Abstract
Melatonin is involved in regulation of a variety of physiological functions through activation of specific G-protein coupled receptors. However, the neuromodulatory role of melatonin, released from photoreceptors in the retina, is poorly understood. Here we show that melatonin enhances the sensitivity of the rod signal pathway by potentiating signal transfer from rod photoreceptors to ON bipolar cells (Rod-ON-BCs). Whole-cell patch-clamp recordings showed that melatonin induced a sustained inward current from Rod-ON-BCs, through activation of the melatonin MT2 receptor, which was identified as one mediated by a cGMP-dependent cation channel. Consistent with this, melatonin was found, using immunocytochemistry, to increase intracellular cGMP levels, which was identified due to an inhibition of phosphodiesterase. Physiologically, melatonin potentiated responses of Rod-ON-BCs to simulated light flashes (brief puffs of CPPG, an mGluR6 antagonist, in the presence of l-AP4, an mGluR6 agonist), which was mediated by cGMP-dependent kinase, and increased the amplitude of the scotopic electroretinographic b-wave, a reflection of Rod-ON-BC activity. These results suggest that melatonin, being at a higher level at night, may improve the signal/noise ratio for rod signals in the outer retina by enhancing signal transfer from rods to BCs.
Bipolar cells (BCs) that relay signals from photoreceptors to ganglion cells occupy a pivotal position in retinal information processing. It is at the BC level that separation of ON and OFF information first appears for afferent inputs from photoreceptors (Dowling, 1987). ON- and OFF-BCs are depolarized and hyperpolarized by illumination of their receptive field centres, respectively. In teleost retina ON- and OFF-BCs are either rod- or cone-dominant (Kato et al. 1991). Rod-dominant ON-BCs (Rod-ON-BCs) express the metabotropic glutamate receptor mGluR6 (Nakajima et al. 1993), activation of which leads to a closure of cGMP-dependent non-specific cation channels (Nawy & Jahr, 1990; Shiells & Falk, 1990). The signalling cascade linking the activation of the mGluR6 to the closure of the cGMP-dependent channel is not well understood. The channel closure may be induced by a phosphodiesterase (PDE)-mediated decrease of intracellular cGMP levels ([cGMP]i) due to the activation of mGluR6 (Nawy & Jahr, 1990; Shiells & Falk, 1990) and/or by a direct interaction of the G-protein Go (Vardi, 1998; Nawy, 1999). It is of interest that the concentration of cGMP in the retina changes in a circadian manner (Faillace et al. 1995) and cGMP-dependent cation channels in photoreceptors show a circadian change in activity (Ko et al. 2001).
Involvement of melatonin (5-methoxy-N-acetyltryptamine), a hormone originally discovered in the pineal gland, in the regulation of various physiological processes has been extensively investigated (Vanecek, 1998). Most recently, it has been reported that melatonin suppresses night-time memory formation in zebrafish (Rawashdeh et al. 2007). The effects of melatonin are mediated through activation of specific melatonin receptors, which are currently classified into MT1 (Mel1a), MT2 (Mel1b) and MT3 (ML2) subtypes (Reppert, 1997). These subtypes are all G-protein coupled (Reppert et al. 1994, 1995; Kunwar et al. 2003; Zhang et al. 2006), activation of which results in changes of cGMP, cAMP and/or other second messengers (Vanecek, 1998). In the vertebrate retina, synthesis and release of melatonin in photoreceptors change in a circadian manner, being at higher levels at night and at lower levels during the daytime (Hamm & Menaker, 1980; Cahill, 1996; Iigo et al. 1997). The existence of melatonin receptors in the retina has also been demonstrated in various species (Reppert et al. 1995; Savaskan et al. 2002; Wiechmann et al. 2004; Sallinen et al. 2005). In addition to the implication of melatonin in many retinal processes, such as retinomotor responses, rod disc shedding and dopamine release (Vanecek, 1998), this hormone is involved in modulating activities of retinal neurons (Wiechmann et al. 1988; Ribelayga et al. 2004). A previous work in this laboratory demonstrated that melatonin modulates glutamatergic transmission from cones to cone-driven horizontal cells in carp retina via a reduction of [cGMP]i caused by activation of the MT1 receptor (Huang et al. 2005).
Here we show that melatonin induces a current mediated by cGMP-dependent cation channels via activation of the MT2 receptor in carp Rod-ON-BCs and causes an increase in [cGMP]i through suppressing the activity of PDE. We further show that melatonin potentiates responses to simulated light flashes of Rod-ON-BCs by stimulating cGMP-dependent kinase (cGK) and scotopic electroretinographic b-waves. These findings suggest that melatonin enhances the sensitivity of the pathway for rod signals in the outer retina.
Methods
Retinal slice preparation
Retinal slices of carp (Carassius carassius) were prepared following a procedure basically similar to Shen et al. (2005). The animals, maintained under a 12 h: 12 h light: dark cycle for at least 1 week, were dark-adapted for 20 min prior to an experiment. They were deeply anaesthetized by immersion in a 0.05% solution of tricaine methylsulphonate (MS-222), before being decapitated and pithed. The eyecup was treated for 5 min with 1 mg hyaluronidase in 1 ml Ringer solution to remove the vitreous humour under room illumination. Animal treatments were in accordance with the NIH guidelines for animal experimentation and the guidelines of Fudan University on the ethical use of animals. The isolated retina was placed on a filter paper with the ganglion cell layer down and sliced at 200 μm intervals using a manual cutter (ST-20, Narishige, Japan). Retinal slices were then transferred into a recording chamber, placed with the cut side up, and viewed through a fixed-stage upright microscope (BX51WI, Olympus, Japan) equipped with a 60× water-immersion ceramic objective and DIC optics. The slices were perfused continuously with carbogen-bubbled Ringer solution, which contained (in mm): NaCl 126, KCl 2.4, CaCl2 2, MgCl2 1.2, NaHCO3 28, glucose 10. We used picrotoxin (100 μm), strychnine (1 μm) and CoCl2 (500 μm), replacing CaCl2 (2 mm), to block synaptic transmission in the slices except for the experiments with simulated light responses. Rod-ON-BCs were identified according to the well-established criteria. ON BCs exhibit a soma in the distal part of the inner nuclear layer (INL), closely apposed to the outer plexiform layer (OPL), and an axon heading down to sublamina b of the inner plexiform layer (IPL) (Saito et al. 1985; Wong et al. 2005). Morphologically, Rod-ON-BCs have relatively larger somata (> 10 μm) than Cone-ON-BCs. Moreover, the Rod-ON-BC possesses a single enlarged axon terminal and flourishing and arborous dendrites, which are clearly different from Cone-ON-BCs in carp retina (Saito & Kujiraoka, 1982). Further identification was provided by checking responses of these cells to glutamate. Whilst both Rod- and Cone-ON-BCs respond to glutamate applied to the dendrites with outward currents, the currents of Rod-ON-BCs, but not Cone-ON-BCs, are blocked by 1 mm Cd2+ (Becchetti & Roncaglia, 2000). Moreover, due to their relatively larger somata, Rod-ON-BCs commonly have a larger capacitance than Cone-ON-BCs. Lucifer yellow-filled BCs were visualized using a mercury light source and a FITC filter set, and the pictures were taken by a cool CCD (Photometrics, CoolSNAP ES, USA).
Whole-cell recording
Whole-cell responses of BCs were conventionally recorded with pipettes of 6–10 MΩ resistance in both voltage-clamp and current-clamp modes, when filled with a solution containing (in mm): potasium gluconate 95, KCl 10, CaCl2 0.5, MgCl2 1, EGTA 10, Hepes 10, ATP 4, GTP 0.5 and Lucifer yellow 0.5, pH 7.3 adjusted with KOH. In the experiments with simulated light responses, CaCl2 (0.5 mm) and EGTA (10 mm) were replaced by 20 mm BAPTA and the concentration of potassium gluconate was reduced from 95 mm to 85 mm (Snellman & Nawy, 2004). The pipettes were mounted on a motor-driven micromanipulator (MP-285, Sutter, USA), and connected to an EPC10 patch-clamp amplifier (Heka, Germany). The series resistances were under 50 MΩ and compensated for 80%. Cells were discarded when they exhibited series resistances exceeding 50 MΩ or showed large fluctuations during the recording. The holding current was often checked and only the data obtained in cells with the holding current of less than 80 pA during the first application of drugs were included in the present work. The average cell capacitance of Rod-ON-BCs was 11.68 ± 1.07 pF (n = 62). Data were filtered at 2 kHz, sampled at 5 kHz, and then stored for further analysis. In the text current densities (pA pF−1), instead of current amplitudes, were used for comparing data obtained in different cells to avoid fluctuations of current amplitudes due to different cell sizes. Drug-containing Ringer solutions were either focally applied through a puff pipette (tip diameter ∼2 μm) by a pressure of 35 kPa (5 p.s.i.) with a PicoSprizer II unit (General Value Co., USA), or administrated in the bath medium through another inlet by a peristaltic pump (Rainin Instrument Co. Inc., USA), according to the purpose of the experiment. Impermeant drugs (cGMP, Lucifer yellow, KT5823), HS-142-1 (a kind gift from Prof. Chiming Wei, Johns Hopkins University, Baltimore, MD, USA) and IBMX were dialysed into neurons after membrane rupture by including them in the pipette.
ERG recording
ERG recordings were made from the isolated, superfused flat-mounted retina during the subjective daytime, which was dark-adapted for at least 2 h. The retina, isolated from the posterior eyecup, was superfused with oxygenated (95% O2, 5% CO2) Ringer solution, consisting of (in mm): NaCl 116, KCl 2.4, MgCl2 1.2, NaHCO3 30 and glucose 10, buffered to pH 7.5, without addition of CaCl2 to mimic the conditions performing the experiments with simulated light responses (Xu & Yang, 2002). The preparation was illuminated diffusely from the photoreceptor side by a photostimulator, which provided an 8 mm diameter spot around the electrode tip. Light intensity and wavelength of the beam were changed by calibrated neutral density and interference filters, respectively. All light intensities referred to in the text are in log units relative to the maximum intensity (log I = 0), which was 6.4 × 1011 quanta cm−2 s−1. Control and drug-containing perfusates were applied by gravity. ERG responses were recorded between a Ag–AgCl ball electrode in contact with the retina at the photoreceptor side and a similar reference electrode in the bottom of the chamber by an amplifier (FZG-81, Shanghai Institute of Physiology, Chinese Academy of Sciences, Shanghai, China) with a bandpass of 0.1–1000 Hz. The data were recorded by pCLAMP 8.1 and Clampfit (Axon Instruments, Inc., CA, USA) for subsequent off-line analysis. Scotopic PIII responses were obtained by perfusing the retina with 3 mm glutamate-containing Ringer solution.
Immunocytochemistry and confocal microscopy
Procedures for immunocytochemical double-labelling analysis were basically similar to those used in the previous work (Huang et al. 2005). Isolated retinae were first incubated in melatonin (100 nm)-containing or normal Ringer solution for 5 min and then immersion-fixed for 20 min at 4°C in fresh 4% formaldehyde in phosphate buffer solution (PBS, pH 7.4), with or without 100 nm melatonin. The primary antibodies used were rabbit polyclonal antibody against cGMP (1: 500; Chemicon, Temecula, CA, USA) and mouse monoclonal antibody against PKC (1: 2000; Sigma, St Louis, MO, USA), whereas the secondary antibodies were Texas red-conjugated donkey anti-rabbit IgG and fluorescein isothiocyanate (FITC)-conjugated donkey anti-mouse IgG (1: 200; Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The preparations were sequentially cryoprotected at 4°C in 0.1 m PBS with 10%, 20% and 30% sucrose for 2 h, 2 h, and overnight, respectively. They were embedded in OCT (Miles Inc., Elkhart, IN, USA) and frozen by liquid nitrogen. Vertical sections, made at 14 μm thickness on a freezing microtome (Leica, Nussloch, Germany), were collected on gelatin chromium-coated slides. The sections were blocked and permeabilized with 6% normal donkey serum, 1% normal bovine serum albumin and 0.2% Triton X-100 in PBS overnight at 4°C, followed by incubation with the primary antibodies. Binding sites were revealed by the secondary antibodies. The sections were incubated sequentially in the primary and secondary antibodies at 4°C for 3 days and 2 h, respectively. Washed with PBS and coverslipped, fluorescently labelled sections were imaged with a Leica SP2 confocal laser scanning microscope (Leica, Mannheim, Germany) using a 63× oil-immersion objective lens. Single optical sections were made through the preparation at intervals of 1.0 μm. To assess the changes in [cGMP]i quantitatively, confocal micrographs of retinal sections doubled-labelled by PKC and cGMP in Ringer solution and after incubation of 100 nm melatonin were taken, and red fluorescence intensities for cGMP under these two conditions were transformed into grey values by the system. Grey values of the labelled Rod-ON-BC somata were then determined after subtracting the background value, which was the grey value of a non-cGMP-immunostained area (20 μm × 20 μm) in the micrograph, by Photoshop CS2 software. Commonly, two Rod-ON-BCs in a single section were picked up for data collection and all data were pooled from 10 preparations. For comparison, such determination was also made for Cone-ON-BCs.
Statistical analysis
All the data were presented as means ± s.e.m. and statistical analysis was made using paired or unpaired Student's t test. We used the t tests to measure differences between trial types (P < 0.05).
Chemicals
All chemicals were obtained from Sigma Chemical Co. (St Louis, MO, USA), unless otherwise specified. Melatonin, luzindole, 4-phenyl-2-propionamidotetralin (4-P-PDOT; Tocris Bioscience), HS-142-1, 1H-[1,2,4]oxiiazolo[4,3-a]quinoxaline-1-one (ODQ), 3-isobutyl-1-methylxanthine (IBMX) and KT5823 were first dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO was less than 0.1% that had no effects on current and voltage responses of ON-BCs.
Results
Melatonin induces cGMP-dependent cation currents in Rod-ON-BCs
Using whole-cell recording, we first investigated effects of melatonin on Rod-ON-BCs. Figure 1A shows a representative Rod-ON-BC intracellularly stained by Lucifer yellow in a retinal slice. The cell exhibited bush-like dendrites in the outer plexiform layer (OPL) and a long axon with a unique enlarged terminal in the proximal part of the inner plexiform layer (IPL). Perfusion of 100 nm melatonin induced an inward current with a long delay (∼50 s) from the cell and reached a peak in 1–2 min (Fig. 1B). The current was accompanied by an increase in noise level, probably due to fluctuations in the underlying channel activity. Such inward currents were recorded in most of Rod-ON-BCs tested (20 out of 24 cells), with an averaged maximal density of 15.24 ± 0.54 pA pF−1 (n = 20), and they showed little sign of desensitization for a period as long as 20 min. Inward currents could be induced, but with smaller amplitudes, even by 10 nm melatonin (data not shown). Currents recorded in all four cells tested were blocked by 100 nm luzindole, a competitive antagonist of both MT1 and MT2 receptors (Fig. 1B). Melatonin-induced currents were also completely suppressed by addition of 100 nm 4-P-PDOT (n = 10), a specific antagonist of the MT2 receptor (Fig. 1C), suggesting the involvement of the MT2 receptor.
Figure 1. Melatonin induces a cGMP-dependent cation current from carp retinal Rod-ON-BCs.
A, a Lucifer yellow-filled Rod-ON-BC that is characterized by an enlarged characteristic terminal bulb (arrowhead). Scale bar, 10 μm. B, melatonin (MEL) of 100 nm induced a sustained inward current from the Rod-ON-BC shown in A, voltage-clamped at −60 mV, with a long delay. The current was almost completely blocked by lunzindole (LUZ) of 100 nm. C, melatonin-induced inward current was blocked by addition of 100 nm 4-P-PDOT. D, current was suppressed by addition of 3 mm Cd2+ in a reversible manner. E, puff of 8-Br-cGMP (1 mm) on the dendrites of a Rod-ON-BC for 50 s induced a sustained inward current (inset; scale bars, 4 pA pF−1, 20 s). I–V relationships of 8-Br-cGMP- (grey) and melatonin- (black) induced currents, from two Rod-ON-BCs, obtained using a voltage ramp from −80 mV to 40 mV for 500 ms. Both curves show slight outward rectification with an Erev of 2.0 mV and 1.5 mV, respectively. F, internal infusion of 2 mm cGMP induced a sustained inward current, and addition of 100 nm melatonin to the bath solution failed to induce any discernable current. G, puff of 3 mm glutamate (Glu) to the dendrites of a Rod-ON-BC induced an outward current. In the presence of 100 nm melatonin, the glutamate current much increased in size.
To characterize the melatonin-induced currents, we studied effects of external Cd2+, which is supposed to block cGMP-dependent cation channels, and found that these currents were completely blocked by addition of 3 mm Cd2+ in a reversible way (n = 5; Fig. 1D). Figure 1E shows the current–voltage (I–V) relationship of the melatonin (100 nm)-induced current from a Rod-ON-BC, using a voltage ramp from −80 mV to 40 mV for 500 ms (black curve), which showed slight outward rectification, exhibiting a reversal potential (Erev) of 1.5 mV (with a mean of 1.7 ± 3.2 mV, n = 5). Application of 8-bromoguanosine-3′,5′-cyclomonophosphate (8-Br-cGMP) mimicked the effects of melatonin. The inset of Fig. 1E shows the inward current of a Rod-ON-BC, induced by 1 mm 8-Br-cGMP applied to the soma. Similarly, the current did not show desensitization, and the I–V curve of the current (grey curve in Fig. 1E) almost coincided with the curve for the melatonin-induced current, yielding an Erev of 2 mV (with an average of 2.5 ± 1.9 mV, n = 5). Internal infusion with 2 mm cGMP induced a similar sustained current with a slow rise, exhibiting an average steady current density of 15.62 ± 0.93 pA pF−1 (n = 6), and during the internal infusion, 100 nm melatonin failed to induce any currents (Fig. 1F).
All the above data suggest that the melatonin current was mediated by a cGMP-dependent channel. Figure 1G shows how the melatonin-induced inward current interacted with the glutamate-induced outward current in a Rod-ON-BC. Glutamate of 3 mm was first applied alone for 5 s, which induced a sustained outward current (a). Following washout with Ringer solution application of 100 nm melatonin induced a sustained inward current (b), and in the presence of melatonin 3 mm glutamate induced a current of much larger density (c), compared to that obtained in Ringer solution. Actually, the current (c) obtained in the presence of melatonin was approximately an algebraic sum of the glutamate-induced one (a) and the melatonin-induced one (b). On average, 100 nm melatonin caused a 2.35 ± 0.56-fold increase of the amplitudes of the glutamate (3 mm) currents (n = 4).
We also tested effects of melatonin and cGMP on Cone-ON-BCs. Neither perfusion of 100 nm melatonin (n = 6) nor internal infusion of 2 mm cGMP (n = 5) induced any discernable currents from these cells (data not shown).
Melatonin increases intracellular cGMP levels of Rod-ON-BCs
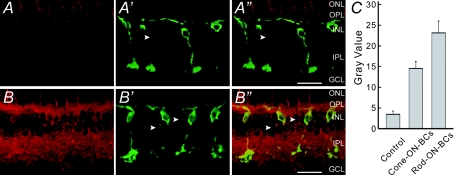
By immunocytochemistry we confirmed that melatonin indeed enhanced [cGMP]i of Rod-ON-BCs. Figure 2A and B shows confocal laser scanning micrographs of the vertical sections of the carp retinae, incubated in normal Ringer solution (A and A″) and melatonin-containing Ringer solution (B and B″), respectively, double-labelled with the antibodies against cGMP and PKC, a specific marker for ON-BCs (Negishi et al. 1988). The retina incubated in normal Ringer solution showed no detectable cGMP-like immunoreactivity, except for faint fluorescence in photoreceptor inner segments (Fig. 2A). As clear from the merged image (Fig. 2A″) of Fig. 2A and Fig. 2A′, showing staining for PKC, there was no detectable cGMP in the Rod-ON-BCs labelled by PKC. In contrast, extensive cGMP immunoreactivity was clearly seen in the retina incubated in 100 nm melatonin for 5 min (Fig. 2B). Both OPL and IPL were strongly labelled. In addition, lots of neurons in the INL were labelled. The merged image (Fig. 2B″) of Fig. 2B and B′ shows that the dendrites, axon terminals and somata of several Rod-ON-BCs labelled by PKC were strongly cGMP-immunoreactive. Similar results were consistently obtained in five preparations. It should be noted that Cone-ON-BCs in the INL (arrowheads), which were lightly labelled by PKC, were also weakly cGMP-positive. Determination of grey values of the PKC-stained ON-BCs (see Methods) showed that 100 nm melatonin induced a ∼6.7-fold increase for the Rod-ON-BCs (10 cells from 5 slices), and a ∼4.2-fold increase for the Cone-ON-BCs (10 cells from 5 slices) (Fig. 2C).
Figure 2. Confocal micrographs of the carp retinae showing an increased [cGMP]i by melatonin.
A, vertical section of the carp retina that was incubated with Ringer solution for 5 min and then labelled with the cGMP antibody. A′, micrograph of the same section labelled with the antibody against PKC. Note that several BCs that were strongly labelled by PKC could be Rod-ON-BCs, whereas one that was faintly labelled could be a Cone-ON-BC (arrowhead). A″, overlapped image of A and A′. B, vertical section of the carp retina that was incubated with 100 nm melatonin for 5 min and then labelled with the cGMP antibody. B′, micrograph of the same section labelled with the antibody against PKC. B″, overlapped image of B and B′, showing that the Rod-ON-BCs are intensively double-labelled, including the dendrites, somata and enlarged characteristic axon terminals. Several BCs, which were lightly labelled by PKC (arrowheads) and could be Cone-ON-BCs, were also cGMP-positive. All the micrograghs were obtained by single optical sectioning at intervals of 1.0 μm. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bars, 20 μm. C, bar chart, showing that melatonin induces an increase in [cGMP]i in both Rod- and Cone-ON-BCs. Grey values were determined in these two BC groups after incubation of 100 nm melatonin and compared to those obtained in normal Ringer solution. P = 0.0003 and 0.0004 (unpaired) for Rod-ON-BCs and Cone-ON-BCs, respectively. Error bars show ± s.e.m.
PDE, but not guanylyl cyclases, is responsible for melatonin-induced increase in [cGMP]i
[cGMP]i is known to be regulated by guanylyl cyclases (GCs), synthesizing cGMP, and PDE, degrading cGMP, in rod-ON-BCs (Shiells & Falk, 1990; Blute et al. 2000; Baldridge & Fischer, 2001). We therefore tested whether the melatonin-caused increase in [cGMP]i could be due to activation of particulate/soluble GCs (pGC/sGC) and/or inhibition of PDE.
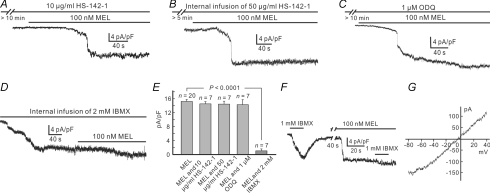
In the presence of HS-142-1 (10 μg ml−1), a selective pGC-A/B antagonist, 100 nm melatonin persisted to induce currents of comparable densities, with an average of 14.49 ± 0.67 pA pF−1 (n = 7) (Fig. 3A and E). Internal infusion of HS-142-1 (50 μg ml−1) yielded a similar result, and the average melatonin (100 nm)-induced current density was 14.45 ± 0.86 pA pF−1 (n = 7) during the infusion (Fig. 3B and E). Similarly, application of 100 nm melatonin induced inward currents after a 10 min preincubation of the sGC antagonist ODQ (1 μm) (Fig. 3C and E), which is not significantly different from that recorded in normal Ringer solution (15.24 ± 0.54 pA pF−1, n = 20) (P = 0.461) with an average current density of 14.31 ± 1.48 pA pF−1 (n = 7). In contrast, the effect of IBMX, a membrane permeant antagonist of PDE, was totally different. As shown in Fig. 3D, internal infusion of 2 mm IBMX induced a sustained inward current (13.5 ± 0.88 pA pF−1, n = 7) after membrane rupture, and during internal infusion of IBMX the melatonin (100 nm) current was greatly reduced in density (1.03 ± 0.54 pA pF−1, n = 7), implying that the effect of melatonin was largely occluded. All the above data are summarized in the bar chart shown in Fig. 3E, in which the melatonin-induced current densities under various conditions are compared to those obtained in normal Ringer solution. We also studied how a puff of 1 mm IBMX affected melatonin-induced currents. A puff of 1 mm IBMX to the Rod-ON-BC soma induced a sustained inward current with slow rise (Fig. 3F). The IBMX-induced current was likely to be mediated by a cGMP-dependent cation channel, as evidenced by an Erev of −0.92 ± 4.6 mV (n = 5) (Fig. 3G), which is very close to those of melatonin- or 8-Br-cGMP-induced currents. After washout with Ringer solution, 100 nm melatonin induced a sustained inward current. It is worth noting that 1 mm IBMX only induced a much smaller current (0.93 ± 0.43 pA pF−1, n = 5) (Fig. 3F) in the presence of 100 nm melatonin.
Figure 3. Melatonin increases [cGMP]i most likely via inhibiting PDE.
A, melatonin of 100 nm induced a current, which was comparable in density to that recorded in Ringer solution when the cell was incubated with 10 μg ml−1 HS-142-1 for 10 min. B, during internal infusion of 50 μg ml−1 HS-142-1, melatonin persisted to induce a current of comparable density. C, following preincubation of 1 μm ODQ for 10 min, melatonin still induced an inward current with comparable density. D, internal infusion of 2 mm IBMX induced an inward current. When the current became steady, addition of 100 nm melatonin induced a current of much less density. E, bar chart, showing that neither HS-142-1 nor ODQ changed the melatonin currents, but IBMX did. P = 0.518 and 0.488 for perfusion and internal infusion of HS-142-1, respectively; P = 0.461 for ODQ; P < 0.0001 for IBMX. F, puff of 1 mm IBMX to the dendrites of a Rod-ON-BC for 15 s induced an inward current. When incubated with 100 nm melatonin for about 1.5 min, the current induced by IBMX was much smaller in density. G, I–V curve of the IBMX current induced from a Rod-ON-BC, using a voltage ramp from −80 mV to 40 mV for 500 ms. The curve yields an Erev of –4.2 mV.
Effects of melatonin on responses of Rod-ON-BCs
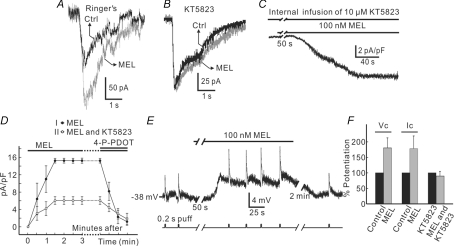
Several studies have demonstrated that cGMP increases responses of Rod-ON-BCs to glutamate mediated by mGluR6 and potentiates their response to dim flash (Nawy & Jahr, 1990; Shiells & Falk, 1990, 2002; Snellman & Nawy, 2004). Now that melatonin causes an increase in [cGMP]i of Rod-ON-BCs, one may ask whether melatonin would modulate light responses of these cells. To simulate light flashes in retinal slices, we used the experimental protocol proposed by Snellman & Nawy (2004), which could avoid presynaptic and lateral interference from photoreceptors and horizontal cells. That is, slices were continuously perfused with saturating concentrations (4 μm) of l-AP4, an mGluR6 agonist, to mimic darkness. During the perfusion of l-AP4, the mGluR6 antagonist (R,S)-α-cyclopropyl-4-phosphonophenylglycine (CPPG) was briefly puffed (0.2 s at intervals of ∼25 s) to dendrites of Rod-ON-BCs by the PicoSprizer to simulate light stimulation. The current response of a Rod-ON-BC to such a simulated light flash under the above conditions is shown in Fig. 4A. This inward current of the cell, like that to a real light flash, reached a peak in a very short time and then sagged to the base line. When the slice was perfused by melatonin (100 nm) for 3 min, which induced a small (∼30 pA) sustained inward current, the current response to 1 mm CPPG puffed to the dendrites was increased from 110 pA in Ringer solution to 195 pA, with an average relative increase of 80.6 ± 32.4% (n = 4) (Fig. 4F). As Rod-ON-BCs express cGK and phosphorylation by cGK may be necessary for cGMP-induced enhancement of Rod-ON-BC responses (Snellman & Nawy, 2004), we investigated if KT5823, a broad spectrum cGK antagonist, could occlude the melatonin-induced potentiation of CPPG-induced currents. Internal infusion of KT5823 (10 μm) induced a small outward current (1.64 ± 0.36 pA pF−1, n = 5), which could be due to the blockade of the activity of cGK in normal Ringer solution. During the internal infusion, the melatonin (100 nm)-induced current was greatly reduced (Fig. 4C). Figure 4D shows a comparison of the melatonin currents obtained in normal Ringer solution (group I, n = 10) and in the presence of 10 μm KT5823 (group II, n = 5). The average steady-state current density was reduced from 15.31 ± 0.61 pA pF−1 (n = 10) to 6.06 ± 0.87 pA pF−1 (n = 5) (P = 0.0004, unpaired). Again, both the currents were completely suppressed by 100 nm 4-P-PDOT. As 10 μm KT5823 was internally infused, the currents of the Rod-ON-BC induced by CPPG puffed to the dendrites tended to decrease slightly (< 10%) just after membrane rupture and then became stable. Addition of 100 nm melatonin then failed to potentiate the response to simulated light flash (Fig. 4B). The data regarding the melatonin-induced potentiation of simulated light responses of Rod-ON-BCs (n = 4) and the elimination of the melatonin effect by KT5823 are summarized in Fig. 4F. We also used current-clamp recording (or voltage-follow mode, with no current injection) to reinforce the result obtained by voltage-clamp recording. A representative example is shown in Fig. 4E. In the presence of 4 μm l-AP4, 1 mm CPPG repetitively puffed to the dendrites at intervals of about 25 s elicited depolarizing responses. Perfusion of 100 nm melatonin caused a depolarization of 4 mV, which was in association with an increase of the voltage response amplitude (from 5 mV to 9 mV). The average relative potentiation was 78.7 ± 40.5% (n = 5), which is in reasonable agreement with the current potentiation (80.6 ± 32.4%, n = 4) obtained by voltage-clamp recording (Fig. 4F).
Figure 4. Effects of melatonin on responses of Rod-ON-BCs.
A, responses of a Rod-ON-BC to simulated light flash in Ringer solution and in the presence of 100 nm melatonin. During the experiment l-AP4 (4 μm) was continuously applied and CPPG (1 mm) was briefly puffed to the dendrites to simulate light flash. CPPG puff induced an inward current in Ringer solution (black trace, Ctrl), and the current increased in size when 100 nm melatonin was applied for 3 min (grey trace, MEL). B, in this Rod-ON-BC filled with 10 μm KT5823, the response was almost unchanged by melatonin. C, with internal infusion of 10 μm KT5823, melatonin still induced an inward current. D, a comparison of averaged melatonin-induced current densities (pA pF−1), in Ringer solution (•) and during internal infusion of KT5823 (○). Both the currents were blocked by 4-P-PDOT (100 nm). E, melatonin potentiated responses of the Rod-ON-BC, recorded in current-clamp mode. CPPG was, respectively, puffed to the dendrites for 0.2 s in the presence of 4 μm l-AP4, which induced a depolarizing response. Application of 100 nm melatonin caused a depolarization of the cell (4 mV) and increased the response amplitude. F, bar chart, showing the statistical analysis of the results regarding melatonin-caused potentiation of Rod-ON-BC responses and the blockade effect of KT5823. P = 0.0005 for patch-clamp results (Vc), P = 0.002 for current-clamp results (Ic) and P = 0.172 for patch-clamp results with addition of KT5823.
Effects of melatonin on electroretinograms
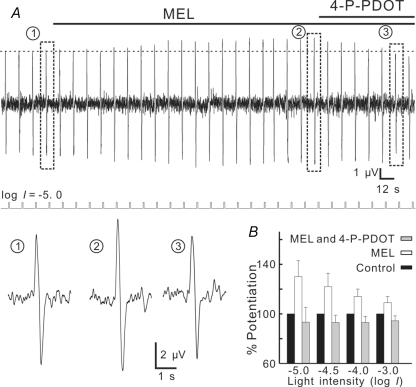
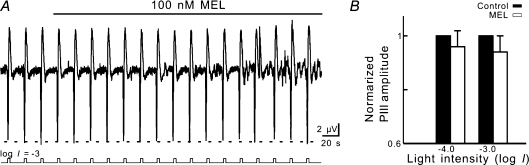
We further tested effects of melatonin on electroretinographic (ERG) b-wave, which reflects the activity of ON-BCs (Stockton & Slaughter, 1989), recorded under dark adapted conditions. Figure 5A shows that 100 nm melatonin increased the b-wave amplitude in response to a dim (log I =−5.0), 500 nm flash of 500 ms from 4.9 μV to 6.3 μV in 2 min, and the effect of melatonin was completely blocked by addition of 100 nm 4-P-PDOT. The melatonin-caused potentiation of the b-wave at this light intensity was 30.0 ± 13.0% (n = 5, Fig. 5B) (P = 0.0003). Such potentiation was also observed with b-waves in response to flashes of increasing intensities (log I =−4.5, −4.0, −3.0) (Fig. 5B). While the increases of the b-waves following melatonin application were all statistically significant (P < 0.0001 for log I =−4.5; P = 0.0003 for log I =−4.0; P = 0.002 for log I =−3.0), the potentiation extent tended to decrease with the increase of light intensity. No effects of melatonin on scotopic PIII components, which are believed to reflect the activity of rod photoreceptors (Witkovsky et al. 1975), were observed (Fig. 6A and B). This suggests that the melatonin effect on b-waves was directly mediated by an activation of MT2 receptors on Rod-ON-BCs, but not a consequence of possible effects on rod photoreceptors.
Figure 5. Melatonin potentiates scotopic b-wave in carp retina.
A, ERG responses induced by a dim (log I =−5.0) flash of 500 nm, presented at intervals of 12 s. When 100 nm melatonin was bath-applied, the b-wave steadily increased in amplitude, and the melatonin effect was blocked by addition of 100 nm 4-P-PDOT. Light signals are shown below the continuous response trace. Representative responses recorded at different times (1, 2, 3) are shown in a faster time scale below the light signal trace. B, bar chart, summarizing the effects of 100 nm melatonin on b-waves induced by flashes of different light intensities. Note that all potentiation effects were blocked by 4-P-PDOT and the potentiation extent tended to decrease with the increase of light intensity.
Figure 6. Effects of melatonin on ERG PIII responses.
A, PIII responses were induced by a dim (log I =−3.0), 500 nm flash of 500 ms, presented at intervals of 20 s. When 100 nm melatonin was bath-applied, the PIII responses were hardly changed. Light signals are shown below the continuous response trace. B, bar chart, summarizing the effects of 100 nm melatonin on PIII responses induced by flashes of two different light intensities. P = 0.269 for log I =−3.0 (n = 4) and P = 0.143 for log I =−4.0 (n = 5).
Discussion
Melatonin induces cGMP-dependent currents by activating MT2 receptors in Rod-ON-BCs
Melatonin induced inward currents from carp Rod-ON-BCs. These currents were exclusively mediated by MT2 receptors, as they were completely blocked by 4-P-PDOT. Expression of the MT2 receptor in the retina has been reported in both mammals and non-mammals (Reppert et al. 1995; Wiechmann et al. 2004). It was reported that in Xenopus laevis this receptor is extensively expressed in the OPL and INL, but its cellular localization is still uncertain (Wiechmann et al. 2004). Most recently, MT2 receptors were found to be functionally expressed in human BCs (Savaskan et al. 2007). Our electrophysiological data strongly suggest that the MT2 receptor is functionally expressed on carp Rod-ON-BCs. Immunocytochemical confirmation of the presence of this receptor will depend on the availability of specific antibodies against the MT2 receptor of this species.
The melatonin-induced currents were blocked by Cd2+ and exhibited an Erev that is very close to the Erev for cGMP-induced currents. These currents were reminiscent in characteristics, including waveform, slow rise and no desensitization, of those induced by bath application of 8-Br-cGMP and internal infusion of cGMP. Moreover, during internal infusion of cGMP melatonin failed to induce any currents. All these results are indicative that they may be mediated by cGMP-dependent cation channels. Indeed, our immunocytochemical data demonstrated a significant increase in [cGMP]i of the Rod-ON-BCs. While melatonin has been found to activate outward potassium currents in suprachiasmatic neurons (Jiang et al. 1995) and oscillatory inward currents in Xenopus oocytes injected with rat spinal cord mRNA (Zahn et al. 2003), there are no data showing that melatonin could elicit a cGMP-dependent cation channel-mediated current. It is noteworthy that KT5823 greatly suppressed melatonin currents of the Rod-ON-BCs (Fig. 4C and D). This result suggests that melatonin-induced currents may not only be dependent on [cGMP]i, but also positively related to the activity of cGK. In other words, the cGMP-dependent channels of the Rod-ON-BCs may be largely modulated by cGK. Whether cGK modulates the cGMP-dependent channels of Rod-ON-BCs directly or indirectly (via modification of coupling between mGluR6 and G protein) remains to be further explored. In salamander ON-BCs there is evidence that shows the regulation of cGMP-dependent currents by calcium/calmodulin-dependent protein kinase II (CaMKII) (Walters et al. 1998) and in cultured hippocampal neurons CaMKII is regulated by cGK (Ninan & Arancio, 2004).
There are two possible explanations for the absence of the melatonin effects in Cone-ON-BCs. First, these cells may not express the MT2 receptor. Alternatively, the MT2 receptor may be present on these cells, but there are no cGMP-activated cation channels on the membrane so that no cGMP-gated currents could be induced. While it is hard to make clear distinction between the two possibilities, we prefer the second one, because melatonin appeared to also cause a slight increase of [cGMP]i in Cone-ON-BCs (Fig. 2B), but internal infusion of cGMP did not induce any currents from these cells (data not shown).
PDE, but not GC, is involved in melatonin-induced increase in [cGMP]i
Activation of melatonin receptors may increase or decrease [cGMP]i, which is cell type dependent (Vacas et al. 1981; Vanecek & Vollrath, 1989; Faillace et al. 1996; Petit et al. 1999; Saenz et al. 2002; Huang et al. 2005; Rimler et al. 2007). In the golden hamster retina melatonin significantly increases cGMP accumulation, which is due to an increase in GC activity and a decrease in PDE activity (Faillace et al. 1996). Our data show, however, that when pGC and sGC were, respectively, blocked by HS-142-1 and ODQ (Fig. 3A, B and C), 100 nm melatonin still could induce currents from Rod-ON-BCs with the densities comparable to those recorded in Ringer solution (14.54 ± 0.65, 14.46 ± 0.84, and 14.30 ± 1.44 versus 15.24 ± 0.54 pA pF−1). In contrast, IBMX induced a sustained inward current with similar characteristics, and in the presence of IBMX the melatonin current was much smaller in size. These results suggest that the increased [cGMP]i of Rod-ON-BCs by melatonin was unlikely owing to the activation of GCs, but may be mainly modulated by an inhibition of PDE. In this context, there is evidence showing that PDE is not involved in mGluR6 signal transduction (Nawy, 1999). This is supported by our data shown in Fig. 1G. That is, it would not have happened that glutamate- and melatonin-induced currents were algebraically summed, if the former current was due to a decrease of [cGMP]i through inhibiting PDE. The mechanism underlying the melatonin-induced changes in [cGMP]i is quite different from that for the changes induced by NO, an important modulator of cGMP levels in the retina. Exposure to NO increases [cGMP]i in Rod-ON-BCs by activating the NO-sensitive sGC expressed in Rod-ON-BCs (Baldridge & Fischer, 2001).
Neuromodulatory role of melatonin in the outer retina
Several groups have reported that Rod-ON-BC responses to dim flashes are potentiated by elevating [cGMP]i either by a dim background or by internal infusion of cGMP or a non-hydrolysable cGMP analogue (Shiells & Falk, 1990, 2002; Snellman & Nawy, 2004). There is evidence that the potentiation may be mediated by cGK, which decreases coupling of the mGluR to the downstream cascade (Snellman & Nawy, 2004). In the present work we showed that the responses of carp Rod-ON-BCs to simulated flashes were significantly potentiated by melatonin. Consistent with it, melatonin also potentiated the scotopic b-wave, a reflection of Rod-ON-BC activity. Such potentiation must be due to the increased [cGMP]i. Our data further support the notion proposed by Snellman & Nawy (2004) that melatonin-induced potentiation of the Rod-ON-BC response to simulated light flash may be mediated by activation of cGK, as it could be almost blocked by KT5823. Nevertheless, melatonin-induced inward currents themselves may be also involved in regulation of response sensitivity of Rod-ON-BCs. As shown in Fig. 4E, melatonin slightly (4 mV) depolarized the Rod-ON-BC, which should have reduced the driving force responsible for the depolarizing response of the Rod-ON-BC, an effect that is opposite to the potentiation effect of melatonin. It means that the melatonin-induced potentiation might have been larger if there were no such changes in the driving force.
Circadian changes in visual sensitivity have been extensively studied in a variety of vertebrates, and the results are species dependent and photoreceptor type related (Brandenburg et al. 1983; Fowlkes et al. 1984; Bassi & Powers, 1987; Dearry & Barlow, 1987; Schaeffel et al. 1991). Specifically, in regard to the rod activity, the visual sensitivity in fish behaviourally determined is higher at dusk than at dawn (for goldfish, Bassi & Powers, 1987; for zebrafish, Li & Dowling, 1998). The data presented in this work therefore suggest that melatonin, which is released more at night, may be implicated in enhancing the response sensitivity during the night-time so that rod signals induced by dim light could be boosted above background noise. It is of interest to note that in the carp outer retina melatonin suppresses responses of cone-driven horizontal cells by activating the MT1 receptor (Huang et al. 2005). It is indeed intriguing that melatonin provides local modulation in the outer retina, which enhances signal transfer from rods to BCs that are located in the direct line of information, but decreases signal transfer from cones to horizontal cells that are involved in lateral inhibition, through two different melatonin receptor subtypes, with one increasing [cGMP]i and the other decreasing it.
Acknowledgments
This work was supported by grants from the National Program of Basic Research sponsored by the Ministry of Science and Technology of China (2006CB500800, 2007CB512200), the Natural Science Foundation of China (90408003, 30570593), and the 211 Project sponsored by the Ministry of Education of China.
References
- Baldridge WH, Fischer AJ. Nitric oxide donor stimulated increase of cyclic GMP in the goldfish retina. Vis Neurosci. 2001;18:849–856. [PubMed] [Google Scholar]
- Bassi CJ, Powers MK. Circadian rhythm in goldfish visual sensitivity. Invest Ophthalmol Vis Sci. 1987;28:1811–1815. [PubMed] [Google Scholar]
- Becchetti A, Roncaglia P. Cyclic nucleotide-gated channels: intra- and extracellular accessibility to Cd2+ of substituted cysteine residues within the P-loop. Pflugers Arch. 2000;440:556–565. doi: 10.1007/s004240000324. [DOI] [PubMed] [Google Scholar]
- Blute TA, Lee HK, Huffmaster T, Haverkamp S, Eldred WD. Localization of natriuretic peptides and their activation of particulate guanylate cyclase and nitric oxide synthase in the retina. J Comp Neurol. 2000;424:689–700. [PubMed] [Google Scholar]
- Brandenburg J, Bobbert AC, Eggelmeyer F. Circadian changes in the response of the rabbits retina to flashes. Behav Brain Res. 1983;7:113–123. doi: 10.1016/0166-4328(83)90008-6. [DOI] [PubMed] [Google Scholar]
- Cahill GM. Circadian regulation of melatonin production in cultured zebrafish pineal and retina. Brain Res. 1996;708:177–181. doi: 10.1016/0006-8993(95)01365-2. [DOI] [PubMed] [Google Scholar]
- Dearry A, Barlow RB., Jr Circadian rhythms in the green sunfish retina. J Gen Physiol. 1987;89:745–770. doi: 10.1085/jgp.89.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE. The Retina: An Approachable Part of the Brain. Cambridge, MA, USA: Belknap Press/Harvard University Press; 1987. [Google Scholar]
- Faillace MP, de las Heras MA, Sarmiento MI, Rosenstein RE. Daily variations in 2-[125I]melatonin specific binding in the golden hamster retina. Neuroreport. 1995;7:141–144. [PubMed] [Google Scholar]
- Faillace MP, Keller Sarmiento MI, Rosenstein RE. Melatonin effect on the cyclic GMP system in the golden hamster retina. Brain Res. 1996;711:112–117. doi: 10.1016/0006-8993(95)01405-5. [DOI] [PubMed] [Google Scholar]
- Fowlkes DH, Karwoski CJ, Proenza LM. Endogenous circadian rhythm in electroretinogram of free-moving lizards. Invest Ophthalmol Vis Sci. 1984;25:121–124. [PubMed] [Google Scholar]
- Hamm HE, Menaker M. Retinal rhythms in chicks: circadian variation in melantonin and serotonin N-acetyltransferase activity. Proc Natl Acad Sci U S A. 1980;77:4998–5002. doi: 10.1073/pnas.77.8.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Lee SC, Yang XL. Modulation by melatonin of glutamatergic synaptic transmission in the carp retina. J Physiol. 2005;569:857–871. doi: 10.1113/jphysiol.2005.098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iigo M, Hara M, Ohtani-Kaneko R, Hirata K, Tabata M, Aida K. Photic and circadian regulations of melatonin rhythms in fishes. Biol Signals. 1997;6:225–232. doi: 10.1159/000109132. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Nelson CS, Allen CN. Melatonin activates an outward current and inhibits Ih in rat suprachiasmatic nucleus neurons. Brain Res. 1995;687:125–132. doi: 10.1016/0006-8993(95)00478-9. [DOI] [PubMed] [Google Scholar]
- Kato S, Negishi K, Teranishi T, Ishita S. The use of the carp retina in neurobiology: its uniqueness and application for neural network analyses of the inner retina. Prog Neurobiol. 1991;37:287–327. doi: 10.1016/0301-0082(91)90021-r. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- Kunwar PS, Starz-Gaiano M, Bainton RJ, Heberlein U, Lehmann R. Tre1, a G protein-coupled receptor, directs transepithelial migration of Drosophila germ cells. Plos Biol. 2003;1:E80. doi: 10.1371/journal.pbio.0000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Dowling JE. Zebrafish visual sensitivity is regulated by a circadian clock. Vis Neurosci. 1998;15:851–857. doi: 10.1017/s0952523898155050. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Nawy S. The metabotropic receptor mGluR6 may signal through Go, but not phosphodiesterase, in retinal bipolar cells. J Neurosci. 1999;19:2938–2944. doi: 10.1523/JNEUROSCI.19-08-02938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346:269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Negishi K, Kato S, Teranishi T. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci Lett. 1988;94:247–252. doi: 10.1016/0304-3940(88)90025-0. [DOI] [PubMed] [Google Scholar]
- Ninan I, Arancio O. Presynaptic CaMKII is necessary for synaptic plasticity in cultured hippocampal neurons. Neuron. 2004;42:129–141. doi: 10.1016/s0896-6273(04)00143-6. [DOI] [PubMed] [Google Scholar]
- Petit L, Lacroix I, de Coppet P, Strosberg AD, Jockers R. Differential signaling of human Mel1a and Mel1b melatonin receptors through the cyclic guanosine 3′-5′-monophosphate pathway. Biochem Pharmacol. 1999;58:633–639. doi: 10.1016/s0006-2952(99)00134-3. [DOI] [PubMed] [Google Scholar]
- Rawashdeh O, de Borsetti NH, Roman G, Cahill GM. Melatonin suppresses nighttime memory formation in zebrafish. Science. 2007;318:1144–1146. doi: 10.1126/science.1148564. [DOI] [PubMed] [Google Scholar]
- Reppert SM. Melatonin receptors: molecular biology of a new family of G protein-coupled receptors. J Biol Rhythms. 1997;12:528–531. doi: 10.1177/074873049701200606. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A. 1995;92:8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13:1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC. A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. J Physiol. 2004;554:467–482. doi: 10.1113/jphysiol.2003.053710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimler A, Jockers R, Lupowitz Z, Zisapel N. Gi and RGS proteins provide biochemical control of androgen receptor nuclear exclusion. J Mol Neurosci. 2007;31:1–12. doi: 10.1007/BF02686113. [DOI] [PubMed] [Google Scholar]
- Saenz DA, Turjanski AG, Sacca GB, Marti M, Doctorovich F, Sarmiento MI, Estrin DA, Rosenstein RE. Physiological concentrations of melatonin inhibit the nitridergic pathway in the Syrian hamster retina. J Pineal Res. 2002;33:31–36. doi: 10.1034/j.1600-079x.2002.01880.x. [DOI] [PubMed] [Google Scholar]
- Saito T, Kujiraoka T. Physiological and morphological identification of two types of on-center bipolar cells in the carp retina. J Comp Neurol. 1982;205:161–170. doi: 10.1002/cne.902050207. [DOI] [PubMed] [Google Scholar]
- Saito T, Kujiraoka T, Yonaha T, Chino Y. Reexamination of photoreceptor-bipolar connectivity patterns in carp retina: HRP-EM and Golgi-EM studies. J Comp Neurol. 1985;236:141–160. doi: 10.1002/cne.902360202. [DOI] [PubMed] [Google Scholar]
- Sallinen P, Saarela S, Ilves M, Vakkuri O, Leppaluoto J. The expression of MT1 and MT2 melatonin receptor mRNA in several rat tissues. Life Sci. 2005;76:1123–1134. doi: 10.1016/j.lfs.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Jockers R, Ayoub M, Angeloni D, Fraschini F, Flammer J, Eckert A, Muller-Spahn F, Meyer P. The MT2 melatonin receptor subtype is present in human retina and decreases in Alzheimer's disease. Curr Alzheimer Res. 2007;4:47–51. doi: 10.2174/156720507779939823. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Wirz-Justice A, Olivieri G, Pache M, Krauchi K, Brydon L, Jockers R, Muller-Spahn F, Meyer P. Distribution of melatonin MT1 receptor immunoreactivity in human retina. J Histochem Cytochem. 2002;50:519–526. doi: 10.1177/002215540205000408. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Rohrer B, Lemmer T, Zrenner E. Diurnal control of rod function in the chicken. Vis Neurosci. 1991;6:641–653. doi: 10.1017/s0952523800002637. [DOI] [PubMed] [Google Scholar]
- Shen Y, Chen L, Ping Y, Yang XL. Glycine modulates the center response of ON type rod-dominant bipolar cells in carp retina. Brain Res Bull. 2005;67:492–497. doi: 10.1016/j.brainresbull.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proc Biol Sci. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Potentiation of ‘on’ bipolar cell flash responses by dim background light and cGMP in dogfish retinal slices. J Physiol. 2002;542:211–220. doi: 10.1113/jphysiol.2002.019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellman J, Nawy S. cGMP-dependent kinase regulates response sensitivity of the mouse on bipolar cell. J Neurosci. 2004;24:6621–6628. doi: 10.1523/JNEUROSCI.1474-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton RA, Slaughter MM. B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J Gen Physiol. 1989;93:101–122. doi: 10.1085/jgp.93.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacas MI, Sarmiento MI, Cardinali DP. Melatonin increases cGMP and decreases cAMP levels in rat medial basal hypothalamus in vitro. Brain Res. 1981;225:207–211. doi: 10.1016/0006-8993(81)90332-2. [DOI] [PubMed] [Google Scholar]
- Vanecek J. Cellular mechanisms of melatonin action. Physiol Rev. 1998;78:687–721. doi: 10.1152/physrev.1998.78.3.687. [DOI] [PubMed] [Google Scholar]
- Vanecek J, Vollrath L. Melatonin inhibits cyclic AMP and cyclic GMP accumulation in the rat pituitary. Brain Res. 1989;505:157–159. doi: 10.1016/0006-8993(89)90129-7. [DOI] [PubMed] [Google Scholar]
- Vardi N. Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. J Comp Neurol. 1998;395:43–52. [PubMed] [Google Scholar]
- Walters RJ, Kramer RH, Nawy S. Regulation of cGMP-dependent current in On bipolar cells by calcium/calmodulin-dependent kinase. Vis Neurosci. 1998;15:257–261. doi: 10.1017/s0952523898152057. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Udin SB, Summers Rada JA. Localization of Mel1b melatonin receptor-like immunoreactivity in ocular tissues of Xenopus laevis. Exp Eye Res. 2004;79:585–594. doi: 10.1016/j.exer.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Yang XL, Wu SM, Hollyfield JG. Melatonin enhances horizontal cell sensitivity in salamander retina. Brain Res. 1988;453:377–380. doi: 10.1016/0006-8993(88)90182-5. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Dudek FE, Ripps H. Slow PIII component of the carp electroretinogram. J Gen Physiol. 1975;65:119–134. doi: 10.1085/jgp.65.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY, Cohen ED, Dowling JE. Retinal bipolar cell input mechanisms in giant danio. II. Patch-clamp analysis of on bipolar cells. J Neurophysiol. 2005;93:94–107. doi: 10.1152/jn.00270.2004. [DOI] [PubMed] [Google Scholar]
- Xu HP, Yang XL. Different effects of low Ca2+ on signal transmission from rods and cones to bipolar cells in carp retina. Brain Res. 2002;957:136–143. doi: 10.1016/s0006-8993(02)03615-6. [DOI] [PubMed] [Google Scholar]
- Zahn PK, Lansmann T, Berger E, Speckmann EJ, Musshoff U. Gene expression and functional characterization of melatonin receptors in the spinal cord of the rat: implications for pain modulation. J Pineal Res. 2003;35:24–31. doi: 10.1034/j.1600-079x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Devries ME, Skolnick J. Structure modeling of all identified G protein-coupled receptors in the human genome. PLoS Comput Biol. 2006;2:e13. doi: 10.1371/journal.pcbi.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]