Abstract
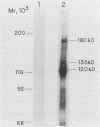
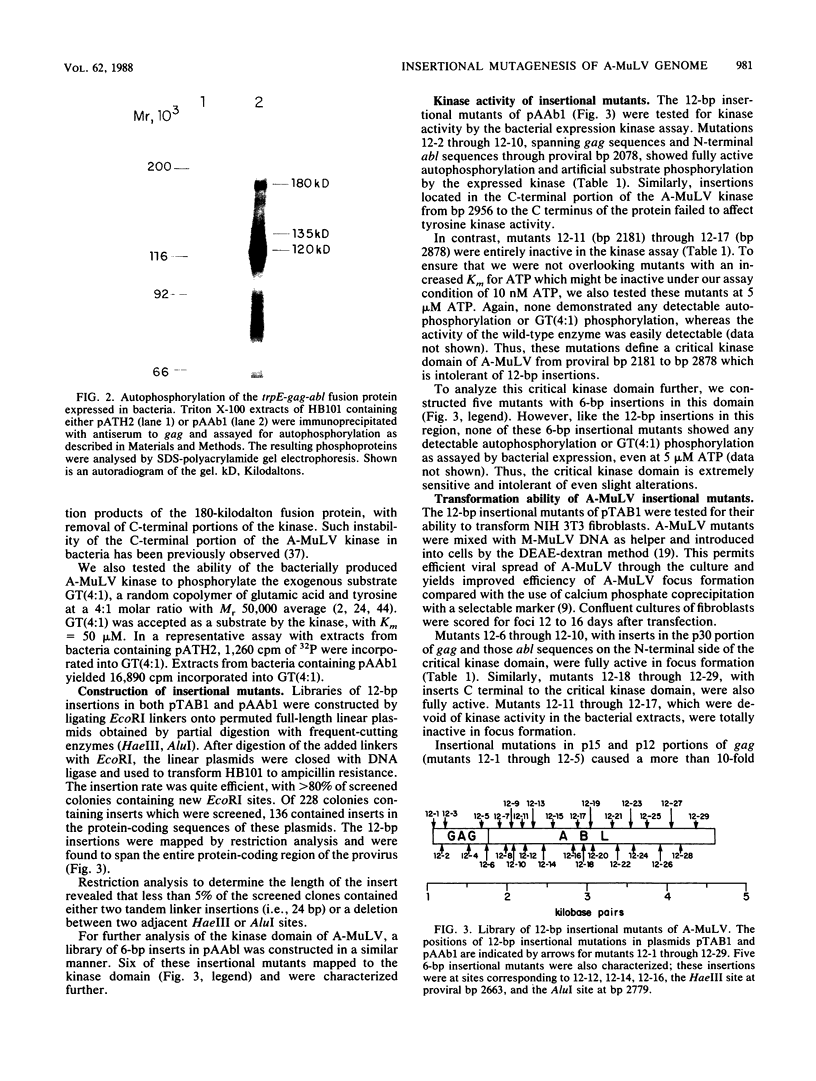
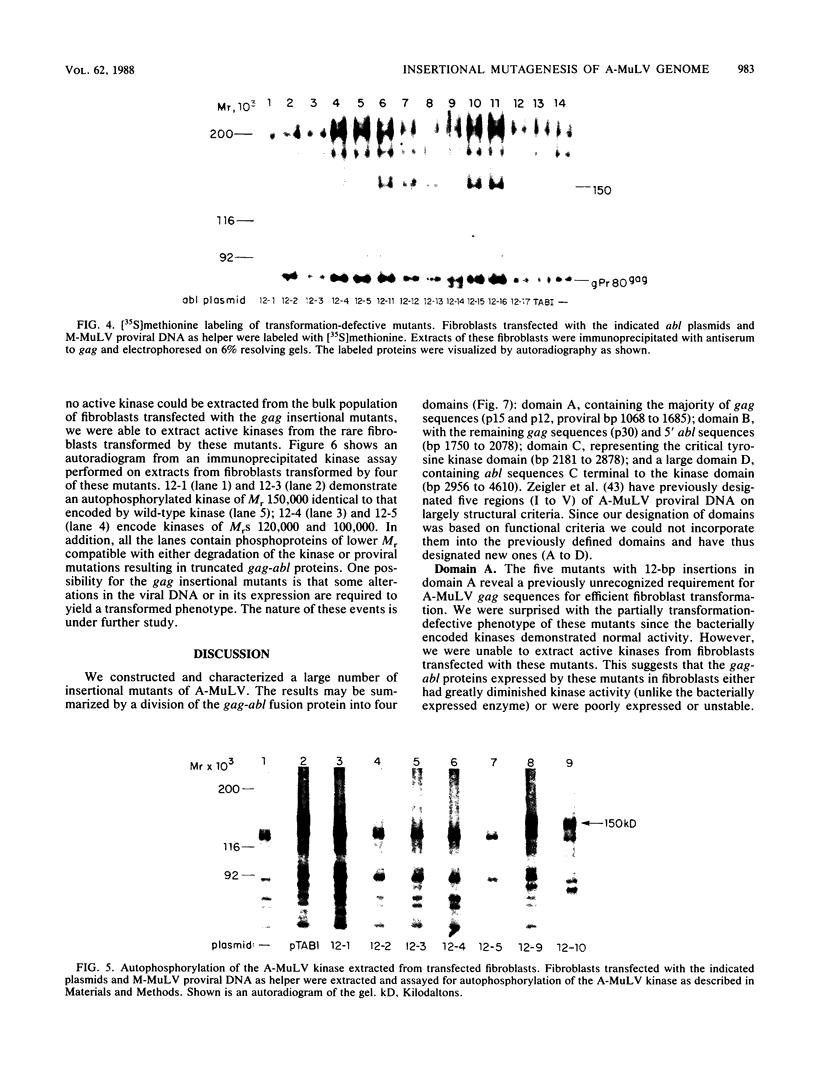
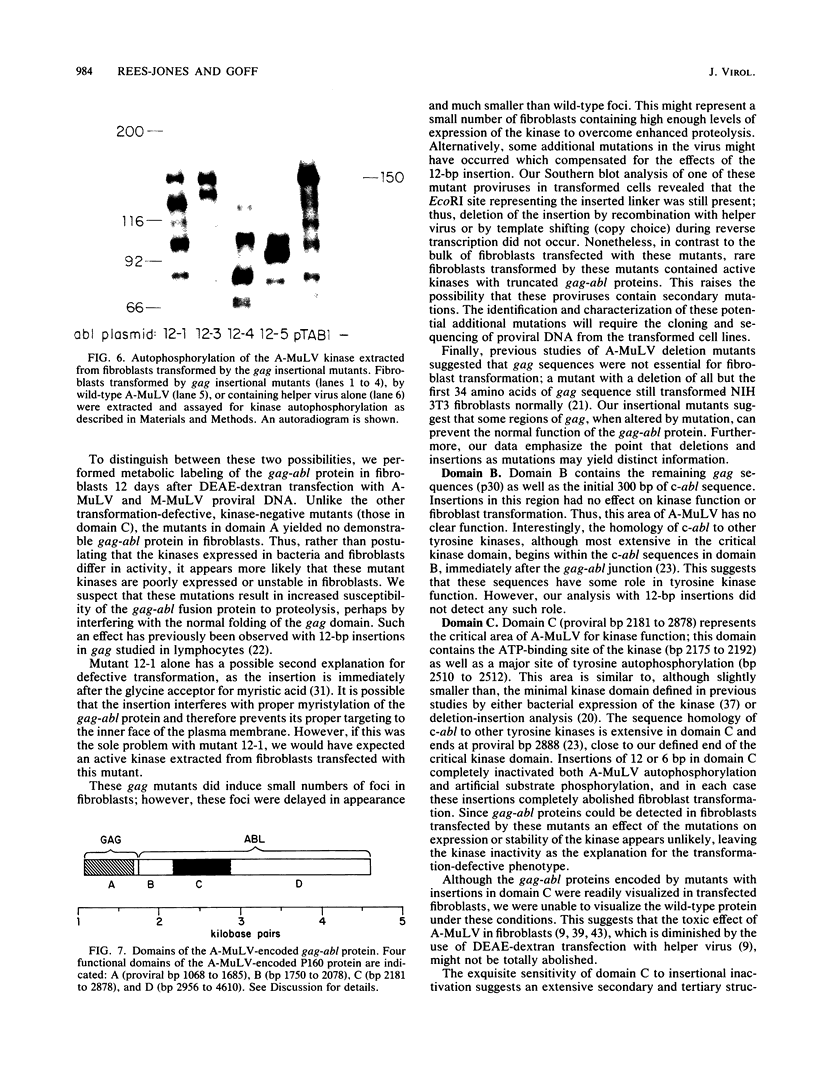
A library of Abelson murine leukemia virus (A-MuLV) proviral DNAs with 12- or 6-base-pair (bp) insertional mutations was constructed. The 29 mutations characterized spanned the entire protein-coding region of the provirus. We tested the effects of these mutations both on the kinase activity of the gag-abl fusion protein encoded by the provirus and on the ability of the provirus to transform NIH 3T3 fibroblasts. To simplify assessment of the mutant kinases, we expressed the A-MuLV-encoded kinase in the bacterial expression vector pATH2, resulting in production of a trpE-gag-abl fusion protein in Escherichia coli. We used an immunoprecipitation kinase assay to measure both autophosphorylation and artificial substrate phosphorylation by the mutant kinases. To assay transformation ability of the mutant proviruses, we transfected NIH 3T3 fibroblasts with the mutants and with helper virus (Moloney MuLV) by the DEAE-dextran method. Our analysis of these A-MuLV insertional mutants allows the division of the protein-coding region of the provirus into four domains: domain A (proviral bp 1068 to 1685), in which insertions have no effect on the bacterially expressed kinase, but diminish both kinase activity and transformation efficiency in fibroblasts; domain B (bp 1750 to 2078), in which insertions have no effect on the provirus; domain C (bp 2181 to 2878), the critical kinase domain, in which 12-bp or even 6-bp insertions completely inactivate the A-MuLV kinase and result in transformation-defective proviruses; and domain D (bp 2956 to 4610), the large C-terminal domain in which mutations are silent.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S., Raymond W. E., Racker E. Synthetic tyrosine polymers as substrates and inhibitors of tyrosine-specific protein kinases. J Biol Chem. 1984 Feb 25;259(4):2051–2054. [PubMed] [Google Scholar]
- Colicelli J., Lobel L. I., Goff S. P. A temperature-sensitive mutation constructed by "linker insertion" mutagenesis. Mol Gen Genet. 1985;199(3):537–539. doi: 10.1007/BF00330771. [DOI] [PubMed] [Google Scholar]
- Ferguson B., Pritchard M. L., Feild J., Rieman D., Greig R. G., Poste G., Rosenberg M. Isolation and analysis of an Abelson murine leukemia virus-encoded tyrosine-specific kinase produced in Escherichia coli. J Biol Chem. 1985 Mar 25;260(6):3652–3657. [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Ross A. H., Eisen H. N. Characterization and use of monoclonal antibodies for isolation of phosphotyrosyl proteins from retrovirus-transformed cells and growth factor-stimulated cells. Mol Cell Biol. 1983 Aug;3(8):1343–1352. doi: 10.1128/mcb.3.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., D'Eustachio P., Ruddle F. H., Baltimore D. Chromosomal assignment of the endogenous proto-oncogene C-abl. Science. 1982 Dec 24;218(4579):1317–1319. doi: 10.1126/science.6293057. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Tabin C. J., Wang J. Y., Weinberg R., Baltimore D. Transfection of fibroblasts by cloned Abelson murine leukemia virus DNA and recovery of transmissible virus by recombination with helper virus. J Virol. 1982 Jan;41(1):271–285. doi: 10.1128/jvi.41.1.271-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P. The Abelson murine leukemia virus oncogene. Proc Soc Exp Biol Med. 1985 Sep;179(4):403–412. doi: 10.3181/00379727-179-42115. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Witte O. N., Gilboa E., Rosenberg N., Baltimore D. Genome structure of Abelson murine leukemia virus variants: proviruses in fibroblasts and lymphoid cells. J Virol. 1981 May;38(2):460–468. doi: 10.1128/jvi.38.2.460-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipreos E. T., Lee G. J., Wang J. Y. Isolation of temperature-sensitive tyrosine kinase mutants of v-abl oncogene by screening with antibodies for phosphotyrosine. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1345–1349. doi: 10.1073/pnas.84.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Davis R. L., Watanabe S. M., Ponticelli A. S., Schiff-Maker L., Rosenberg N., Witte O. N. Only site-directed antibodies reactive with the highly conserved src-homologous region of the v-abl protein neutralize kinase activity. J Virol. 1984 Jul;51(1):223–232. doi: 10.1128/jvi.51.1.223-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lobel L. I., Goff S. P. Construction of mutants of Moloney murine leukemia virus by suppressor-linker insertional mutagenesis: positions of viable insertion mutations. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4149–4153. doi: 10.1073/pnas.81.13.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Baltimore D. The minimum transforming region of v-abl is the segment encoding protein-tyrosine kinase. J Virol. 1985 Apr;54(1):114–122. doi: 10.1128/jvi.54.1.114-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Rosenberg N., Baltimore D. Sequences of the A-MuLV protein needed for fibroblast and lymphoid cell transformation. Cell. 1983 Sep;34(2):569–579. doi: 10.1016/0092-8674(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Prywes R., Hoag J., Rosenberg N., Baltimore D. Protein stabilization explains the gag requirement for transformation of lymphoid cells by Abelson murine leukemia virus. J Virol. 1985 Apr;54(1):123–132. doi: 10.1128/jvi.54.1.123-132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees-Jones R. W., Hendricks S. A., Quarum M., Roth J. The insulin receptor of rat brain is coupled to tyrosine kinase activity. J Biol Chem. 1984 Mar 25;259(6):3470–3474. [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Van de Ven W. J., Stephenson J. R. Abelson murine leukemia virus transformation-defective mutants with impaired P120-associated protein kinase activity. J Virol. 1980 Nov;36(2):374–386. doi: 10.1128/jvi.36.2.374-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg N. E., Clark D. R., Witte O. N. Abelson murine leukemia virus mutants deficient in kinase activity and lymphoid cell transformation. J Virol. 1980 Dec;36(3):766–774. doi: 10.1128/jvi.36.3.766-774.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D., Scher C. D. In vitro transformation of lymphoid cells by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1932–1936. doi: 10.1073/pnas.72.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Witte O. N. Abelson murine leukemia virus mutants with alterations in the virus-specific P120 molecule. J Virol. 1980 Jan;33(1):340–348. doi: 10.1128/jvi.33.1.340-348.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Schultz A., Oroszlan S. Myristylation of gag-onc fusion proteins in mammalian transforming retroviruses. Virology. 1984 Mar;133(2):431–437. doi: 10.1016/0042-6822(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Raschke W. C. Evidence that the Abelson virus protein functions in vivo as a protein kinase that phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1552–1556. doi: 10.1073/pnas.78.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A., Dunn C. Y., Yuasa Y., Devare S. G., Reddy E. P., Aaronson S. A. Abelson murine leukemia virus: structural requirements for transforming gene function. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5508–5512. doi: 10.1073/pnas.79.18.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori T., Miyazoe I., Shirasawa T., Taniguchi M., Graf T. A temperature-sensitive mutant of Abelson murine leukemia virus confers inducibility of IgM expression to transformed lymphoid cells. EMBO J. 1987 Apr;6(4):951–956. doi: 10.1002/j.1460-2075.1987.tb04844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Characterization of the Abelson murine leukemia virus-encoded tyrosine-specific protein kinase. Methods Enzymol. 1983;99:373–378. doi: 10.1016/0076-6879(83)99073-0. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Localization of tyrosine kinase-coding region in v-abl oncogene by the expression of v-abl-encoded proteins in bacteria. J Biol Chem. 1985 Jan 10;260(1):64–71. [PubMed] [Google Scholar]
- Wang J. Y., Ledley F., Goff S., Lee R., Groner Y., Baltimore D. The mouse c-abl locus: molecular cloning and characterization. Cell. 1984 Feb;36(2):349–356. doi: 10.1016/0092-8674(84)90228-9. [DOI] [PubMed] [Google Scholar]
- Watanabe S. M., Witte O. N. Site-directed deletions of Abelson murine leukemia virus define 3' sequences essential for transformation and lethality. J Virol. 1983 Mar;45(3):1028–1036. doi: 10.1128/jvi.45.3.1028-1036.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Goff S., Rosenberg N., Baltimore D. A transformation-defective mutant of Abelson murine leukemia virus lacks protein kinase activity. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4993–4997. doi: 10.1073/pnas.77.8.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N. Molecular and cellular biology of Abelson virus transformation. Curr Top Microbiol Immunol. 1983;103:127–146. doi: 10.1007/978-3-642-68943-7_6. [DOI] [PubMed] [Google Scholar]
- Zick Y., Rees-Jones R. W., Grunberger G., Taylor S. I., Moncada V., Gorden P., Roth J. The insulin-stimulated receptor kinase is a tyrosine-specific casein kinase. Eur J Biochem. 1983 Dec 15;137(3):631–637. doi: 10.1111/j.1432-1033.1983.tb07872.x. [DOI] [PubMed] [Google Scholar]
- Ziegler S. F., Whitlock C. A., Goff S. P., Gifford A., Witte O. N. Lethal effect of the Abelson murine leukemia virus transforming gene product. Cell. 1981 Dec;27(3 Pt 2):477–486. doi: 10.1016/0092-8674(81)90389-5. [DOI] [PubMed] [Google Scholar]