies, and used without further purification. All reactions were carried under argon gas.
2,6-Bis(diethanolamino)-4,8-disubstituted-pyrimido[5,4-d]pyrimidine, or 2,6-diethanolamino-4,8-disubstituted-pyrimido[5,4-d]pyrimidine. General procedure I
To a solution of 2,4,6,8-tetra-chloro-pyrimido[5,4-d]pyrimidine (TCPP) (0.27 g, 1 mmole) in anhydrous THF (10 ml), appropriate amine (4.2 mmole) was added in this first step. The reaction was stirred on an ice-water bath for 20 min, and then water (100 ml) was added to precipitate the reaction intermediate. After drying over P2O5, the intermediate was dissolved in DMSO (3 ml), and an appropriate amine (diethanolamine, ethanolamine or morpholine) (3 ml) was added and the reaction was heated at 150 °C for 6 hours with stirring. Then, the product was purified by flash silica gel chromatography.
2,6-Bis(diethanolamino)-4,8-disubstituted-pyrimido[5,4-d]pyrimidine. General procedure II
To a solution of 2,4,6,8-tetra-chloro-pyrimido[5,4-d]pyrimidine (TCPP) (0.27 g, 1 mmole) in anhydrous THF (10 ml), appropriate Grignard reagent (2.1 mmole) was added at this first step. The reaction was stirred in ice-water bath for 20min, and then water (100 ml) was added to precipitate the reaction intermediate. After drying over P2O5, the intermediate was dissolved in DMSO (3 ml), and diethanolamine (3 ml) was added; and the reaction was heated at 150 °C for 6 hours with stirring. Then, the product was purified by flash silica gel chromatography.
2,6-Bis(dialkoxylethylamino)-4,8-disubstituted-pyrimido[5,4-d]pyrimidine. General procedure III
NaH (60% in mineral oil, 0.28 g, 7 mmole) was added to a solution of dipyridamole (0.35 g, 0.69 mmole) in anhydrous DMF (10 ml), and the reaction was stirred at room temperature for 2 hours; and then appropriate alkyl halide (32 mmole) was added, and the reaction was stirred for overnight. The reaction mixture was participated between CH2Cl2 (60 ml) and H2O (50 ml), and the organic layer was separated, the left aqueous solution was extracted with CH2Cl2 (20 ml × 2), and all organic solutions were incorporated and dried over anhydrous Na2SO4. Then the CH2Cl2 was removed under reduced pressure, and the residue was subjected to flash silica gel chromatography for purification of the product.
2,6-Diethanolamino-4,8-dipiperidino-pyrimido[5,4-d]pyrimidine (1)
Compound 1 was prepared by general procedure I with piperidine (0.41 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. The product was purified by flash silica gel chromatography (CH2Cl2/MeOH=16/1) to give a yellow powdery solid (162 mg, 39%). Mp: 152-153 °C; MS (ESI) m/z 417 (M + H)+, 439 (M + Na)+; 1H NMR (DMSO-d6) δ 6.016 (t, 2H, 2 × NH, disappeared after D2O, J = 5.5 Hz), 4.606 (t, 2H, 2 × OH, disappeared after D2O, J = 5.5 Hz), 4.057 (br s, 8H, 2 × N(CH2CH2)2CH2), 3.513 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.269 (q, 4H, 2 × NHCH2CH2OH, J1 = 5.5 Hz, J2 = 6 Hz), 1.641 (br d, 4H, 2 × N(CH2CH2)2CH2, J = 4.5 Hz), 1.592 (br d, 8H, 2 × N(CH2CH2)2CH2, J = 4.5 Hz); Anal. (C20H32N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-dipyrrolidinyl-pyrimido[5,4-d]pyrimidine (2)
Compound 2 was prepared by general procedure I with pyrroline (0.35 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=15/1) to give a yellow power solid (252 mg, 53%). Mp: 212-213 °C; MS (ESI) m/z 477 (M + H)+; 1H NMR (DMSO-d6) δ 4.688 (m, 4H, 4 × OH, disappeared after D2O exchange), 4.119 (br s, 8H, 2 × N(CH2CH2)2), 3.592 (br s, 16H, 2 × N(CH2CH2OH)2), 1.877 (br s, 8H, 2 × N(CH2CH2)2); Anal. Calcd (C22H36N8O4): C, H, N.
2,6-Diethanolamino-4,8-dipyrrolidinyl-pyrimido[5,4-d]pyrimidine (3)
Compound 3 was prepared by general procedure I with pyrroline (0.35 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=15/1) to give a yellow power solid (176 mg, 45%). Mp: 219-220 °C; MS (ESI) m/z 389 (M + H)+, 411 (M + Na)+; 1H NMR (DMSO-d6) δ 5.774 (t, 2H, 2 × NH, disappeared after D2O), 4.591 (t, 2H, 2 × OH, disappeared after D2O exchange), 4.006 (br s, 8H, 2 × N(CH2CH2)2), 3.505 (q, 4H, 2 × NHCH2CH2OH, J = 6 Hz), 3.292 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz), 1.863 (br s, 8H, 2 × N(CH2CH2)2); Anal. (C18H28N8O2) C, H, N.
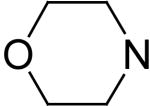
2,6-Bis(diethanolamino)-4,8-dimorpholino-pyrimido[5,4-d]pyrimidine (4)
Compound 4 was prepared by general procedure I with morpholine (0.37 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=16/1) to give a yellow power solid (274 mg, 54%). Mp: 205-206 °C; MS (ESI) m/z 509 (M + H)+, 531 (M + Na)+; 1 H NMR (DMSO-d6) δ 4.689 (t, 4H, 4 × OH, disappeared after D2O), 4.121 (br s, 8H, 2 × N(CH2CH2)2O), 3.715 (t, 8H, 2 × N(CH2CH2)2O), 3.573 (br s, 16H, 2 × N(CH2CH2OH)2); Anal. (C22H36N8O6 · 0.5 H2O) C, H, N.
2,6-Diethanolamino-4,8-dimorpholino-pyrimido[5,4-d]pyrimidine (5)
Compound 5 was prepared by general procedure I with morpholine (0.37 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=15/1) to give a yellow power solid (211 mg, 50%). Mp: 203-204 °C; MS (ESI) m/z 421 (M + H)+, 443 (M + Na)+; 1H NMR (DMSO-d6) δ 6.186 (t, 2H, 2 × NH, disappeared after D2O), 4.619 (t, 2H, 2 × OH, disappeared after D2O), 4.128 (br s, 8H, 2 × N(CH2CH2)2O), 3.708 (t, 8H, 2 × N(CH2CH2)2O), 3.504 (q, 4H, 2 × NHCH2CH2OH), 3.254 (q, 4H, 2 × NHCH2CH2OH); Anal. (C18H18N8O4 · 0.5 H2O) C, H, N.
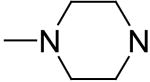
2,6-Bis(diethanolamino)-4,8-di-(N-methyl-piperazino)-pyrimido[5,4-d]pyrimidine (6)
Compound 6 was prepared by general procedure I with 1-methylpiperazine (0.47 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=1/1) to give a yellow power solid (273 mg, 51%). Mp: 199-200 °C; MS (ESI) m/z 535 (M + H)+, 557 (M + Na)+; 1H NMR (DMSO-d6) δ 4.719 (t, 4H, 4 × OH, disappeared after D2O), 4.122 (br s, 8H, 2 × N(CH2CH2)2NCH3), 3.591 (br s, 16H, 2 × N(CH2CH2OH)2), 2.434 (t, 8H, 2 × N(CH2CH2)2NCH3), 2.219 (s, 6H, 2 × CH3); Anal. (C24H42N10O4) C, H, N.
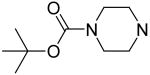
2,6-Bis(diethanolamino)-4,8-di-(N-BOC-piperazino)-pyrimido[5,4-d]pyrimidine (7)
Compound 7 was prepared by general procedure I with N-BOC-piperazine (0.78 g, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=5/1) to give a yellow power solid (304 mg, 43%). Mp: 223-224 °C; MS (ESI) m/z 707 (M + H)+, 729 (M + Na)+; 1H NMR (DMSO-d6) δ 4.749 (br t, 4H, 4 × OH, disappeared after D2O), 4.121 (br s, 8H, 2 × N(CH2CH2)2N-BOC), 3.611 (s, 16H, 2 × N(CH2CH2OH)2), 3.489 (br s, 8H, 2 × N(CH2CH2)2N-BOC), 1.465 (s, 18H, 6 × CH3). Anal. (C32H54N10O8) C, H, N.
2,6-Bis(diethanolamino)-4-piperazino-8-(N-Cbz-piperazino)-pyrimido[5,4-d]pyrimidine (8)
Compound 8 was prepared by general procedure I with benyl piperazine-1-carboxylate (0.93 g, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=1.5/1) to give a yellow power solid (77 mg, 12%). Mp: 133-134 °C; MS (ESI) m/z 641 (M + H)+, 663 (M + Na)+; 1H NMR (DMSO-d6) δ 7.388 (d, 3H, Ar-H-3, Ar-H-4, Ar-H-5), 7.335 (m, 2H, Ar-H-2, Ar-H-6), 5.121 (s, 2H, PhCH2), 4.699 (t, 4H, 4 × OH, disappeared after D2O), 4.119 (br s, 4H, N(CH2CH2)2NH), 4.031 (br s, 4H, N(CH2CH2)2NCbz), 3.570 (br s, 21H, 2 × N(CH2CH2OH)2, N(CH2CH2)2NH), 2.789 (br s, 4H, N(CH2CH2)2NCbz); Anal. (C30H44N10O6) C, H, N.
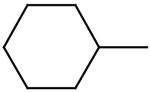
2,6-Bis(diethanolamino)-4,8-dicyclohexyl-pyrimido[5,4-d]pyrimidine (9)
Compound 9 was prepared by general procedure II with cyclehexylmagnesium chloride solution (2.0 M in diethyl ether, 1.05 ml, 2.1 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=24/1) to give a yellow power solid (226 mg, 45%). Mp: 226-228 °C; MS (ESI) m/z 503 (M + H)+, 525 (M + Na)+; 1H NMR (DMSO-d6) δ 4.782 (t, 4H, 4 × OH, disappeared after D2O), 3.717 (br s, 8H, 2 × N(CH2CH2OH)2), 3.675 (br s, 8H, 2 × N(CH2CH2OH)2), 3.602 (m, 2H, 2 × CH(CH2CH2)2CH2), 1.892 − 1.815 (m, 8H, 2 × CH(CH2CH2)2CH2), 1.753 (d, 2H, 2 × CH(CH2CH2)2CHAHB), 1.589 − 1.397 (m, 8H, 2 × CH(CH2CH2)2CH2), 1.283 (m, 2H, 2 × CH(CH2CH2)2CHAHB); Anal. (C26H42N6O4) C, H, N.
2,6-Bis(diethanolamino)-4,8-diphenyl-pyrimido[5,4-d]pyrimidine (10)
Compound 10 was prepared by general procedure II with phenylmagnesium chloride solution (2.0 M in tetrahydrofuran, 1.05 ml, 2.1 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=9/1) to give a red power solid (29 mg, 5.9%). Mp: 208-209 °C; MS (ESI) m/z 491 (M + H)+, 513 (M + Na)+; 1H NMR (DMSO-d6) δ 8.470 (m, 4H, 2 × Ar-H-2, 2 × Ar-H-6), 7.557 (m, 6H, 2 × Ar-H-3, 2 × Ar-H-4, 2 × Ar-H-5), 4.805 (t, 4H, 4 × OH, disappeared after D2O, J = 5Hz), 3.793 (br s, 8H, 2 × N(CH2CH2OH)2), 3.708 (t, 8H, 2 × N(CH2CH2OH)2, J = 5Hz); Anal. (C26H30N6O4) C, H, N.
2,6-Bis(diethanolamino)-4,8-dihexamethyleneimino-pyrimido[5,4-d]pyrimidine (11)
Compound 11 was prepared by general procedure I with hexamethyleneimine (0.48 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (Hexane/Acetone=2/1) to give a yellow power solid (213 mg, 40%). Mp: 212-213 °C; MS (ESI) m/z 533 (M + H)+; 1H NMR (DMSO-d6) δ 4.677 (br t, 4H, 4 × OH, disappeared after D2O), 4.129 (br, s, 8H, 2 × N(CH2CH2CH2)2), 3.572 (s, 16H, 2 × N(CH2CH2OH)2), 1.775 (br s, 8H, 2 × N(CH2CH2CH2)2), 1.511 (br s, 8H, 2 × N(CH2CH2CH2)2); Anal. (C26H44N8O4) C, H, N.
2,6-Diethanolamino-4,8-dihexamethyleneimino-pyrimido[5,4-d]pyrimidine (12)
Compound 12 was prepared by general procedure I with hexamethyleneimine (0.48 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=20/1) to give a yellow power solid (106 mg, 24%). Mp: 166-167 °C; MS (ESI) m/z 445 (M + H)+, 467 (M + Na)+; 1H NMR (DMSO-d6) δ 5.787 (q, 2H, 2 × NH, disappeared after D2O, J = 5.5 Hz), 4.592 (t, 2H, 2 × OH, disappeared after D2O, J = 5.5 Hz), 4.112 (br s, 8H, 2 × N(CH2CH2CH2)2), 3.514 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.246 (t, 4H, 2 × NHCH2CH2OH, J1 = 5.5 Hz, J2 = 6 Hz), 1.776 (br s, 8H, 2 × N(CH2CH2CH2)2), 1.500 (br s, 8H, 2 × N(CH2CH2CH2)2); Anal. (C22H36N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-diheptamethyleneimino-pyrimido[5,4-d]pyrimidine (13)
Compound 13 was prepared by general procedure I with heptamethyleneimine (0.53 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (Hexane/Acetone=3/1) to give a yellow power solid (219 mg, 39%). Mp: 204-205 °C; MS (ESI) m/z 561 (M + H)+; 1H NMR (DMSO-d6) δ 4.681 (t, 4H, 4 × OH, disappeared after D2O), 4.091 (br, s, 8H, 2 × N(CH2CH2CH2)2CH2), 3.576 (br s, 16H, 2 × N(CH2CH2OH)2), 1.782 (br s, 8H, 2 × N(CH2CH2CH2)2CH2), 1.541 (br s, 8H, 2 × N(CH2CH2CH2)2CH2), 1.479 (br s, 4H, 2 × N(CH2CH2CH2)2CH2); Anal. (C28H48N8O4) C, H, N.
2,6-Diethanolamino-4,8-diheptamethyleneimino-pyrimido[5,4-d]pyrimidine (14)
Compound 14 was prepared by general procedure I with heptamethyleneimine (0.53 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (Hexane/Acetone=2/1) to give a yellow power solid (246 mg, 43%). Mp: 150-151 °C; MS (ESI) m/z 573 (M + H)+, 495 (M + Na)+, 511 (M + K)+; 1H NMR (DMSO-d6) δ 5.751 (t, 2H, 2 × NH, disappeared after D2O, J = 6 Hz), 4.592 (t, 2H, 2 × OH, disappeared after D2O, J = 5.5 Hz), 4.077 (br, s, 8H, 2 × N(CH2CH2CH2)2CH2), 3.508 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.256 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 6 Hz), 1.786 (br s, 8H, 2 × N(CH2CH2CH2)2CH2), 1.527 (br s, 8H, 2 × N(CH2CH2CH2)2CH2), 1.468 (br s, 4H, 2 × N(CH2CH2CH2)2CH2); Anal. (C24H40N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-di-octomethyleneimino-pyrimido[5,4-d]pyrimidine (15)
Compound 15 was prepared by general procedure I with octomethyleneimine (0.54 g, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (Hexane/Acetone=2.5/1) to give a yellow power solid (65 mg, 11%). Mp: 213-214 °C; MS (ESI) m/z 589 (M + H)+; 1H NMR (DMSO-d6) δ 4.686 (t, 4H, 4 × OH, disappeared after D2O), 4.055 (br, s, 8H, 2 × N(CH2CH2CH2CH2)2), 3.602 (m, 16H, 2 × N(CH2CH2OH)2), 1.811 (s, 8H, 2 × N(CH2CH2CH2CH2)2), 1.648 (s, 8H, 2 × N(CH2CH2CH2CH2)2), 1.451 (s, 8H, 2 × N(CH2CH2CH2CH2)2); Anal. Calcd (C30H52N8O4): C 61.20, H 8.90, N 19.03; Found: C 60.73, H 8.84, N 18.87.
2,6-Diethanolamino-4,8-di-octomethyleneimino-pyrimido[5,4-d]pyrimidine (16)
Compound 16 was prepared by general procedure I with octomethyleneimine (0.54 g, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (Hexane/Acetone=2.5/1) to give a yellow power solid (60 mg, 12%). Mp: 167 °C; MS (ESI) m/z 501 (M + H)+, 523 (M + Na)+; 1H NMR (DMSO-d6) δ 5.746 (t, 2H, 2 × NH, disappeared after D2O, J = 6 Hz), 4.601 (t, 2H, 2 × OH, disappeared after D2O, J = 5.5 Hz), 4.039 (br, s, 8H, 2 × N(CH2CH2CH2CH2)2), 3.514 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.284 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 1.822 (br s, 8H, 2 × N(CH2CH2CH2CH2)2), 1.645 (br s, 8H, 2 × N(CH2CH2CH2CH2)2), 1.440 (s, 8H, 2 × N(CH2CH2CH2CH2)2); Anal. (C26H44N8O2) C, H, N.
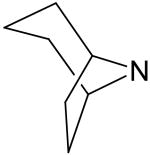
2,6-Bis(diethanolamino)-4,8-dinontropano-pyrimido[5,4-d]pyrimidine (17)
Compound 17 was prepared by general procedure I with nontropane54 (0.47 g, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=10/1) to give a yellow power solid (250 mg, 45%). Mp: 244-245 °C; MS (ESI) m/z 557 (M + H)+, 579 (M + Na)+; 1H NMR56 (DMSO-d6) δ 6.203 (br s, 2H), 4.908 (br s, 2H), 4.695 (t, 4H, 4 × OH, disappeared after D2O), 3.581 (br s, 16H, 2 × N(CH2CH2OH)2), 1.942-1.455 (series of br s, 20H); Anal. (C28H44N8O4) C, H, N.
2,6-Diethanolamino-4,8-dinontropanopyrimido[5,4-d]pyrimidine (18)
Compound 18 was prepared by general procedure I with nontropane54 (0.47 g, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 16/1) to give a yellow power solid compound 7 (128 mg, 27%). Mp: 254-255 °C; MS (ESI) m/z 469 (M + H)+, 491 (M + Na)+; 1H NMR57 (DMSO-d6) δ 6.319 (br s, 2H), 5.901 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5.5 Hz), 4.909 (br s, 2H), 4.584 (t, 2H, 2 × OH, disappeared after D2O, J = 5.5 Hz), 3.515 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.243 (d, 4H, 2 × NHCH2CH2OH, J = 5.5 Hz), 1.938-1.430 (series of br s, 20H); Anal. (C24H36N8O2) C, H, N.
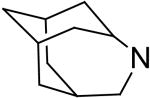
2,6-Bis(diethanolamino)-4,8-di-(4-azatricyclo[4.3.1.13,8]undecane)-pyrimido[5,4-d]pyrimidine (19)
Compound 19 was prepared by general procedure I with 4-azatricyclo[4.3.1.13,8]undecane55 (0.64 g, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=20/1) to give a yellow power solid (262 mg, 41%). Mp: 252-253 °C; MS (ESI) m/z 637 (M + H)+, 659 (M + Na)+; 1H NMR57 (DMSO-d6) δ 5.765 (br s, 2H), 4.680 (t, 4H, 4 × OH, disappeared after D2O), 3.890 (br s, 4H), 3.589 (br s, 16H, 2 × N(CH2CH2OH)2), 2.299 (br s, 2H), 1.959 (t, 4H), 1.929 (br s, 8H), 1.759-1.733 (br d, 4H), 1.604-1.516 (m, 8H); Anal. (C34H52N8O4 · 0.5 H2O) C, H, N.
2,6-Diethanolamino-4,8-di-(4-azatricyclo[4.3.1.13,8]undecane)-pyrimido[5,4-d]pyrimidine (20)
Compound 20 was prepared by general procedure I with 4-azatricyclo[4.3.1.13,8]undecane55 (0.64 g, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (Hexane/Acetone=2.5/1) to give a yellow power solid (198 mg, 36%). Mp: 194-196 °C; MS (ESI) m/z 549 (M + H)+, 571 (M + Na)+; 1H NMR55 (DMSO-d6) δ 5.746 (t, 2H, 2 × NH, disappeared after D2O, J = 6 Hz), 5.720 (br s, 2H), 4.580 (t, 2H, 2 × OH, disappeared after D2O, J = 5.5 Hz), 3.834 (br s, 4H), 3.505 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.289 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 6 Hz), 2.287 (br s, 2H), 1.951-1.926 (m, 12H), 1.793-1.767 (br d, 4H), 1.605-1.510 (m, 8H); Anal. (C30H44N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-di-(dimethylamino)-pyrimido[5,4-d]pyrimidine (21)
Compound 21 was prepared by general procedure I with dimethylamine solution (2.0 M in tetrahydrofuran, 2.1 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=12/1) to give a yellow power solid (174 mg, 41%). Mp: 207-208 °C; MS (ESI) m/z 425 (M + H)+, 447 (M + Na)+; 1H NMR (DMSO-d6) δ 4.704 (t, 4H, 4 × OH, disappeared after D2O, J = 5.5Hz), 3.597 (m, 16H, 2 × N(CH2CH2OH)2, J = 5.5Hz), 3.409 (br s, 12H, 4 × CH3); Anal. (C18H32N8O4) C, H, N.
2,6-Diethanolamino-4,8-di-(dimethylamino)-pyrimido[5,4-d]pyrimidine (22)
Compound 22 was prepared by general procedure I with dimethylamine solution (2.0 M in tetrahydrofuran, 2.1 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=9/1) to give a yellow power solid (40 mg, 12%). Mp: 159-161 °C; MS (ESI) m/z 337 (M + H)+, 359 (M + Na)+; 1H NMR (DMSO-d6) δ 5.954 (br s, 2H, 2 × NH, disappeared after D2O), 4.634 (t, 2H, 2 × OH, disappeared after D2O), 3.509 (q, 4H, 2 × NHCH2CH2OH), 3.371 (br s, 12H, 4 × CH3), 3.284 (t, 4H, 2 × NHCH2CH2OH); Anal. (C14H24N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-di-(diethylamino)-pyrimido[5,4-d]pyrimidine (23)
Compound 23 was prepared by general procedure I with diethylamine (0.44 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=10/1) to give a yellow power solid (187 mg, 39%). Mp: 165-166 °C; MS (ESI) m/z 481 (M + H)+, 503 (M + Na)+; 1H NMR (DMSO-d6) δ 4.692 (t, 4H, 4 × OH, disappeared after D2O), 3.914 (br s, 8H, 4 × CH2CH3), 3.589 (br s, 16H, 2 × N(CH2CH2OH)2), 1.205 (t, 12H, 4 × CH2CH3); Anal. (C22H40N8O4) C, H, N.
2,6-Diethanolamino-4,8-di-(diethylamino)-pyrimido[5,4-d]pyrimidine (24)
Compound 24 was prepared by general procedure I with diethylamine (0.44 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=16/1) to give a yellow power solid (122 mg, 31%). Mp: 127 °C; MS (ESI) m/z 393 (M + H)+, 415 (M + Na)+; 1H NMR (DMSO-d6) δ 5.787 (t, 2H, 2 × NH, disappeared after D2O, J = 5.5 Hz), 4.599 (t, 2H, 2 × OH, disappeared after D2O, J = 6 Hz), 3.902 (br s, 8H, 4 × CH2CH3), 3.506 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 6 Hz), 3.265 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 1.198 (t, 12H, 4 × CH2CH3); Anal. (C18H32N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-di-(dipropylamino)-pyrimido[5,4-d]pyrimidine (25)
Compound 25 was prepared by general procedure I with dipropylamine (0.58 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=18/1) to give a yellow power solid (81 mg, 15%). Mp: 150-151 °C; MS (ESI) m/z 537 (M + H)+, 559 (M + Na)+, 575 (M + K)+; 1H NMR (DMSO-d6) δ 4.707 (br s, 4H, 4 × OH, disappeared after D2O), 3.846 (br s, 8H, 4 × CH2CH2CH3), 3.586 (br s, 16H, 2 × N(CH2CH2OH)2), 1.637 (q, 8H, 4 × CH2CH2CH3, J = 7.5 Hz), 0.878 (t, 12H, 4 × CH2CH2CH3, J = 7.5 Hz); Anal. (C26H48N8O4) C, H, N.
2,6-Diethanolamino-4,8-di-(dipropylamino)-pyrimido[5,4-d]pyrimidine (26)
Compound 26 was prepared by general procedure I with dipropylamine (0.58 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=12/1) to give a yellow power solid (85 mg, 19%). Mp: 144-145 °C; MS (ESI) m/z 449 (M + H)+, 471 (M + Na)+; 1H NMR (DMSO-d6) δ 5.723 (t, 2H, 2 × NH, disappeared after D2O, J = 6 Hz), 4.618 (t, 2H, 2 × OH, disappeared after D2O, J = 5.5 Hz), 3.837 (br s, 8H, 4 × CH2CH2CH3), 3.518 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.288 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 6 Hz), 1.644 (m, 8H, 4 × CH2CH2CH3, J = 7.5 Hz), 0.876 (t, 12H, 4 × CH2CH2CH3, J = 7.5 Hz); Anal. (C22H40N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-di-(dibutylamino)-pyrimido[5,4-d]pyrimidine (27)
Compound 27 was prepared by general procedure I with dibutylamine (0.71 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 27/1) to give a yellow power solid (157 mg, 27%). Mp: 126-127 °C; MS (ESI) m/z 593 (M + H)+, 615 (M + Na)+; 1H NMR (DMSO-d6) δ 4.705 (t, 4H, 4 × OH, disappeared after D2O, J = 5 Hz), 3.876 (br s, 8H, 4 × CH2CH2CH2CH3), 3.581 (br s, 16H, 2 × N(CH2CH2OH)2), 1.591 (m, 8H, 4 × CH2CH2CH2CH3, J = 8 Hz), 1.308 (m, 8H, 4 × CH2CH2CH2CH3, J1 = 8 Hz, J2 = 7.5 Hz), 0.903 (t, 12H, 4 × CH3, J = 7.5 Hz); Anal. (C30H56N8O4) C, H, N.
2,6-Diethanolamino-4,8-di-(dibutylamino)-pyrimido[5,4-d]pyrimidine (28)
Compound 28 was prepared by general procedure I with dibutylamine (0.71 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (Hexane/Acetone = 5/1) to give a yellow power solid (124 mg, 22%). Mp: 129-130 °C; MS (ESI) m/z 505 (M + H)+; 1H NMR (DMSO-d6) δ 5.687 (t, 2H, 2 × NH, disappeared after D2O, J = 5.5 Hz), 4.615 (t, 2H, 2 × OH, disappeared after D2O, J = 5.5 Hz), 3.870 (br s, 8H, 4 × CH2CH2CH2CH3), 3.505 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.267 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 6 Hz), 1.596 (m, 8H, 4 × CH2CH2CH2CH3, J = 7.5 Hz), 1.308 (m, 8H, 4 × CH2CH2CH2CH3, J = 7.5 Hz), 0.907 (t, 12H, 4 × CH3, J = 7.5 Hz); Anal. (C26H48N8O2 · 0.5 H2O) C, H, N.
2,6-Bis(diethanolamino)-4,8-di-(diisobutylamino)-pyrimido[5,4-d]pyrimidine (29)
Compound 29 was prepared by general procedure I with diisobutylamine (0.73 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=14/1) to give a yellow power solid (59 mg, 10%). Mp: 169-171°C; MS (ESI) m/z 593 (M + H)+, 615 (M + Na)+; 1H NMR (DMSO-d6) δ 4.669 (br s, 4H, 4 × OH, disappeared after D2O), 3.808 (br s, 8H, 4 × CH2CH(CH3)2), 3.528 (br s, 16H, 2 × N(CH2CH2OH)2), 1.890 (br s, 4H, 4 × CH2CH(CH3)2), 0.752 (br s, 24H, 4 × CH2CH(CH3)2); Anal. (C30H56N8O4) C, H, N.
2,6-Diethanolamino-4,8-di-(diisobutylamino)-pyrimido[5,4-d]pyrimidine (30)
Compound 30 was prepared by general procedure I with diisobutylamine (0.73 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=18/1) to give a yellow power solid (212 mg, 42%). Mp: 154°C; MS (ESI) m/z 505 (M + H)+, 527 (M + Na)+; 1H NMR (DMSO-d6) δ 5.821 (t, 2H, 2 × NH, disappeared after D2O, J = 5.5 Hz), 4.634 (t, 2H, 2 × OH, disappeared after D2O, J = 5.5 Hz), 3.881 (br s, 8H, 4 × CH2CH(CH3)2), 3.532 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.276 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 1.983 (m, 4H, 4 × CH2CH(CH3)2, J = 6.5 Hz), 0.840 (d, 24H, 4 × CH2CH(CH3)2, J = 6.5 Hz); Anal. (C26H48N8O2 · 0.5 H2O) C, H, N.
2,6-Bis(diethanolamino)-4,8-di-(dipentylamino)-pyrimido[5,4-d]pyrimidine (31)
Compound 31 was prepared by general procedure I with dipentylamine (0.85 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 20/1) to give a yellow power solid (145 mg, 22%). Mp: 130-131 °C; MS (ESI) m/z 649 (M + H)+, 671 (M + Na)+; 1H NMR (DMSO-d6) δ 4.706 (t, 4H, 4 × OH, disappeared after D2O), 3.865 (br s, 8H, 4 × CH2(CH2)3CH3), 3.578 (q, 16H, 2 × N(CH2CH2OH)2), 1.604 (m, 8H, 4 × CH2CH2(CH2)2CH3), 1.332 - 1.229 (m, 16H, 4 × CH2CH2(CH2)2CH3), 0.869 (t, 12H, 4 × CH3); Anal. (C34H64N8O4 · 0.5 H2O) C, H, N.
2,6-Diethanolamino-4,8-di-(dipentylamino)-pyrimido[5,4-d]pyrimidine (32)
Compound 32 was prepared by general procedure I with dipentylamine (0.85 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (Hexane/Acetone = 15/1) to give a yellow power solid (26 mg, 4.6%). Mp: 128-129 °C; MS (ESI) m/z 561 (M + H)+, 583 (M + Na)+; 1H NMR (DMSO-d6) δ 5.659 (t, 2H, 2 × NH, disappeared after D2O, J = 5.5 Hz), 4.619 (t, 2H, 2 × OH, disappeared after D2O, J = 5.5 Hz), 3.860 (br s, 8H, 4 × CH2(CH2)3CH3), 3.504 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.269 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 1.609 (m, 8H, 4 × CH2CH2(CH2)2CH3, J1 = 7.5 Hz, J2 = 7 Hz), 1.255 (m, 16H, 4 × CH2CH2(CH2)2CH3), 0.871 (t, 12H, 4 × CH3, J = 7 Hz); Anal. (C30H56N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-di-(diisopentylamino)-pyrimido[5,4-d]pyrimidine (33)
Compound 33 was prepared by general procedure I with diisopentylamine (0.86 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (Hexane/Acetone = 7/1) to give a yellow power solid (156 mg, 24%). Mp: 129°C; MS (ESI) m/z 671 (M + Na)+; 1H NMR (DMSO-d6) δ 4.705 (t, 4H, 4 × OH, disappeared after D2O, J = 5.5 Hz), 3.864 (br s, 8H, 4 × CH2CH2CH(CH3)2), 3.578 (br s, 16H, 2 × N(CH2CH2OH)2), 1.603 (m, 8H, 4 × CH2CH2CH(CH3)2), 1.301 (m, 16H, 4 × CH2CH2CH(CH3)2, 4 × CH3), 0.868 (t, 12H, 4 × CH3, J = 7 Hz); Anal. (C34H64N8O4) C, H, N.
2,6-Diethanolamino-4,8-di-(diisopentylamino)-pyrimido[5,4-d]pyrimidine (34)
Compound 34 was prepared by general procedure I with diisopentylamine (0.86 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (Hexane/Acetone = 15/1) to give a yellow power solid (125 mg, 22%). Mp: 97-98°C; MS (ESI) m/z 561 (M + H)+; 1H NMR (DMSO-d6) δ 5.727 (m, 2H, 2 × NH, disappeared after D2O, J = 5.5 Hz), 4.621 (m, 2H, 2 × OH, disappeared after D2O, J = 5.5 Hz), 3.858 (br d, 8H, 4 × CH2CH2CH(CH3)2), 3.510 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.269 (q, 4H, 2 × NHCH2CH2OH, J1 = 5.5 Hz, J2 = 6 Hz), 1.841 - 1.335 (m, 8H, 4 × CH2CH2CH(CH3)2), 1.321 - 1.058 (m, 12H, 4 × CH3), 0.884 - 0.778 (m, 16H, 4 × CH2CH2CH(CH3)2), 4 × CH3); Anal. (C30H56N8O2) C, H, N.
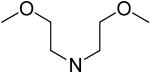
2,6-Bis(diethanolamino)-4,8-di-(bis(2-methoxyethyl)amino)-pyrimido[5,4-d]pyrimidine (35)
Compound 35 was prepared by general procedure I with bis(2-methoxyethyl)amine (0.65 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 10/1) to give a yellow power solid (260 mg, 43%). Mp: 104-105 °C; MS (ESI) m/z 601 (M + H)+, 623 (M + Na)+; 1H NMR (DMSO-d6) δ 4.685 (br s, 4H, 4 × OH, disappeared after D2O), 4.145 (br s, 8H, 4 × CH2CH2OCH3), 3.594 (t, 8H, 4 × CH2CH2OCH3), 3.557 (br s, 16H, 2 × N(CH2CH2OH)2), 3.260 (s, 12H, 4 × CH3); Anal. (C26H48N8O8) C, H, N.
2,6-Diethanolamino-4,8-di-(bis(2-methoxyethyl)amino)-pyrimido[5,4-d]pyrimidine (36)
Compound 36 was prepared by general procedure I with bis(2-methoxyethyl)amine (0.65 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 14/1) to give a yellow power solid (100 mg, 20%). Mp: 68-69 °C; MS (ESI) m/z 513 (M + H)+, 535 (M + Na)+; 1H NMR (DMSO-d6) δ 5.905 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 4.598 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5 Hz), 4.125 (br s, 8H, 4 × CH2CH2OCH3), 3.595 (t, 8H, 4 × CH2CH2OCH3), 3.496 (q, 4H, 2 × NHCH2CH2OH, J = 5 Hz), 3.257 (s, 12H, 4 × CH3), 3.235 (br s, 4H, 2 × NHCH2CH2OH); Anal. (C22H40N8O6) C, H, N.
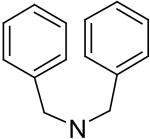
2,6-Bis(diethanolamino)-4,8-bis(dibenzylamino)-pyrimido[5,4-d]pyrimidine (37)
Compound 37 was prepared by general procedure I with dibenzylamine (0.83g, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 16/1) to give a yellow power solid compound 9 (332 mg, 46%). Mp: 199 °C; MS (ESI) m/z 729 (M + H)+, 751 (M + Na)+; 1H NMR (DMSO-d6) δ 7.324 (t, 8H, 4 × Ar-H-3, 4 × Ar-H-5, J1 = 7.5 Hz, J2 = 7 Hz), 7.261 - 7.224 (m, 12H, 4 × Ar-H-2, 4 × Ar-H-6, 4 × Ar-H-4), 5.317 (br s, 8H, 4 × CH2Ph), 4.564 (t, 4H, 4 × OH, disappeared after D2O, J = 5 Hz), 3.259 (br d, 16H, 2 × N(CH2CH2OH)2); Anal. (C42H48N8O4) C, H, N.
2,6-Diethanolamino-4,8-bis(dibenzylamino)-pyrimido[5,4-d]pyrimidine (38)
Compound 38 was prepared by general procedure I with dibenzylamine (0.83g, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 20/1) to give a yellow power solid compound 8 (125 mg, 20%). Mp: 215-216 °C; MS (ESI) m/z 641 (M + H)+, 663 (M + Na)+; 1H NMR (DMSO-d6) δ 7.327 (t, 8H, 4 × Ar-H-3, 4 × Ar-H-5, J1 = 7 Hz, J2 = 7.5 Hz), 7.289 (d, 8H, 4 × Ar-H-2, 4 × Ar-H-6, J = 7 Hz), 7.247 (t, 4H, 4 × Ar-H-4, J1 = 7.5 Hz, J2 = 7 Hz), 5.970 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5.5 Hz), 4.279 (br s, 8H, 4 × CH2Ph), 4.419 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5.5 Hz), 3.243 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.927(br s, 4H, 2 × NHCH2CH2OH); Anal. (C38H40N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-diamino-Pyrimido[5,4-d]pyrimidine (39)
Compound 39 was prepared by general procedure I with ammonia solution (7N in methanol, 0.6 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=7/1) to give a yellow power solid (63 mg, 17%). Mp: 225-226°C; MS (ESI) m/z 369 (M + H)+, 391 (M + Na)+; 1H NMR (DMSO-d6) δ 7.194 (br s, 2H, 2 × NHAHB, disappeared after D2O), 6.663 (br s, 2H, 2 × NHAHB, disappeared after D2O), 4.675 (t, 4H, 4 × OH, disappeared after D2O, J = 4.5 Hz), 3.625 (t, 8H, 2 × N(CH2CH2OH)2, J = 5 Hz), 3.584 (t, 8H, 2 × N(CH2CH2OH)2, J1 = 4.5 Hz, J2 = 5 Hz); Anal. Calcd (C14H24N8O4) C 45.64, H 6.57, N 30.42; Found: C 45.29, H 6.73, N 29.58.
2,6-Bis(diethanolamino)-4,8-dimethylamino-pyrimido[5,4-d]pyrimidine (40)
Compound 40 was prepared by general procedure I with methylamine solution (2M in tetrahydrofuran, 2.1 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=7/1) to give a yellow power solid (190 mg, 48%). Mp: 213-214°C; MS (ESI) m/z 397 (M + H)+, 419 (M + Na)+, 435 (M + K)+; 1H NMR (DMSO-d6) δ 7.154 (q, 2H, 2 × NHCH3, disappeared after D2O, J = 4.5 Hz), 4.675 (br s, 4H, 4 × OH, disappeared after D2O), 3.676 (t, 8H, 2 × N(CH2CH2OH)2), 3.619 (t, 8H, 2 × N(CH2CH2OH)2), 2.949 (d, 6H, 2 × NHCH3, J = 4.5 Hz); Anal. (C16H28N8O4) C, H, N.
2,6-Diethanolamino-4,8-dimethylamino-pyrimido[5,4-d]pyrimidine (41)
Compound 41 was prepared by general procedure I with methylamine solution (2M in tetrahydrofuran, 2.1 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=15/1) to give a yellow power solid (176 mg, 57%). Mp: 212°C; MS (ESI) m/z 309 (M + H)+, 331 (M + Na)+; 1H NMR (DMSO-d6) δ 7.120 (q, 2H, 2 × NHCH3, disappeared after D2O, J = 5 Hz), 5.947 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 6 Hz), 4.610 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5.5 Hz), 3.530 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.402 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 6 Hz), 2.920 (d, 6H, 2 × NHCH3, J = 5 Hz); Anal. (C12H20N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-diethylamino-pyrimido[5,4-d]pyrimidine (42)
Compound 42 was prepared by general procedure I with ethylamine solution (2M in tetrahydrofuran, 2.1 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=10/1) to give a yellow power solid (174 mg, 41%). Mp: 188-189°C; MS (ESI) m/z 425 (M + H)+, 447 (M + Na)+, 463 (M + K)+; 1H NMR (DMSO-d6) δ 7.151 (br s, 2H, 2 × NHCH2CH3, disappeared after D2O), 4.690 (s, 4H, 4 × OH, disappeared after D2O), 3.668 (br s, 8H, 2 × N(CH2CH2OH)2), 3.622 (br s, 8H, 2 × N(CH2CH2OH)2), 3.469 (br s, 4H, 2 × CH2CH3), 1.190 (t, 6H, 2 × CH2CH3); Anal. (C18H32N8O4) C, H, N.
2,6-Diethanolamino-4,8-diethylamino-pyrimido[5,4-d]pyrimidine (43)
Compound 43 was prepared by general procedure I with ethylamine solution (2M in tetrahydrofuran, 2.1 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=24/1) to give a yellow power solid (205 mg, 61%). Mp: 175-176°C; MS (ESI) m/z 337 (M + H)+, 357 (M + Na)+, 375 (M + K)+; 1H NMR (DMSO-d6) δ 7.057 (br s, 2H, 2 × NHCH2CH3, disappeared after D2O), 5.941 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 6 Hz), 4.621 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5.5 Hz), 3.532 (q, 4H, 2 × NHCH2CH2OH, J = 5.5 Hz), 3.442 (m, 4H, 2 × CH2CH3, J = 6 Hz), 3.392 (q, 4H, 2 × NHCH2CH2OH, J = 6 Hz), 1.174 (t, 6H, 2 × CH2CH3, J = 6 Hz); Anal. (C14H24N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-dipropylamino-pyrimido[5,4-d]pyrimidine (44)
Compound 44 was prepared by general procedure I with propylamine (0.35 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=8/1) to give a yellow power solid (217 mg, 48%). Mp: 145-146°C; MS (ESI) m/z 453 (M + H)+, 475 (M + Na)+; 1H NMR (DMSO-d6) δ 7.154 (t, 2H, 2 × NHCH2CH2CH3, disappeared after D2O), 4.701 (t, 4H, 4 × OH, disappeared after D2O), 3.670 (br d, 8H, 2 × N(CH2CH2OH)2), 3.631 (t, 8H, 2 × N(CH2CH2OH)2), 3.400 (m, 4H, 2 × CH2CH2CH3), 1.607 (m, 4H, 2 × CH2CH2CH3, J = 7.5 Hz), 0.910 (t, 6H, 2 × CH2CH2CH3, J = 7.5 Hz); Anal. (C20H36N8O4) C, H, N.
2,6-Diethanolamino-4,8-dipropylamino-pyrimido[5,4-d]pyrimidine (45)
Compound 45 was prepared by general procedure I with propylamine (0.35 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=10/1) to give a yellow power solid (196 mg, 54%). Mp: 147-148°C; MS (ESI) m/z 365 (M + H)+, 387 (M + Na)+; 1H NMR (DMSO-d6) δ 7.055 (br s, 2H, 2 × NHCH2CH2CH3, disappeared after D2O), 5.957 (br s, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 4.636 (br s, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 3.544 (br s, 4H, 2 × NHCH2CH2OH), 3.376 (br s, 8H, 2 × NHCH2CH2OH, 2 × CH2CH2CH3), 1.591 (m, 4H, 2 × CH2CH2CH3, J = 7.5 Hz), 0.901 (t, 6H, 2 × CH2CH2CH3, J = 7.5 Hz); Anal. (C16H28N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-diisopropylamino-pyrimido[5,4-d]pyrimidine (46)
Compound 46 was prepared by general procedure I with isopropylamine (0.36 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=8/1) to give a yellow power solid (201 mg, 44%). Mp: 188-190°C; MS (ESI) m/z 453 (M + H)+, 475 (M + Na)+; 1H NMR (DMSO-d6) δ 6.547 (d, 2H, 2 × NHCH(CH3)2, disappeared after D2O), 4.706 (br s, 4H, 4 × OH, disappeared after D2O), 4.218 (m, 2H, 2 × NHCH(CH3)2), 3.662 (t, 8H, 2 × N(CH2CH2OH)2), 3.620 (br s, 8H, 2 × N(CH2CH2OH)2), 1.264 (d, 12H, 2 × NHCH(CH3)2); Anal. (C20H36N8O4) C, H, N.
2,6-Diethanolamino-4,8-diisopropylamino-pyrimido[5,4-d]pyrimidine (47)
Compound 47 was prepared by general procedure I with isopropylamine (0.36 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=14/1) to give a yellow power solid (165 mg, 45%). Mp: 167°C; MS (ESI) m/z 365 (M + H)+, 387 (M + Na)+, 403 (M + K)+; 1H NMR (DMSO-d6) δ 6.590 (d, 2H, 2 × NHCH(CH3)2, disappeared after D2O), 6.008 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 6 Hz), 4.646 (br s, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 4.241 (m, 2H, 2 × NHCH(CH3)2, J = 6.5 Hz), 3.535 (br s, 4H, 2 × NHCH2CH2OH), 3.382 (m, 4H, 2 × NHCH2CH2OH, J = 6 Hz), 1.235 (d, 12H, 2 × NHCH(CH3)2 J = 6.5 Hz); Anal. (C16H28N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-dibutylamino-pyrimido[5,4-d]pyrimidine (48)
Compound 48 was prepared by general procedure I with butylamine (0.42 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=10/1) to give a yellow power solid (82 mg, 17%). Mp: 117-118°C; MS (ESI) m/z 481 (M + H)+, 503 (M + Na)+, 519 (M + K)+; 1H NMR (DMSO-d6) δ 7.135 (br s, 2H, 2 × NHCH2CH2CH2CH3, disappeared after D2O), 4.693 (br s, 4H, 4 × OH, disappeared after D2O), 3.658 (br s, 8H, 2 × N(CH2CH2OH)2), 3.617 (br s, 8H, 2 × N(CH2CH2OH)2), 3.431 (br s, 4H, 2 × NHCH2CH2CH2CH3), 1.580 (m, 4H, 2 × NHCH2CH2CH2CH3, J = 7 Hz), 1.350 (m, 4H, 2 × NHCH2CH2CH2CH3, J1 = 7 Hz, J2 = 7.5 Hz), 0.919 (t, 6H, 2 × NHCH2CH2CH2CH3, J = 7.5 Hz); Anal. Calcd (C22H40N8O4 · 0.5 H2O): C 53.86, H 8.63, N 22.84; Found: C 54.32, H 8.34, N 22.72.
2,6-Diethanolamino-4,8-dibutylamino-pyrimido[5,4-d]pyrimidine (49)
Compound 49 was prepared by general procedure I with butylamine (0.42 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=14/1) to give a yellow power solid (70 mg, 18%). Mp: 127-128°C; MS (ESI) m/z 393 (M + H)+, 415 (M + Na)+; 1H NMR (DMSO-d6) δ 7.018 (br s, 2H, 2 × NHCH2CH2CH2CH3, disappeared after D2O), 5.938 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5.5 Hz), 4.618 (br s, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 3.529 (m, 4H, 2 × NHCH2CH2OH), 3.404 (m, 4H, 2 × NHCH2CH2CH2CH3, J = 7 Hz), 3.380 (m, 4H, 2 × NHCH2CH2OH, J = 5.5 Hz), 1.556 (m, 4H, 2 × NHCH2CH2CH2CH3, J1 = 7 Hz, J2 = 7.5 Hz), 1.345 (m, 4H, 2 × NHCH2CH2CH2CH3, J1 = 7.5 Hz, J2 = 7 Hz), 0.921 (t, 6H, 2 × NHCH2CH2CH2CH3, J = 7 Hz); Anal. (C18H32N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-diisobutylamino-pyrimido[5,4-d]pyrimidine (50)
Compound 50 was prepared by general procedure I with isobutylamine (0.36 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=9/1) to give a yellow power solid (58 mg, 12%). Mp: 162-163°C; MS (ESI) m/z 481 (M + H)+, 503 (M + Na)+, 519 (M + K)+; 1H NMR (DMSO-d6) δ 7.145 (br s, 2H, 2 × NHCH2CH(CH3)2, disappeared after D2O), 4.703 (br s, 4H, 4 × OH, disappeared after D2O), 3.649 (d, 8H, 2 × N(CH2CH2OH)2), 3.619 (d, 8H, 2 × N(CH2CH2OH)2), 3.278 (t, 4H, 2 × NHCH2CH(CH3)2, J = 6.5 Hz), 1.957 (m, 2H, 2 × NHCH2CH(CH3)2, J1 = 6.5 Hz, J2 = 7 Hz), 0.917 (d, 12H, 2 × NHCH2CH(CH3)2, J = 7 Hz); Anal. (C22H40N8O4) C, H, N.
2,6-Diethanolamino-4,8-diisobutylamino-pyrimido[5,4-d]pyrimidine (51)
Compound 51 was prepared by general procedure I with isobutylamine (0.36 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=12/1) to give a yellow power solid (78 mg, 20%). Mp: 141-142°C; MS (ESI) m/z 393 (M + H)+, 415 (M + Na)+, 431 (M + K)+; 1H NMR (DMSO-d6) δ 7.019 (t, 2H, 2 × NHCH2CH(CH3)2, disappeared after D2O), 5.980 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5.5 Hz), 4.631 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5.5 Hz), 3.537 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.377 (q, 4H, 2 × NHCH2CH2OH, J1 = 5.5 Hz, J2 = 6 Hz), 3.255 (t, 4H, 2 × NHCH2CH(CH3)2, J = 6.5 Hz), 1.954 (m, 2H, 2 × NHCH2CH(CH3)2, J1 = 6.5 Hz, J2 = 7 Hz), 0.914 (d, 12H, 2 × NHCH2CH(CH3)2, J = 7 Hz); Anal. Calcd (C18H32N8O2): C 55.08, H 8.22, N 28.55; Found; C 54.46, H 8.15, N 28.22.
2,6-Bis(diethanolamino)-4,8-di(tert-butylamino)-pyrimido[5,4-d]pyrimidine (52)
Compound 52 was prepared by general procedure I with tert-butylamine (0.44 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=6/1) to give a yellow power solid (173 mg, 36%). Mp: 244-245°C; MS (ESI) m/z 481 (M + H)+, 503 (M + Na)+; 1H NMR (DMSO-d6) δ 6.475 (s, 2H, 2 × NHC(CH3)3, disappeared after D2O), 4.748 (t, 4H, 4 × OH, disappeared after D2O), 3.643 (br s, 16H, 2 × N(CH2CH2OH)2), 1.474 (s, 18H, 2 × NHC(CH3)3); Anal. (C22H40N8O4) C, H, N.
2,6-Diethanolamino-4,8-di(tert-butylamino)-pyrimido[5,4-d]pyrimidine (53)
Compound 53 was prepared by general procedure I with tert-butylamine (0.44 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=9/1) to give a yellow power solid (190 mg, 48%). Mp: 154°C; MS (ESI) m/z 393 (M + H)+, 415 (M + Na)+; 1H NMR (DMSO-d6) δ 6.456 (s, 2H, 2 × NHC(CH3)3, disappeared after D2O), 6.135 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 6 Hz), 4.652 (br s, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 3.536 (q, 4H, 2 × NHCH2CH2OH, J = 5.5 Hz), 3.338 (q, 4H, 2 × NHCH2CH2OH, J1 = 5.5 Hz, J2 = 6 Hz), 1.477 (s, 18H, 2 × NHC(CH3)3); Anal. (C18H32N8O2) C, H, N.
2,6-Bis(diethanolamino)-4,8-diamylamino-pyrimido[5,4-d]pyrimidine (54)
Compound 54 was prepared by general procedure I with amylamine (0.49 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=12/1) to give a yellow power solid (190 mg, 37%). Mp: 128°C; MS (ESI) m/z 509 (M + H)+, 531 (M + Na)+, 547 (M + K)+; 1H NMR (DMSO-d6) δ 7.139 (br s, 2H, 2 × NHCH2CH2(CH2)2CH3, disappeared after D2O), 4.690 (br s, 4H, 4 × OH, disappeared after D2O), 3.662 (br s, 8H, 2 × N(CH2CH2OH)2), 3.624 (d, 8H, 2 × N(CH2CH2OH)2), 3.423 (br s, 4H, 2 × NHCH2CH2(CH2)2CH3), 1.598 (m, 4H, 2 × NHCH2CH2(CH2)2CH3), 1.320 (m, 8H, 2 × NHCH2CH2(CH2)2CH3), 0.882 (t, 6H, 2 × NHCH2CH2(CH2)2CH3); Anal. (C24H44N8O4) C, H, N.
2,6-Diethanolamino-4,8-diamylamino-pyrimido[5,4-d]pyrimidine (55)
Compound 55 was prepared by general procedure I with amylamine (0.49 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=17/1) to give a yellow power solid (155 mg, 37%). Mp: 147-148°C; MS (ESI) m/z 421 (M + H)+, 443 (M + Na)+; 1H NMR (DMSO-d6) δ 7.032 (t, 2H, 2 × NHCH2CH2(CH2)2CH3, disappeared after D2O), 5.931 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 4.620 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 3.530 (q, 4H, 2 × NHCH2CH2OH), 3.382 (m, 8H, 2 × NHCH2CH2(CH2)2CH3, 2 × NHCH2CH2OH), 1.582 (m, 4H, 2 × NHCH2CH2(CH2)2CH3), 1.309 (m, 8H, 2 × NHCH2CH2(CH2)2CH3), 0.882 (t, 6H, 2 × NHCH2CH2(CH2)2CH3); Anal. (C20H36N8O2 · 0.5 H2O) C, H, N.
2,6-Bis(diethanolamino)-4,8-diisopentylamino-pyrimido[5,4-d]pyrimidine (56)
Compound 56 was prepared by general procedure I with isopentylamine (0.49 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=22/1) to give a yellow power solid (346 mg, 68%). Mp: 124-125°C; MS (ESI) m/z 509 (M + H)+, 531 (M + Na)+, 547 (M + K)+; 1H NMR (DMSO-d6) δ 7.131 (t, 2H, 2 × NHCH2CH2CH(CH3)2, disappeared after D2O), 4.679 (t, 4H, 4 × OH, disappeared after D2O), 3.666 (t, 8H, 2 × N(CH2CH2OH)2), 3.621 (d, 8H, 2 × N(CH2CH2OH)2), 3.454 (q, 4H, 2 × NHCH2CH2CH(CH3)2), 1.621 (m, 2H, 2 × NHCH2CH2CH(CH3)2), 1.505 (m, 4H, 2 × NHCH2CH2CH(CH3)2), 0.936 (d, 12H, 2 × NHCH2CH2CH(CH3)2); Anal. (C24H44N8O4 · 0.5 H2O) C, H, N.
2,6-Diethanolamino-4,8-diisopentylamino-pyrimido[5,4-d]pyrimidine (57)
Compound 57 was prepared by general procedure I with isopentylamine (0.49 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=30/1) to give a yellow power solid (235 mg, 56%). Mp: 132-133°C; MS (ESI) m/z 421 (M + H)+, 443 (M + Na)+; 1H NMR (DMSO-d6) δ 6.999 (br s, 2H, 2 × NHCH2CH2CH(CH3)2, disappeared after D2O), 5922 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 4.621 (br s, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 3.538 (q, 4H, 2 × NHCH2CH2OH), 3.422 (q, 4H, 2 × NHCH2CH2OH), 3.384 (m, 4H, 2 × NHCH2CH2CH(CH3)2), 1.614 (m, 2H, 2 × NHCH2CH2CH(CH3)2), 1.490 (m, 4H, 2 × NHCH2CH2CH(CH3)2), 0.931 (d, 12H, 2 × NHCH2CH2CH(CH3)2); Anal. (C20H36N8O2 · H2O) C, H, N.
2,6-Bis(diethanolamino)-4,8-di(tert-amylamino)-pyrimido[5,4-d]pyrimidine (58)
Compound 58 was prepared by general procedure I with tert-amylamine (0.49 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=14/1) to give a yellow power solid (245 mg, 48%). Mp: 212-213°C; MS (ESI) m/z 509 (M + H)+, 531 (M + Na)+, 547 (M + K)+; 1H NMR (DMSO-d6) δ 6.432 (s, 2H, 2 × NHC(CH3)2CH2CH3, disappeared after D2O), 4.757 (d, 4H, 4 × OH, disappeared after D2O), 3.637 (s, 16H, 2 × N(CH2CH2OH)2), 1.841 (q, 4H, 2 × NHC(CH3)2CH2CH3, J = 7.5 Hz), 1.415 (s, 12H, 2 × NHC(CH3)2CH2CH3), 0.833 (t, 6H, 2 × NHC(CH3)2CH2CH3, J = 7.5 Hz); Anal. (C24H44N8O4) C, H, N.
2,6-Diethanolamino-4,8-di(tert-amylamino)-pyrimido[5,4-d]pyrimidine (59)
Compound 59 was prepared by general procedure I with tert-amylamine (0.49 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=18/1) to give a yellow power solid (196 mg, 47%). Mp: 167-168°C; MS (ESI) m/z 421 (M + H)+, 443 (M + Na)+; 1H NMR (DMSO-d6) δ 6.391 (s, 2H, 2 × NHC(CH3)2CH2CH3, disappeared after D2O), 6.143 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 6 Hz), 4.653 (br s, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 3.552 (q, 4H, 2 × NHCH2CH2OH, J = 5.5 Hz), 3.328 (q, 4H, 2 × NHCH2CH2OH, J1 = 5.5 Hz, J2 = 6 Hz), 1.881 (q, 4H, 2 × NHC(CH3)2CH2CH3, J = 7.5 Hz), 1.414 (s, 12H, 2 × NHC(CH3)2CH2CH3), 0.814 (t, 6H, 2 × NHC(CH3)2CH2CH3, J = 7.5 Hz); Anal. Calcd (C20H36N8O2 · 0.5 H2O): C 55.92, H 8.68, N 26.09; Found: C 56.02, H 8.67, N 25.62.
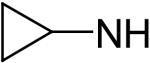
2,6-Bis(diethanolamino)-4,8-dicyclopropylaminopyrimido[5,4-d]pyrimidine (60)
Compound 60 was prepared by general procedure I with cyclopropylamine (0.31 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 14/1) to give a yellow power solid (220 mg, 49%). Mp: 225 °C; MS (ESI) m/z 449 (M + H)+, 471 (M + Na)+; 1H NMR (DMSO-d6) δ 7.060 (d, 2H, 2 × NHCH(CH2)2, disappeared after D2O, J = 3.5 Hz), 4.688 (s, 4H, 4 × OH, disappeared after D2O), 3.681 (t, 8H, 2 × N(CH2CH2OH)2), 3.627 (d, 8H, 2 × N(CH2CH2OH)2), 2.796 (m, 2H, 2 × NHCH(CH2)2), 0.783 (m, 4H, 2 × NHCH(CH2)2-H2a,3a), 0.622 (m, 4H, 2 × NHCH(CH2)2-H2e,3e); Anal. (C20H32N8O4 · 0.25 H2O) C, H, N.
2,6-Diethanolamino-4,8-dicyclopropylaminopyrimido[5,4-d]pyrimidine (61)
Compound 61 was prepared by general procedure I with cyclopropylamine (0.31 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 17/1) to give a yellow power solid (156 mg, 43%). Mp: 199 °C; MS (ESI) m/z 361 (M + H)+, 383 (M + Na)+; 1H NMR (DMSO-d6) δ 6.983 (d, 2H, 2 × NHCH(CH2)2, disappeared after D2O, J = 3.5 Hz), 5.003 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 6 Hz), 4.633 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J1 = 5 Hz, J2 = 5.5 Hz), 3.525 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.396 (q, 4H, 2 × NHCH2CH2OH, J = 6 Hz), 2.869 (m, 2H, 2 × NHCH(CH2)2), 0.748 (m, 4H, 2 × NHCH(CH2)2-H2a,3a), 0.633 (m, 4H, 2 × NHCH(CH2)2-H2e,3e); Anal. (C16H24N8O2 · 0.25 H2O) C, H, N.
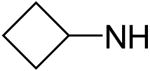
2,6-Bis(diethanolamino)-4,8-dicyclobutylaminopyrimido[5,4-d]pyrimidine (62)
Compound 62 was prepared by general procedure I with cyclobutylamine (0.37 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 16/1) to give a yellow power solid (212 mg, 45%). Mp: 222-223 °C; MS (ESI) m/z 477 (M + H)+, 499 (M + Na)+; 1H NMR (DMSO-d6) δ 6.992 (br s, 2H, 2 × NHCH(CH2)2CH2, disappeared after D2O), 4.701 (s, 4H, 4 × OH, disappeared after D2O), 4.467 (br s, 2H, 2 × NHCH(CH2)2CH2), 3.615 (br d, 16H, 2 × N(CH2CH2OH)2, 2.295 (m, 4H, 2 × NHCH(CH2)2CH2-H2a,4a), 2.196 (m, 4H, 2 × NHCH(CH2)2CH2-H2e,4e), 1.729 (m, 4H, 2 × NHCH(CH2)2CH2); Anal. (C22H36N8O4) C, H, N.
2,6-Diethanolamino-4,8-dicyclobutylaminopyrimido[5,4-d]pyrimidine (63)
Compound 63 was prepared by general procedure I with cyclobutylamine (0.37 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 19/1) to give a yellow power solid (183 mg, 47%). Mp: 189-190 °C; MS (ESI) m/z 389 (M + H)+, 411 (M + Na)+; 1H NMR (DMSO-d6) δ 7.052 (d, 2H, 2 × NHCH(CH2)2CH2, disappeared after D2O, J = 8 Hz), 5.971 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5.5 Hz), 4.645 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J1 = 4.5 Hz, J2 = 5.5 Hz), 4.555 (m, 2H, 2 × NHCH(CH2)2CH2, J1 = 8 Hz, J2 = 8.5 Hz), 3.533 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.405 (q, 4H, 2 × NHCH2CH2OH, J1 = 5.5 Hz, J2 = 6 Hz), 2.272 (m, 4H, 2 × NHCH(CH2)2CH2-H2a,4a), 2.097 (m, 4H, 2 × NHCH(CH2)2CH2-H2e,4e), 1.675 (m, 4H, 2 × NHCH(CH2)2CH2); Anal. Calcd (C18H28N8O2): C, H, N.
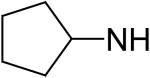
2,6-Bis(diethanolamino)-4,8-dicyclopentylamino-pyrimido[5,4-d]pyrimidine (64)
Compound 64 was prepared by general procedure I with cyclopentylamine (0.42 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=10/1) to give a yellow power solid (97 mg, 19%). Mp: 211-212 °C; MS (ESI) m/z 505 (M + H)+, 527 (M + Na)+; 1H NMR (DMSO-d6) δ 6.619 (d, 2H, 2 × NHCH(CH2CH2)2, disappeared after D2O), 4.704 (br s, 4H, 4 × OH, disappeared after D2O), 4.296 (m, 2H, 2 × NHCH(CH2CH2)2), 3.657 (t, 8H, 2 × N(CH2CH2OH)2), 3.615 (d, 8H, 2 × N(CH2CH2OH)2), 2.016 (m, 4H, 2 × NHCH(CHaxHeqCH2)2), 1.720 (m, 4H, 2 × NHCH(CH2CHaxHeq)2), 1.587 (m, 8H, 2 × NHCH(CH2CHaxHeq)2, 2 × NHCH(CHaxHeqCH2)2); Anal. (C24H40N8O4) C, H, N.
2,6-Diethanolamino-4,8-dicyclopentylamino-pyrimido[5,4-d]pyrimidine (65)
Compound 65 was prepared by general procedure I with cyclopentylamine (0.42 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=18/1) to give a yellow power solid (175 mg, 42%). Mp: 203-204 °C; MS (ESI) m/z 417 (M + H)+, 439 (M + Na)+; 1H NMR (DMSO-d6) δ 6.678 (d, 2H, 2 × NHCH(CH2CH2)2, disappeared after D2O), 6.028 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 6 Hz), 4.651 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5 Hz), 4.337 (q, 2H, 2 × NHCH(CH2CH2)2), 3.534 (q, 4H, 2 × NHCH2CH2OH, J = 5 Hz), 3.380 (q, 4H, 2 × NHCH2CH2OH, J = 6 Hz), 1.998 (m, 4H, 2 × NHCH(CHaxHeqCH2)2), 1.708 (m, 4H, 2 × NHCH(CH2CHaxHeq)2), 1.559 (m, 8H, 2 × NHCH(CH2CHaxHeq)2, 2 × NHCH(CHaxHeqCH2)2); Anal. (C20H32N8O2 · 0.5 H2O) C, H, N.
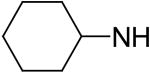
2,6-Bis(diethanolamino)-4,8-dicyclohexylamino-pyrimido[5,4-d]pyrimidine (66)
Compound 66 was prepared by general procedure I with cyclohexylamine (0.48 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=12/1) to give a yellow power solid (320 mg, 60%). Mp: 198-199 °C; MS (ESI) m/z 533 (M + H)+, 555 (M + Na)+; 1H NMR (DMSO-d6) δ 6.586 (d, 2H, 2 × NHCH(CH2CH2)2CH2, disappeared after D2O), 4.708 (t, 4H, 4 × OH, disappeared after D2O), 3.891 (m, 2H, 2 × NHCH(CH2CH2)2CH2), 3.637 (m, 16H, 2 × N(CH2CH2OH)2), 1.916 (m, 4H, 2 × NHCH(CHaxHeqCH2)2CH2), 1.729 (m, 4H, 2 × NHCH(CH2CHaxHeq)2CH2), 1.593 (m, 2H, 2 × NHCH(CH2CH2)2CHaxHeq), 1.442-1.330 (m, 8H, 2 × NHCH(CH2CHaxHeq)2CH2, 2 × NHCH(CHaxHeqCH2)2CH2), 1.248 (m, 2H, 2 × NHCH(CH2CH2)2CHaxHeq); Anal. (C26H44N8O4) C, H, N.
2,6-Diethanolamino-4,8-dicyclohexylamino-pyrimido[5,4-d]pyrimidine (67)
Compound 67 was prepared by general procedure I with cyclohexylamine (0.48 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=15/1) to give a yellow power solid (44 mg, 10%). Mp: 163 °C; MS (ESI) m/z 445 (M + H)+, 467 (M + Na)+; 1H NMR (DMSO-d6) δ 6.589 (d, 2H, 2 × NHCH(CH2CH2)2CH2, disappeared after D2O), 6.033 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 4.632 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 3.915 (q, 2H, 2 × NHCH(CH2CH2)2CH2), 3.525 (q, 4H, 2 × NHCH2CH2OH), 3.355 (q, 4H, 2 × NHCH2CH2OH), 1.901 (m, 4H, 2 × NHCH(CHaxHeqCH2)2CH2), 1.735 (m, 4H, 2 × NHCH(CH2CHaxHeq)2CH2), 1.600 (m, 2H, 2 × NHCH(CH2CH2)2CHaxHeq), 1.399-1.304 (m, 8H, 2 × NHCH(CH2CHaxHeq)2CH2, 2 × NHCH(CHaxHeqCH2)2CH2), 1.225 (m, 2H, 2 × NHCH(CH2CH2)2CHaxHeq); Anal. (C22H36N8O2) C, H, N.
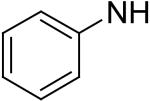
2,6-Bis(diethanolamino)-4,8-diphenylamino-pyrimido[5,4-d]pyrimidine (68)
Compound 68 was prepared by general procedure I with aniline (0.39 ml, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH=14/1) to give a yellow power solid (186 mg, 36%). Mp: 209-210 °C; MS (ESI) m/z 519 (M - H)-; 1H NMR (DMSO-d6) δ 8.918 (s, 2H, 2 × NHAr, disappeared after D2O), 7.916 (d, 4H, 2 × Ar-H-2, 2 × Ar-H-6, J = 8 Hz), 7.404 (t, 4H, 2 × Ar-H-3, 2 × Ar-H-5, J = 8 Hz), 7.105 (t, 2H, 2 × Ar-H-4), 4.753 (t, 4H, 4 × OH, disappeared after D2O, J = 5.5 Hz), 3.789 (br s, 8H, 2 × N(CH2CH2OH)2), 3.689 (q, 8H, 2 × N(CH2CH2OH)2, J = 5.5 Hz); Anal. (C26H32N8O4) C, H, N.
2,6-Diethanolamino-4,8-diphenylaminopyrimido[5,4-d]pyrimidine (69)
Compound 69 was prepared by general procedure I with aniline (0.39 ml, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 16/1) to give a yellow power solid compound 4 (138 mg, 32%). Mp: 233-234 °C; MS (ESI) m/z 433 (M + H)+, m/z 455 (M + Na)+; 1H NMR (DMSO-d6) δ 8.937 (s, 2H, 2 × NHAr, disappeared after D2O), 8.010 (d, 4H, 2 × Ar-H-2, 2 × Ar-H-6, J = 8 Hz), 7.388 (t, 4H, 2 × Ar-H-3, 2 × Ar-H-5, J1 = 7.5 Hz, J2 = 8.5 Hz), 7.092 (t, 2H, 2 × Ar-H-4, J1 = 7.5 Hz, J2 = 7 Hz), 6.564 (br s, 2H, 2 × NHCH2CH2OH, disappeared after D2O), 4.694 (t, 2H, 2 × OH, disappeared after D2O, J = 5.5 Hz), 3.613 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.526 (q, 4H, 2 × NHCH2CH2OH, J1 = 5.5 Hz, J2 = 6 Hz); Anal. Calcd (C22H24N8O2 · 0.5 H2O): C 59.85, H 5.71, N 25.38; Found; C 60.31, H 5.69, N 25.23.
2,6-Bis(diethanolamino)-4,8-bis(dibenzylamino)-pyrimido[5,4-d]pyrimidine (70)
Compound 70 was prepared by general procedure I with dibenzylamine (0.83g, 4.2 mmole) at the first step, and diethanolamine (3 ml, 30 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 16/1) to give a yellow power solid compound 9 (332 mg, 46%). Mp: 199 °C; MS (ESI) m/z 729 (M + H)+, 751 (M + Na)+; 1H NMR (DMSO-d6) δ 7.324 (t, 8H, 4 × Ar-H-3, 4 × Ar-H-5, J1 = 7.5 Hz, J2 = 7 Hz), 7.261 - 7.224 (m, 12H, 4 × Ar-H-2, 4 × Ar-H-6, 4 × Ar-H-4), 5.317 (br s, 8H, 4 × CH2Ph), 4.564 (t, 4H, 4 × OH, disappeared after D2O, J = 5 Hz), 3.259 (br d, 16H, 2 × N(CH2CH2OH)2); Anal. (C42H48N8O4) C, H, N.
2,6-Diethanolamino-4,8-bis(dibenzylamino)-pyrimido[5,4-d]pyrimidine (71)
Compound 71 was prepared by general procedure I with dibenzylamine (0.83g, 4.2 mmole) at the first step, and ethanolamine (3 ml, 50 mmole) at the second step. Product was purified by flash silica gel chromatography (CH2Cl2/MeOH = 20/1) to give a yellow power solid compound 8 (125 mg, 20%). Mp: 215-216 °C; MS (ESI) m/z 641 (M + H)+, 663 (M + Na)+; 1H NMR (DMSO-d6) δ 7.327 (t, 8H, 4 × Ar-H-3, 4 × Ar-H-5, J1 = 7 Hz, J2 = 7.5 Hz), 7.289 (d, 8H, 4 × Ar-H-2, 4 × Ar-H-6, J = 7 Hz), 7.247 (t, 4H, 4 × Ar-H-4, J1 = 7.5 Hz, J2 = 7 Hz), 5.970 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5.5 Hz), 4.279 (br s, 8H, 4 × CH2Ph), 4.419 (t, 2H, 2 × NHCH2CH2OH, disappeared after D2O, J = 5.5 Hz), 3.243 (q, 4H, 2 × NHCH2CH2OH, J1 = 6 Hz, J2 = 5.5 Hz), 3.927(br s, 4H, 2 × NHCH2CH2OH); Anal. (C38H40N8O2) C, H, N.
2,6-Di-(2′-hydroxyethoxy)-4,8-dipiperidinopyrimido[5,4-d]pyrimidine (72)
Compound 72 was prepared by a literature procedure.43
2,6-Dimorpholino-4,8-dipiperidino-pyrimido[5,4-d]pyrimidine (73)
Compound 73 was prepared by general procedure I with piperidine (0.41 ml, 4.2 mmole) at the first step, and morpholine (3 ml, 34 mmole) at the second step. Product was purified by flash silica gel chromatography (Hexane/Acetone=10/1) to give a yellow power solid (173 mg, 37%). Mp: 203-204 °C; MS (ESI) m/z 469 (M + H)+, 491 (M + Na)+; 1H NMR (DMSO-d6) δ 4.076 (br s, 8H, 2 × N(CH2CH2)2CH2), 3.663 (t, 8H, 2 × N(CH2CH2)O, J = 5 Hz), 3.542 (t, 8H, 2 × N(CH2CH2)O, J = 5 Hz), 1.664 (br d, 4H, 2 × N(CH2CH2)2CH2, J = 4.5 Hz), 1.608 (br d, 8H, 2 × N(CH2CH2)2CH2, J = 4.5 Hz); Anal. (C24H36N8O2) C, H, N.
2,6-Bis[N,N-di-(2′-formyloxy)ethylamino]-4,8-dipiperidinopyrimido[5,4-d]pyrimidine (74)
Dipyridamole (0.51 g, 1 mmole) was dissolved in formic acid (10 ml, 0.25 mole); the reaction was stirred at reflux for 6 hours, and then the solvent was evaporated under reduced pressure. The residue was dissolved in methylene chloride (50 ml) and washed with 10% NaHCO3 solution, and then the organic layer was separated and dried by anhydrous Na2SO4. After the solvent was removed, The residue was subjected to flash silica gel chromatography (Hexane:Acetone = 6:1) to give g yellow fluorescent power compound 74 (0.567 g, 92%). mp 129-130 °C (lit:25 128-130 °C). MS (ESI) m/z 639 (M + Na)+, m/z 617 (M + H)+; 1H NMR (DMSO-d6) δ 8.225 (s, 4H, 4 × CHO), 4.289 (t, 8H, 2 × N(CH2CH2OCHO)2, J = 5.5 Hz), 4.052 (m, 8H, 2 × N(CH2CH2)2CH2), 3.784 (t, 8H, 2 × N(CH2CH2OCHO)2, J = 5.5 Hz), 1.646 (m, 4H, 2 × N(CH2CH2)2CH2), 1.599 (m, 8H, 2 × N(CH2CH2)2CH2); Anal. (C28H40N8O8) C, H, N.
2,6-Bis[N,N-di-(2′-acetoxy)ethylamino]-4,8-dipiperidinopyrimido[5,4-d]pyrimidine (75)
In an ice-water bath, acetyl chrolide (1.45 ml, 20 mmole) was added to a solution dipyridamole (0.51 g, 1 mmole) and a catalytic amount of DMAP in anhydrous THF (30 ml). The reaction was stirred for 3 hours, and then the solvent was evaporated under reduced pressure. The residue was dissolved in methylene chloride (50 ml) and washed with 10% NaHCO3 solution, and then the organic layer was separated and dried by anhydrous Na2SO4. After the solvent was removed, the residue was subjected to flash silica gel chromatography (Hexane:Acetone = 10:1) to give g yellow fluorescent needle-like compound 75 (0.64 g, 95%). mp 121-122 °C (lit:25 123-124 °C). MS (ESI) m/z 695 (M + Na)+, m/z 673 (M + H)+; 1H NMR (DMSO-d6) δ 4.191 (t, 8H, 2 × N(CH2CH2OCOCH3)2, J = 5.5 Hz), 4.046 (m, 8H, 2 × N(CH2CH2)2CH2), 3.746 (t, 8H, 2 × N(CH2CH2OCOCH3)2, J = 5.5 Hz), 1.982 (s, 12H, 4 × CH3), 1.649 (m, 4H, 2 × N(CH2CH2)2CH2), 1.598 (m, 8H, 2 × N(CH2CH2)2CH2); Anal. (C32H48N8O8) C, H, N.
2,6-Bis[N,N-di-(2′-methoxy)ethylamino]-4,8-dipiperidinopyrimido[5,4-d]pyrimidine (76)
Compound 76 was prepared by general procedure III with MeI (2 ml, 32 mmole) as alkyl halide. The residue was subjected to flash silica gel chromatography (Hexane:Acetone = 8:1) to give yellow fluorescent power compound 56 (247 mg, 64%). Mp: 64 – 65 °C. MS (ESI) m/z 583 (M + Na)+, 561 (M + H)+; 1H NMR (DMSO-d6) δ 4.052 (br s, 8H, 2 × N(CH2CH2)2CH2), 3.766 (t, 8H, 2 × N(CH2CH2OCH3)2, J = 6 Hz), 3.574 (t, 8H, 2 × N(CH2CH2OCH3)2, J = 6 Hz), 3.351 (s, 12H, 4 × CH3), 1.683 (s, 12H, 2 × N(CH2CH2)2CH2); Anal. (C28H48N8O4) C, H, N.
2,6-Bis[N,N-di-(2′-ethoxy)ethylamino]-4,8-dipiperidinopyrimido[5,4-d]pyrimidine (77)
Compound 77 was prepared by general procedure III with ethyl bromide (2.4 ml, 32 mmole) as alkyl halide. The residue was subjected to flash silica gel chromatography (Hexane:Acetone = 12:1) to give yellow fluorescent power compound 57 (520 mg, 84%). Mp: 58-59 °C; MS (ESI) m/z 639 (M + Na)+, 617 (M + H)+; 1H NMR (DMSO-d6) δ 4.049 (br s, 8H, 2 × N(CH2CH2)2CH2), 3.776 (br s, 8H, 2 × N(CH2CH2OCH2CH3)2), 3.624 (br s, 8H, 2 × N(CH2CH2OCH2CH3)2), 3.500 (q, 8H, 4 × CH2CH3), 1.708 (br s, 12H, 2 × N(CH2CH2)2CH2), 1.194 (t, 12H, 4 × CH2CH3); Anal. (C32H56N8O4) C, H, N.
2,6-Bis[N,N-di-(2′-propoxy)ethylamino]-4,8-dipiperidinopyrimido[5,4-d]pyrimidine (78)
Compound 78 was prepared by general procedure III with propyl bromide (2.9 ml, 32 mmole) as alkyl halide. The residue was subjected to flash silica gel chromatography (Hexane:Acetone = 13:1) to give yellow fluorescent power compound 58 (570 mg, 85%). Mp: 32-34 °C; MS (ESI) m/z 695 (M + Na)+, 673 (M + H)+; 1H NMR (DMSO-d6) δ 4.056 (br s, 8H, 2 × N(CH2CH2)2CH2), 3.779(br s, 8H, 2 × N(CH2CH2OCH2CH2CH3)2), 3.616 (br s, 8H, 2 × N(CH2CH2OCH2CH2CH3)2), 3.399(t, 8H, 4 × CH2CH2CH3, J = 6.5 Hz), 1.699 (br s, 12H, 2 × N(CH2CH2)2CH2), 1.578 (m, 8H, 4 × CH2CH2CH3, J1 = 6.5 Hz, J2 = 7.5 Hz), 0.915 (t, 12H, 4 × CH2CH2CH3, J = 7.5 Hz); Anal. (C36H64N8O4) C, H, N.
2,6-[N-(2-hydroxyethyl)-N-(2-isopropoxyethyl)-amino]-4,8-di-piperidino-pyrimido[5,4-d]pyrimidine (79)
Compound 79 was prepared by general procedure III with isopropyl bromide (3 ml, 32 mmol) as alkyl halide. The residue was subjected to flash silica gel chromatography (Hexane:Acetone = 2:1) to give yellow fluorescent power compound 58 (326 mg, 55%). Mp: 56-58 °C; MS (ESI) m/z 611 (M + Na)+, 589 (M + H)+; 1H NMR (DMSO-d6) δ 4.574 (m, 2H, 2 × OH, disappeared after D2O), 4.058 (br s, 8H, 2 × N(CH2CH2)2CH2), 3.631 (t, 4H, 2 × CH2OH), 3.570 (br s, 8H, 2 × (CH3)2CHOCH2CH2NCH2CH2OH), 3.525 (m, 6H, 2 × (CH3)2CHOCH2), 1.646 (br s, 4H, 2 × N(CH2CH2)2CH2), 1.597 (br s, 8H, 2 × N(CH2CH2)2CH2), 1.075 (d, 12H, 4 × CH3); Anal. (C30H52N8O4) C, H, N.
Flow Cytometric Assays
The compounds were tested to determine their ENT1nucleoside transporter binding ability by a flow cytometric assay.44 Briefly, human leukemia K562 cells growing in RPMI 1640 medium were washed once, resuspended at 1.6 × 106 cells/ml in phosphate-buffered saline at pH 7.4, and incubated with 5-(SAENTA)-X8-fluorescein (30 nM) in the presence or absence of varying concentrations of test compounds at room temperature for 45 mins. Flow cytometric measurements of cell-associated fluorescence were then performed with a FACSCalibur (Becton Dickinson, San Jose, CA) equipped with a 15 mW-argon laser (Molecular Resources Flow Cytometry Facility, University of Tennessee Health Sciences Center). In each assay, 5000 cells were analyzed from suspensions of 4 × 105 cells/ml. The units of fluorescence were arbitrary channel numbers. Percentage (%) of control (i.e., ENT1 transporter-specific fluorescence in the presence of SAENTA-fluorescein without test compounds) was calculated for each sample by the equation below (eq 1).
| (eq 1) |
where SFs is the ENT1 transporter-specific fluorescence of test samples, and SFf is the ENT1 transporter-specific fluorescence of the SAENTA-fluorescein ligand standard in mean channel numbers. The results were fed into the PRISM program (GraphPad, San Diego, CA) to derive concentration-dependent curves. From these curves, the IC50 values were obtained and used to calculate inhibition constants (Ki) values from eq 2:
| (eq 2) |
where [L] and KL are the concentration and the Kd value of the SAENTA-fluorescein, respectively57. The Ki values were used to compare the abilities of the new compounds to displace the ENT1 transporter-specific ligand (5-(SAENTA)-X8-fluorescein24 and, for that matter, their affinity for the ENT1 transporter.
Supplementary Material
Elemental analysis data for C, H and N are available (2 pages).
Acknowledgments
Financial support from the National Cancer Institute (Grant Nos. CA101856 and CA105327) and the National Institute of Allergy and Infectious Diseases (Grant No. AI065372) is hereby acknowledged.
Footnotes
Abbreviations: Cbz, carbobenzoxy; CNTs, concentrative nucleoside transporters; DMSO, dimethylsulfoxide; ENT1, Equilibrative Nucleoside Transporter 1; ENT2, Equilibrative Nucleoside Transporter 2; ENT3, Equilibrative Nucleoside Transporter 3; ENT4, Equilibrative Nucleoside Transporter 4; ESI, electrospray ionization; LC, liquid chromatography; MS, mass spectrometry; NBMPR, nitrobenzylmercaptopurine riboside; NMR, nuclear magnetic resonance; NTIs, nucleoside transporter inhibitors; SAR, structure-activity relationship; CDKs, cyclin dependent kinases; TCPP, 2,4,6,8-tetrachloropyrimido[5,4-d]pyrimidine; TLC, thin-layer chromatography; TMS, tetramethylsilane;
References
- 1.Griffith DA, Jarvis SM. Nucleoside and Nucleobase Transport Systems of Mammalian Cells. Biochim Biophys Acta. 1996;1286:153–181. doi: 10.1016/s0304-4157(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 2.Kong W, Engel K, Wang J. Mammalian Nucleoside Transporters. Curr Drug Metabolism. 2004;5:63–84. doi: 10.2174/1389200043489162. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin SA, Beal PR, Yao SYM, King AE, Cass CE, Young JD. The Equilibrative Nucleoside Transporter Family, SLC29. Pflugers Arch – Eur J Physiol. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- 4.Gray JH, Owen RP, Giacomini KM. The Concentrative Nucleoside Transporter Family, SLC28. Pflugers Arch – Eur J Physiol. 2004;447:728–734. doi: 10.1007/s00424-003-1107-y. [DOI] [PubMed] [Google Scholar]
- 5.Fritzgerald GA. Dipyridamole. N Engl J Med. 1987;316:1247–1257. doi: 10.1056/NEJM198705143162005. [DOI] [PubMed] [Google Scholar]
- 6.Abd-Elfattah AS, Wechsler AS. Separation between Ischemic and Reperfusion Injury by Site Specific Entrapment of Endogenous Adenosine and Inosine Using NBMPR and EHNA. J Card Surg. 1994;9 Suppl:387–396. doi: 10.1111/jocs.1994.9.3s.387. [DOI] [PubMed] [Google Scholar]
- 7.Abd-Elfattah ASA, Hoehner J, Wechsler AS. Identification of Nucleoside Transport Binding Sites in the Human Myocardium. Mol Cell Biochem. 1998;180:105–110. [PubMed] [Google Scholar]
- 8.Van Belle H. Nucleoside Transport Inhibition: A Therapeutic Approach to Cardioprotection via Adenosine? Cardiovasc Res. 1993;27:68–76. doi: 10.1093/cvr/27.1.68. [DOI] [PubMed] [Google Scholar]
- 9.Deckert J, Morgan PF, Marangos PJ. Adenosine Uptake Site Heterogeneity in the CNS? Uptake Inhibitors as Probes and Potential Neuropharmaceuticals. Life Sci. 1988;42:1331–1345. doi: 10.1016/0024-3205(88)90162-2. [DOI] [PubMed] [Google Scholar]
- 10.Geiger JD, Parkinson FE, Kowaluk EA. Regulators of Endogenous Adenosine Levels as Therapeutic Agents. In: Jacobson KA, Jarvis MF, editors. Purinergic Approaches in Experimental Therapeutics. Wiley-Liss; New York: 1997. pp. 55–84. [Google Scholar]
- 11.Ohta A, Stkovski M. The Role of G-Protein-Coupled Adenosine Receptors in Downregulation of Inflammation and Protection from Tissue Damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 12.Tew KD, Houghton PJ, Houghton JA. Preclinical and Clinical Modulation of Anticancer Drugs. Chapter 7. CRC Press; Boca Raton, FL: 1993. [Google Scholar]
- 13.Buolamwini JK. Nucleoside Transport Inhibitors: Structure-Activity Relationships and Potential Therapeutic Applications. Curr Med Chem. 1997;4:35–66. [Google Scholar]
- 14.Williams EF. Radioreceptor Characterization of Cardiovascular Nucleoside Transporters. SAAS Bulletin: Biochem, Biotech. 1996;9:51–56. [PubMed] [Google Scholar]
- 15.Hoehner J, Wechsler AS, Abd-Elfattah AS. Nucleoside Transport in the Human Myocardium. Dev Cardiovasc Med. 1996;181:209–218. [Google Scholar]
- 16.Zhu Z, Furr J, Buolamwini JK. Synthesis and Flow Cytometric Evaluation of Novel 1,2,3,4-Tetrahydroisoquinoline Conformationally Constrained Analogs of Nitrobenzylmercaptopurine Riboside (NBMPR) Designed for Probing Its Conformation When Bound to the es Nucleoside Transporter. J Med Chem. 2003;46:831–837. doi: 10.1021/jm020405p. [DOI] [PubMed] [Google Scholar]
- 17.Gupte A, Buolamwini JK. Novel Halogenated Nitrobenzylthioinosine Analogs as es Nucleoside Transporter Inhibitors. Bioorg Med Chem. 2004;14:2257–2260. doi: 10.1016/j.bmcl.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Hammond JR. Interaction of a Series of Draflazine Analogues With Equilibrative Nucleoside Transporters: Species Differences and Transporter Subtype Selectivity. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:373–382. doi: 10.1007/s002100000214. [DOI] [PubMed] [Google Scholar]
- 19.Cass CE, Muzik H, Paterson RP. Combination Therapy of Mouse Leukemia L1210 by 1-β-D-Arabinofuranosylcytosine and 6-[(4-Nitrobenzyl)thio]-9-β-D-Ribofuranosylpurine. Cancer Res. 1975;35:1187–1193. [PubMed] [Google Scholar]
- 20.Benedict WF, Baker MS, Haroun L, Choi E, Ames BN. Mutagenicity of Cancer Chemotherapeutic Agents in the Salmonella/Microsome Test. Cancer Res. 1977;37:2209–2213. [PubMed] [Google Scholar]
- 21.Elion GB. Biochemistry and Pharmacology of Purine Analogs. Federation Proc. 1967;26:898–904. [PubMed] [Google Scholar]
- 22.Grover GJ, Sleph PG. The Cardioprotective Effects of R 75231 and Lidoflazine Are Not Caused by Adenosine A1 Receptor Activation. J Pharmacol Exp Ther. 1994;268:90–96. [PubMed] [Google Scholar]
- 23.Mustafa SJ, Nakagawa Y. Relaxing Effects of Dipazep and Lidoflazine in Dog Cerebral and Renal Arteries Independent of Adenosine. Pharmacology. 1988;37:75–84. doi: 10.1159/000138450. [DOI] [PubMed] [Google Scholar]
- 24.Kates RA, Dorsey LM, Kaplan JA, Hatcher CR, Guyton RA. Pretreatment with Lidoflazine, A Calcium-channel Blocker. J Thorac Cardiovasc Surg. 1983;85:278–286. [PubMed] [Google Scholar]
- 25.Harker LA, Kadatz RA, Gmbh KT. Mechanism of Action of Dipyridamole. Thromb Res. 1983 IV:39–46. doi: 10.1016/0049-3848(83)90356-0. [DOI] [PubMed] [Google Scholar]
- 26.Rivey MP, Alexander MR, Taylor JW. Dipyridamole: A Critical Evaluation. Drug Intelligence and Clinical Pharmacy. 1984;18:869–880. doi: 10.1177/106002808401801103. [DOI] [PubMed] [Google Scholar]
- 27.Weber G, Prajda N. Targeted and Non-targeted Actions of Anti-cancer Drugs. Advan Enzyme Regul. 1994;34:71–89. doi: 10.1016/0065-2571(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 28.Boyer CR, Karjian PL, Wahi GM, Pegram M, Neuteboom STC. Nucleoside Transport Inhibitors, Dipyridamole and p-Nitrobenzylthioinosine, Selectively Potentiate the Antitumor Activity of NB1011. Anti-Cancer Drugs. 2002;13:29–36. doi: 10.1097/00001813-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Grem JL. Biochemical Modulation of Fluorouracil by Dipyridamole: Preclinical and Clinical Experience. Semin Oncol. 1992;19 3:56–65. [PubMed] [Google Scholar]
- 30.Smith PG, Marshman E, Newell DR, Curtin NJ. Dipyridamole Potentiates the in vitro Activity of MTA ( LY231514) by Inhibition of Thymidine Transport. Br J Cancer. 2000;82:924–930. doi: 10.1054/bjoc.1999.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherman IW. Biochemistry of Plasmodium (Malarial Parasites) Microbiol Rev. 1979;43:453–495. doi: 10.1128/mr.43.4.453-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gero AM, O'Sullivan WJ. Purines and Pyrimidines in Malarial Parasites. Blood Cells. 1990;16:467–484. [PubMed] [Google Scholar]
- 33.el Kouni MH. Potential Chemotherapeutic Targets in the Purine Metabolism of Parasites. Pharmacol Ther. 2003;99:283–309. doi: 10.1016/s0163-7258(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 34.Carter NS, Landfear SM, Ullmam B. Nucleoside Transporters of Parasitic Protozoa. Trends Parasitol. 2001;17:145. doi: 10.1016/s1471-4922(00)01806-7. [DOI] [PubMed] [Google Scholar]
- 35.el Kouni MH, Guarcello V, Al Safarjalani ON, Naguib FNM. Metabolism and Selective Toxicity of 6-Nitrobenzylthioinosine in Toxoplasma gondii. Antimicrob Agents Chemother. 1999;43:2437–2443. doi: 10.1128/aac.43.10.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akaki M, Nakano Y, Ito Y, Nagayasu E, Aikawa M. Effects of Dipyridamole on Plasmodium falciparum-Infected Erythrocytes. Parasitol Res. 2002;88:1044–1050. doi: 10.1007/s00436-002-0690-8. [DOI] [PubMed] [Google Scholar]
- 37.Kadatz R. The Pharmacology of 2,6-bis(diethanolamino)-4,8-dipiperidino-pyrimido-(5,4-d)-pyrimidine, a new compound with coronary dilatory properties. Arzneimittelforsch. 1959;9:39–45. [PubMed] [Google Scholar]
- 38.Fischer FG, Roch W, Roch J, Kottler A. Substituted Pyrimido-[5,4-d]-Pyrimidines. US Pat 3,031,450. 1962
- 39.Roch J, Beisenherz G, an der Riss B. Basic-Substituted Pyrimido-[5,4-d]-Pyrimidines. US Pat 3,074,928. 1963
- 40.Kaminsky D, Lutz WB, Lazarus S. Some Congeners and Analogs of Dipyridamole. J Med Chem. 1966;9:610–612. doi: 10.1021/jm00322a041. [DOI] [PubMed] [Google Scholar]
- 41.Roch J, Erich M, Narr B, Machleidt H. S African pat 6,905,504. 1970
- 42.Northen JS, Boyle FT, Clegg W, Curtin NJ, Edwards AJ, Griffin RJ, Golding BT. Controlled Stepwise Conversion of 2,4,6,8-Tetrachloropyrimido-[5,4-d]pyrimidine into 2,4,6,8-Tetrasubstituted Pyrimido[5,4-d]-Pyrimidines. J Chem Soc, Perkin Trans. 2002;1:108–115. [Google Scholar]
- 43.Curitn NJ, Barlow HC, Bowman KJ, Calvert AH, Davison R, Golding BT, Huang B, Loughlin PJ, Newell DR, Smith PG, Griffin RJ. Resistance-Modifying Agents. 11.1 Pyrimido[5,4-d]pyrimidine Modulators of Antitumor Drug Activity. Synthesis and Structure-Activity Relationships for Nucleoside Transport Inhibition and Binding to α1-Acid Glycoprotein. J Med Chem. 2004;47:4905–4922. doi: 10.1021/jm040772w. [DOI] [PubMed] [Google Scholar]
- 44.Buolamwini JK, Wiley JS, Robins MJ, Craik JD, Cass CE, Gati WP, Paterson ARP. Conjugates of Fluorescein and SAENTA (5′-S-(2-aminoethyl)-N6-(4-nitrobenzyl)-5′-Thioadenosine): Flow Cytometry Probes for the Es Transporter Elements of the Plasma Membrane. Nucleosides Nucleotides. 1994;13:737–751. [Google Scholar]
- 45.Hammond JR. Kinetic Analysis of Ligand Binding to the Ehrlich Cell Nucleoside Transporter: Pharmacological Characterization of Allosteric Interaction with the [3H]Nitrobenzylthioinosine Binding Site. Mol Pharmacol. 1991;39:771–779. [PubMed] [Google Scholar]
- 46.Jones KW, Hammond JR. Heterogeneity of [3H]Dipyridamole Binding to CNS Membrances: Correlation with [3H]Nitrobenzylthioinosine Binding and [3H]Uridine Influx Studies. J Neurochem. 1992;59:1363–1371. doi: 10.1111/j.1471-4159.1992.tb08449.x. [DOI] [PubMed] [Google Scholar]
- 47.Deckert J, Hennemann A, Bereznai B, Fritze J, Vock R, Marangos PJ, Riederer P. (3H)Dipyridamole and (3H)Nitrobenzylthioinosine Binding Sites at the Human Parietal Cortex and Erythrocyte Adenosine Transporter: A Comparison. Life Sci. 1994;55:1675–1682. doi: 10.1016/0024-3205(94)00276-2. [DOI] [PubMed] [Google Scholar]
- 48.Vargas F, Rivas C, Fuentes A, Tse Cheng A, Velutini G. The Photochemistry of Dipyridamole. J Photochem Photobiol. 2002;153:237–243. [Google Scholar]
- 49.Boleti H, Coe IR, Baldwin SA, Young JD, Cass CE. Molecular Identification of the Equilibrative NBMPR-sensitive (es) Nucleoside Transporter and Demonstration of an Equilibrative NBMPR-insensitive (ei) Transport Activity in Human Erythroleukemia (K562) Cells. Neuropharmacology. 1997;36:1167–1179. doi: 10.1016/s0028-3908(97)00136-6. [DOI] [PubMed] [Google Scholar]
- 50.Huang Y, Anderle P, Bussey KJ, Barbacioru C, Shankavaram U, Dai Z, Reinhold WC, Papp A, Weinstein JN, Sadee W. Membrane Transporters and Chennels: Role of the Transportome in Cancer Chemosensitivity and Chemoresistance. Cancer Res. 2004;64:4294–4301. doi: 10.1158/0008-5472.CAN-03-3884. [DOI] [PubMed] [Google Scholar]
- 51.Addo JK, Buolamwini JK. Design, Synthesis, and Evaluation of 5′-S-aminoethyl-N(6)-azidobenzyl-5′-thioadenosine Biotin Conjugate: A Bifunctional Photoaffinity Probe for the es Nucleoside Transporter. Bioconjug Chem. 2004;15:536–540. doi: 10.1021/bc034165j. [DOI] [PubMed] [Google Scholar]
- 52.Huang M, Wang Y, Cogut SB, Mitchell BS, Graves LM. Inhibition of Nucleoside Transport by Protein Kinase Inhibitors. J Pharmacol Exp Ther. 2003;304:753–760. doi: 10.1124/jpet.102.044214. [DOI] [PubMed] [Google Scholar]
- 53.Huang M, Wang Y, Collins M, Gu JJ, Mitchell BS, Graves LM. Inhibition of Nucleoside Transport by p38 MARK Inhibitors. J Biol Chem. 2002;277:28364–28367. doi: 10.1074/jbc.C200321200. [DOI] [PubMed] [Google Scholar]
- 54.Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, Newman AH. Novel N-Substituted 3α-[Bis(4′-fluorophenyl)methoxyltropane Analogs: Selective Ligands for the Dopamine Transporter. J Med Chem. 1997;40:4329–4339. doi: 10.1021/jm970525a. [DOI] [PubMed] [Google Scholar]
- 55.Georgiev VS, Kinsolving CR. Substituted 4-Azatricyclo[4.3.1.13,8]undecane Compounds. US Pat 4,557,865. 1985
- 56.Williams DE, Craig KS, Patrick B, McHardy LM, Soest RV, Roberge M, Andersen RJ. Motuporamines, Anti-Invasion and Anti-Angiogenic Alkaloids from the Marine Sponge Xestospongia exigua (Kirkpatrick): Isolation, Structure Elucidation, Analog Synthesis, and Conformational Analysis. J Org Chem. 2002;67:245–258. doi: 10.1021/jo016101c. [DOI] [PubMed] [Google Scholar]
- 57.Cheng Y, Prusoff WH. Relationship between the Inhibition Constant (Ki) and the Concentration of Inhibitor Which Causes 50 Percent Inhibition (IC50) of an Enzymatic Reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Elemental analysis data for C, H and N are available (2 pages).