Abstract
In humans, the rostral part of the ventral premotor cortex (PMv), the homologue of F5 in monkeys, is known to be critically involved in shaping the hand to grasp objects. How does information about hand posture, that is processed in PMv, give rise to appropriate motor commands for transmission to spinal circuits controlling the hand? Whereas PMv is crucial for skilled visuomotor control of the hand, PMv sends relatively few direct corticospinal projections to spinal segments innervating hand muscles and the most likely route for PMv to contribute to the control of hand shape is through cortico-cortical connections with primary motor cortex (M1). If this is the case, we predicted that PMv–M1 interactions should be modulated specifically during precision grasping in humans. To address this issue, we investigated PMv–M1 connections by means of paired-pulse transcranial magnetic stimulation (TMS) and compared whether they were differentially modulated at rest, and during precision versus power grip. To do so, TMS was applied over M1 either in isolation or after a conditioning stimulus delivered, at different delays, over the ipsilateral PMv. For the parameters of TMS tested, we found that, at rest, PMv exerted a net inhibitory influence on M1 whereas, during power grip, this inhibition disappeared and was converted into a net facilitation during precision grip. The finding that, in humans, PMv–M1 interactions are selectively modulated during specific types of grasp provides further evidence that these connections play an important role in control of the hand.
In the primate, the rostral part of the ventral premotor cortex (PMv) is a key component of the cortical circuit responsible for the visuomotor transformations which result in the adaptation of hand posture appropriate for the object to be grasped (Jeannerod et al. 1995). In monkeys, neurons located in area F5, the probable homologue of the rostral PMv in humans (Rizzolatti et al. 2002), show object-specific activity during grasping (Murata et al. 1997; Raos et al. 2006; Umilta et al. 2007) and reversible inactivation of this area impairs object-specific grasp (Fogassi et al. 2001). In humans, functional imaging studies have shown that PMv is activated during object manipulation (Binkofski et al. 1999; Grezes et al. 2003) and, recently, we have demonstrated that virtual lesions of PMv selectively alter finger positioning on the object during grasp (Davare et al. 2006), suggesting that PMv is critically involved in hand shaping.
However, it is still unclear how information about hand grasp processed in PMv gives rise to appropriate motor commands for transmission to spinal motoneurones innervating hand muscles. Although the role of PMv in precision grasping is well established, in monkeys this cortical area surprisingly sends relatively few direct projections to the cervical enlargement (Dum & Strick, 1991; He et al. 1993; Luppino et al. 1999; Tanne-Gariepy et al. 2002; Dum & Strick, 2005). Therefore, it has been suggested that PMv contributes to the control of hand shape through its cortico-cortical connections with the primary motor cortex (M1) (Cerri et al. 2003; Shimazu et al. 2004). This view has been corroborated by the finding that, in monkeys, F5 stimulation facilitates descending corticospinal (CS) volleys from M1, an effect that is abolished by reversible inactivation of M1 (Shimazu et al. 2004).
In humans, little is known about the nature of PMv–M1 connections at rest and about the possible modulation of these interactions during different types of grasp. To address this issue, we used transcranial magnetic stimulation (TMS) with a paired-pulse protocol, where one coil was used to activate PMv at different delays, while the other was used to apply pulses over M1 and to probe the excitability of CS projections to contralateral hand muscles (Civardi et al. 2001; Koch et al. 2007). We hypothesized that if PMv–M1 interactions are causally involved in hand shaping for grasp, they should be specifically modulated during different types of grasp.
Methods
Participants
Seven right-handed (Oldfield, 1971) volunteers (22–28 years) participated in both experiments of this study after providing informed consent. None of the subjects reported neurological impairments. They were all screened for adverse reactions to TMS by means of the TMS safety screen questionnaire (Keel et al. 2001). The experimental procedure was approved by the Ethics Committee of the Université catholique de Louvain.
Experimental tasks
The first experiment aimed at determining, at rest, the functional connectivity between PMv and M1. Subjects were comfortably seated in an armchair with their hands palm-up and relaxed on a pillow, while TMS pulses were delivered (see below).
The second experiment was performed to determine whether PMv–M1 interactions are modulated during different grasps. The subjects had to perform, with the right hand, either a precision grip (grasping a 20-mm plasticine cube between the thumb and index finger) or a power grip (grasping a tennis ball). The background level of electromyographic (EMG) activity recorded from the first dorsal interosseous (1DI) was continuously monitored so that it equalled 10% of the level recorded during the maximal voluntary contraction (MVC) in both conditions. The MVC was measured with a pinch gauge that subjects had to squeeze between the tip of the thumb and the side of the index finger (key pinch force, Mathiowetz et al. 1986) and was equal to 71.3 ± 10.9 N (n = 7). The MVC was computed as the average of three successive trials. The level of 1DI EMG was controlled visually throughout the experimental session and subjects were instructed to keep it as stable as possible within a window of ±2 s.d. of the level of EMG signal that corresponded to 10% MVC.
Transcranial magnetic stimulation
To investigate PMv–M1 interactions in the left hemisphere, we used two custom-made figure-of-eight coils (7 cm outer diameter) connected to two single-pulse monophasic Magstim model 200 stimulators (Magstim Company, Whitland, UK). The conditioning (C) stimulus was delivered over PMv, with anterior to posterior induced current, through a coil held tangentially to the skull with the handle pointing forward; the test (T) stimulus was delivered over M1, with posterior to anterior induced current, through a coil held perpendicularly to the central sulcus with the handle pointing backwards (Fig. 1). The C and T stimuli were set, respectively, at 80% and 120% of the resting motor threshold (rMT), defined as the minimum intensity that induced motor evoked potentials (MEPs) ≥ 50 μV peak to peak in the 1DI in 5 out of 10 trials (Rossini et al. 1994). The rMT was determined by using a coil connected to a single-pulse Magstim stimulator and equalled on average 42 ± 4% of the maximal stimulator output (mean ± s.d., n = 7).
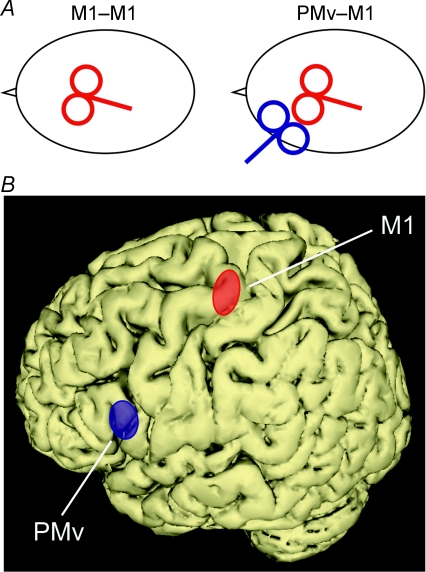
Figure 1. TMS sites.
A, schematic view of the coil positions in the M1–M1 and the PMv–M1 conditions. B, location of the TMS sites as given by the co-registration; PMv is shown in blue (mean MNI coordinates: −58, 13, 19), M1 in red (mean coordinates: −38, −23, 60). The ellipses illustrate the 95% confidence interval centred over the mean calculated for all subjects (n = 7).
In a control condition (M1–M1 condition), both the C and T stimuli were applied over M1, with posterior to anterior induced current, through the same coil connected to two single-pulse monophasic Magstim model 200 stimulators through a Bistim module (Magstim Company, Whitland, UK). This M1–M1 condition was introduced to control for spread of TMS current from PMv to M1. The coil was held tangential to the skull with the handle perpendicular to the central sulcus. Since, when using a Bistim module, the maximal output of the stimulator decreases by 11% (Magstim Company, personal communication), C and T stimulation intensities in the M1–M1 condition were multiplied by 1.12.
Stimulation sites
The coil position was precisely determined, in every subject, by means of a co-registration technique of the stimulation sites onto individual anatomical magnetic resonance images previously gathered for each subject (Davare et al. 2006; see Noirhomme et al. 2004 for details). In order to target PMv, the coil was positioned over the caudal portion of the pars opercularis of the inferior frontal gyrus (BA 44). In the present study, the mean normalized MNI coordinates of PMv were −58 ± 3, 13 ± 5, 19 ± 9 mm (x, y, z, mean ± s.d.; n = 7), close to those reported by functional imaging studies (Binkofski et al. 1999; Ehrsson et al. 2000, 2001; Kuhtz-Buschbeck et al. 2001). Additionally, we have shown that a virtual lesion of this region impairs precision grasping (Davare et al. 2006). In order to target M1, the coil was positioned over the site where TMS induced the largest MEPs in the 1DI muscle. The co-registration procedure confirmed that the M1 site overlapped the hand knob (Yousry et al. 1997; Lotze et al. 2003); its mean normalized MNI coordinates were −38 ± 3, −23 ± 4, 60 ± 10 mm (x, y, z, mean ± s.d.; n = 7), which are also comparable to those reported in functional imaging studies (Fink et al. 1997; Picard & Strick, 2001).
The mean Euclidian distance between PMv and M1 stimulation sites was 58 ± 6 mm (mean ± s.d.; n = 7), a distance which was sufficient to allow positioning of both coils over the same hemisphere (see Fig. 1A).
Experimental procedure
In Experiment 1, subjects had to perform eight blocks of 40 trials, four in the M1–M1 condition and four in the PMv–M1 condition. The C–T interval (ISI) was varied randomly between 1, 2, 4, 6, 8, 10 and 15 ms. T alone was delivered in 1 out of 8 trials and the MEP amplitudes measured in this condition were used as control values. Altogether, for either PMv–M1 or M1–M1 condition, 160 trials were performed: 20 trials for each C–T interval (7 ISI = 140 trials) and 20 trials for the T alone.
Experiment 2 was scheduled on a separate day. A total of 16 blocks of 40 trials were performed for each stimulation condition, namely M1–M1 (8 blocks, 320 trials) and PMv–M1 (8 blocks, 320 trials). In half of the blocks, subjects performed a steady precision grip and, in the other half, a steady power grip. Therefore, for a given stimulation (M1–M1/PMv–M1) and grasp (precision/power grip) condition, 160 trials were performed: 20 trials for each of the 7 ISI and 20 trials for the T alone condition. TMS pulses were delivered while subjects maintained the precision or power grip posture and appropriate level of 1DI EMG activity constant throughout the block. In both experiments, the intertrial interval was randomly distributed between 5 and 7 s.
Data acquisition and analysis
The Magstim stimulators were triggered using Signal software and CED data acquisition interface (Cambridge Electronic Design, Cambridge, UK). EMG activity was recorded with two surface electrodes (Neuroline, Medicotest, Denmark), one positioned over the 1DI muscle belly and the other over the head of the 2nd metacarpal bone. The raw EMG signals were amplified (gain: 1K), band-pass filtered (10–500 Hz, Neurolog, Digitimer Ltd, UK) and digitized at 2 kHz for offline analysis. In addition, in Experiment 2, in order to provide subjects with an online visual feed-back of the 1DI contraction level, the EMG was rectified and low-pass filtered (15 Hz, fourth-order, zero phase-lag Butterworth filter) using Labview software (National Instruments, Austin, TX, USA). This signal was not stored for further analysis.
The peak-to-peak amplitude of each individual MEP was measured and expressed as a percentage of the control (baseline) MEP (T stimulus alone) gathered during the same block. The EMG levels during either precision or power Grip were estimated by computing the area-under-curve of the 500 ms period preceding the TMS pulse and were not different across conditions (t = 0.58, P = 0.46).
Statistical analyses
To analyse data from Experiment 1, a repeated measure ANOVA (ANOVARM) was performed on the relative MEP amplitudes with the Site of C stimulus delivery (M1 or PMv) and C–T Interval (1, 2, 4, 6, 8, 10, 15 ms or T alone) as within-subject factors. For Experiment 2, an ANOVARM on the relative MEP amplitudes was performed, for each C site, with Grip (precision or power grip) and C–T Interval as within-subject factors. Post hoc comparisons were performed using Dunnett's test. Finally, paired t tests were used to assess whether the effects were systematically found in each subject.
Results
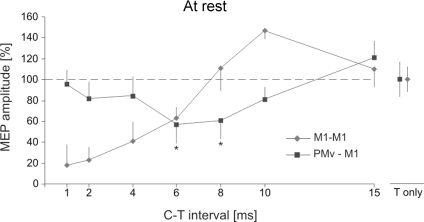
PMv–M1 interactions were investigated at rest by applying a test (T) stimulus over M1 after a conditioning (C) stimulus was delivered, at different intervals, either over ipsilateral PMv (PMv–M1) or M1 (M1–M1). We found a distinct effect of the site of conditioning stimulus application on the MEP amplitude (Site × C–T Interval, F = 4.23, P = 0.032). Indeed, in the PMv–M1 condition, the MEP amplitude was significantly smaller than in controls, but only for C–T Interval of 6 and 8 ms (post hoc, both P < 0.015, Figs 2A and 3). A paired t test showed that this suppression, at 6 and 8 ms, was significant in all seven subjects (all P < 0.024). For other intervals, the MEP amplitude was unaffected by conditioning of PMv (all P > 0.05). In the M1–M1 condition, we corroborated results of previous studies (Kujirai et al. 1993), namely a decrease in MEP amplitude for short intervals (1, 2, 4, 6 ms, short interval cortical inhibition or SICI) and an increase for longer ones (10 ms) (P < 0.003); conditioning stimuli delivered over M1 8 or 15 ms before the test shock had no effect on MEP amplitude (P > 0.05).
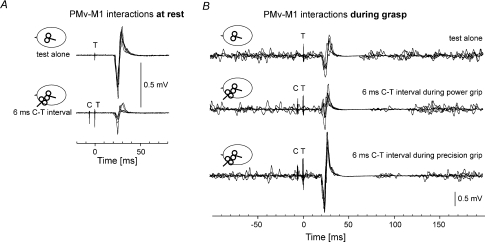
Figure 2. Typical MEPs showing the PMv–M1 interactions at rest and during grasp.
A, at rest, superposition of 5 responses to a single test TMS pulse (T) over M1 (top) and from a test (T) pulse over M1 preceded 6 ms before by a conditioning (C) pulse over PMv (bottom). B, during grasp. The top traces show superposition of 5 responses to a single test (T) pulse over M1. Three responses were recorded during precision grip and 2 during power grip. The middle traces show responses conditioned by a PMv pulse (C) delivered 6 ms before the test M1 pulse during power grip, and the bottom traces, during precision grip.
Figure 3. PMv–M1 interactions at rest.
Relative amplitude of MEPs recorded from the 1DI at rest. The squares (PMv–M1 condition) represent MEP amplitudes resulting from a supra-threshold test (T) stimulus applied over M1 preceded by a subthreshold conditioning (C) stimulus applied over PMv at different intervals (X-axis). A significant suppression was found at both the 6 and 8 ms C–T intervals. Diamonds show MEP amplitude recorded during the M1–M1 condition. The error bars show 1 s.d.
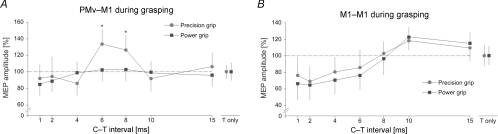
In Experiment 2, we investigated whether the PMv–M1 interactions were modulated differently during a precision or a power grip while subjects kept the same level of muscle contraction during both grasps. We found a specific modulation of the PMv–M1 connections, as shown by a significant Grip × C–T Interval interaction, when the conditioning stimulus was applied over PMv (F = 3.45, P = 0.029). Indeed, during precision grip, C delivered over PMv at a C–T interval of 6 or 8 ms led to a significant MEP facilitation (both P < 0.008, Figs 2B and 4A), whereas no MEP modulation was found during power grip (all P > 0.05). A paired t test showed that the facilitation at 6 and 8 ms during precision grip was significant in all seven subjects (all P < 0.031). These results indicate that the resting PMv–M1 inhibition is released during power grip and turns to facilitation during precision grip. When conditioning was applied over M1, we only found a main effect of C–T Interval (F = 5.73, P = 0.004) confirming the result of Experiment 1, namely a reduction of the MEP amplitude for short intervals (1, 2, 4, 6 ms, all P < 0.05) and an increase at 10 ms (P = 0.03, Fig. 4B). It is noteworthy that SICI during grasping was reduced when compared with SICI at rest (1, 2, 4 ms, all P < 0.05; 6 ms, P = 0.062), in line with previous results (Reynolds & Ashby, 1999). There was no main effect of Grip nor Grip × C–T Interval interaction in the M1–M1 condition (both F < 2.23, both P > 0.082). Finally, when comparing the amplitude of MEPs in response to a test stimulation alone (T) during grasp, we noticed an increase in MEP amplitude during precision grip with respect to power grip (t = 2.55, P = 0.025); this finding corroborates previous studies (Flament et al. 1993; Schieppati et al. 1996) (see Table 1).
Figure 4. PMv–M1 and M1–M1 interactions during grasp.
Relative amplitude of MEPs recorded from the 1DI during a 10% maximum voluntary contraction either during a precision grip (circles) or a power grip (squares). A, during a precision grip, the resting PMv–M1 inhibition turned into facilitation at both the 6 and 8 ms C–T intervals, whereas, during a power grip this inhibition was cancelled. B, during grasp, M1–M1 interactions showed a reduced SICI compared to at rest, irrespective of the type of grasp. The error bars show 1 s.d.
Table 1.
Raw baseline MEP amplitudes in all conditions
| At rest | Precision grip | Power grip | |
|---|---|---|---|
| Test MEP (mV) | 0.33 ± 0.05 | 0.68 ± 0.14 | 0.52 ± 0.09 |
Mean ± s.d. (n = 7) of the raw MEP values gathered from a single TMS pulse over M1 (T) at rest, during precision grip and power grip. Note that the MEP size during precision grip was larger than during power grip (t = 2.55, P = 0.025).
Discussion
The present study shows that, at rest, PMv exerts an inhibitory influence on M1, as reflected in the suppression of MEPs evoked from M1 by TMS. This interaction was selectively modulated during different types of grasp. During power grip, this inhibition was released and, during precision grip – a task known to be associated with a particularly strong activation of PMv (Ehrsson et al. 2000; Umilta et al. 2007) – it turned into facilitation. These results suggest a causal role of PMv–M1 interactions in precision grasping and support the view that connections between PMv and M1 could be critically involved in conveying information used to adapt hand posture appropriate for the object to be grasped (Cerri et al. 2003; Shimazu et al. 2004; Cattaneo et al. 2005; Prabhu et al. 2007).
At rest, we found that the net inhibitory action on M1 was evident for subthreshold stimulation applied over PMv 6 and 8 ms before M1 stimulation. As the time course of this inhibition was strikingly different from that observed when an identical C stimulus was applied over M1, it can be ruled out that this finding resulted from a spread of the conditioning stimulus to M1. This is, of course, crucial because of the small distance between the PMv and M1 stimulation sites (∼6 cm). The present finding is reminiscent of the resting-state inhibition exerted by the middle frontal gyrus and SMA on M1 (Civardi et al. 2001) and the time course of the PMv–M1 inhibition described here is the same as in that study.
We have shown that the interactions between PMv and M1 are modulated during voluntary grasps. Similarly, Civardi et al. (2001) found that a voluntary contraction (10% of MVC) led to a decrease in the inhibition exerted by the middle frontal gyrus on M1. Therefore, this release of inhibition from non-primary motor areas during grasp could be regarded as a necessary – but non-specific – condition to permit voluntary hand movements to be executed. However, the present results extend this finding by showing that PMv–M1 interactions are selectively modulated during precision grasping. Indeed, we found that, whereas the level of activation in 1DI EMG activity, and hence in the descending drive to 1DI motoneurones, were identical in both power and precision grips, only the latter condition led to a net facilitation of M1 by PMv. This view is consistent with results from functional imaging studies showing that PMv is more active during precision grip than during power grip (Ehrsson et al. 2000, 2001). A recent electrophysiological study in monkeys showed that populations of neurons in PMv and M1 fire at higher rates for precision grip than for other types of grasps (Umilta et al. 2007). However, to determine which parameters are critical to reveal this facilitatory interaction, it will be necessary to investigate several other types of grasps with different levels of grip force.
This inhibitory effect at rest of PMv stimulation on M1 output to hand muscles contrasts with previous reports of facilitation in the macaque monkey (Cerri et al. 2003; Shimazu et al. 2004). However, there are some key differences. First, this study was performed in awake subjects, while the macaque studies were done in either a sedated or deeply anaesthetized state. In fact, there are also reports of inhibition of M1 from PMv (Tokuno & Nambu, 2000) and particularly in the awake, behaving monkey (Prabhu et al. 2005). Second, TMS probably has a much less focused action on PMv than the intracortical stimulation used in the monkey studies. In the results reported here, we may see the net effect of PMv projections, some of which are facilitatory and some inhibitory, to M1 pyramidal cells. The balance between these two pathways could also account for the differential effects we found at rest versus during grasp. In line with this view, Civardi et al. (2001) found that, when applying the conditioning stimulation, only the late I-wave components of the descending drive were suppressed, which agrees with the involvement, at rest, of M1 interneurons that are facilitated by projections from PMv and which inhibit M1 pyramidal cells. The appearance of facilitation from PMv during active precision grip, as shown in the present study, suggests in addition that PMv–M1 interactions are task-specific; Prabhu et al. (2005) also found that PMv–M1 interactions suppressed some muscles and facilitated others in a task-dependent manner. The balance between suppression and facilitation may also be dependent on the intensity of the conditioning stimulus and this needs further investigation.
Finally, it is noteworthy that the time course of the PMv–M1 facilitation observed here during precision grip is somewhat longer compared with that found in the monkey. Shimazu et al. (2004) found that the maximum PMv–M1 facilitation was seen at very brief C–T intervals (1–2 ms), rather than at 6–8 ms as in the present study. One explanation for this difference is, of course, the much larger conduction distance in human compared with monkey (60 mm versus 10 mm; see also Civardi et al. 2001). It should be also noted that the time course of the facilitation reported here is consistent with recent TMS studies that showed that M1 outputs were modulated 6–8 ms after conditioning the ipsilateral or contralateral dorsal premotor cortex (Koch et al. 2007; O'Shea et al. 2007), and with studies of cortico-cortical potentials evoked in human M1 by direct stimulation of the exposed inferior prefrontal gyrus (Greenlee et al. 2007). The latencies of these potentials ranged from 2 to 10 ms which may also reflect the relatively indirect pathways that link PMv to M1 corticospinal outputs (Shimazu et al. 2004).
In conclusion, for the TMS parameters tested, we have shown that the interaction between PMv and M1 is modulated by different types of grasp. We suggest that the connections involved play a causal role in precision grip probably by conveying information about how to grasp an object according to its shape. Further research is necessary to investigate how hand posture, object shape and motor goal interact to reveal the facilitation exerted by PMv on M1.
Acknowledgments
We are grateful to Professor L. Fadiga for his comments. This work was supported by grants from the Fonds Spéciaux de Recherche of the Université catholique de Louvain, the Fonds de la Recherche Scientifique Médicale, and the Fondation Médicale Reine Elisabeth. M.D. is supported by the Wellcome Trust.
References
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci. 1999;11:3276–3286. doi: 10.1046/j.1460-9568.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Voss M, Brochier T, Prabhu G, Wolpert DM, Lemon RN. A cortico-cortical mechanism mediating object-driven grasp in humans. Proc Natl Acad Sci U S A. 2005;102:898–903. doi: 10.1073/pnas.0409182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri G, Shimazu H, Maier MA, Lemon RN. Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. J Neurophysiol. 2003;90:832–842. doi: 10.1152/jn.01026.2002. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci. 2006;26:2260–2268. doi: 10.1523/JNEUROSCI.3386-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci. 2005;25:1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren E, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol. 2001;85:2613–2623. doi: 10.1152/jn.2001.85.6.2613. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol. 2000;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- Flament D, Goldsmith P, Buckley CJ, Lemon RN. Task dependence of responses in first dorsal interosseous muscle to magnetic brain stimulation in man. J Physiol. 1993;464:361–378. doi: 10.1113/jphysiol.1993.sp019639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey: a reversible inactivation study. Brain. 2001;124:571–586. doi: 10.1093/brain/124.3.571. [DOI] [PubMed] [Google Scholar]
- Greenlee JD, Oya H, Kawasaki H, Volkov IO, Severson MA, 3rd, Howard MA, 3rd, Brugge JF. Functional connections within the human inferior frontal gyrus. J Comp Neurol. 2007;503:550–559. doi: 10.1002/cne.21405. [DOI] [PubMed] [Google Scholar]
- Grezes J, Armony JL, Rowe J, Passingham RE. Activations related to ‘mirror’ and ‘canonical’ neurones in the human brain: an fMRI study. Neuroimage. 2003;18:928–937. doi: 10.1016/s1053-8119(03)00042-9. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 1993;13:952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18:314–320. [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- Koch G, Franca M, Mochizuki H, Marconi B, Caltagirone C, Rothwell JC. Interactions between pairs of transcranial magnetic stimuli over the human left dorsal premotor cortex differ from those seen in primary motor cortex. J Physiol. 2007;578:551–562. doi: 10.1113/jphysiol.2006.123562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Ehrsson HH, Forssberg H. Human brain activity in the control of fine static precision grip forces: an fMRI study. Eur J Neurosci. 2001;14:382–390. doi: 10.1046/j.0953-816x.2001.01639.x. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Kaethner RJ, Erb M, Cohen LG, Grodd W, Topka H. Comparison of representational maps using functional magnetic resonance imaging and transcranial magnetic stimulation. Clin Neurophysiol. 2003;114:306–312. doi: 10.1016/s1388-2457(02)00380-2. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4) Exp Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Wiemer DM, Federman SM. Grip and pinch strength: norms for 6- to 19-year-olds. Am J Occup Ther. 1986;40:705–711. doi: 10.5014/ajot.40.10.705. [DOI] [PubMed] [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G. Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol. 1997;78:2226–2230. doi: 10.1152/jn.1997.78.4.2226. [DOI] [PubMed] [Google Scholar]
- Noirhomme Q, Ferrant M, Vandermeeren Y, Olivier E, Macq B, Cuisenaire O. Registration and real-time visualization of transcranial magnetic stimulation with 3-D MR images. IEEE Trans Biomed Eng. 2004;51:1994–2005. doi: 10.1109/TBME.2004.834266. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Sebastian C, Boorman ED, Johansen-Berg H, Rushworth MF. Functional specificity of human premotor–motor cortical interactions during action selection. Eur J Neurosci. 2007;26:2085–2095. doi: 10.1111/j.1460-9568.2007.05795.x. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Prabhu G, Shimazu H, Cerri G, Brochier T, Spinks RL, Maier MA, Lemon RN. Modulation of primary motor cortex outputs from ventral premotor cortex during visually-guided grasp in the macaque monkey. J Physiol. 2005;565P:C109. doi: 10.1113/jphysiol.2008.165571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu G, Voss M, Brochier T, Cattaneo L, Haggard P, Lemon R. Excitability of human motor cortex inputs prior to grasp. J Physiol. 2007;581:189–201. doi: 10.1113/jphysiol.2006.123356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raos V, Umilta MA, Murata A, Fogassi L, Gallese V. Functional properties of grasping-related neurons in the ventral premotor area F5 of the macaque monkey. J Neurophysiol. 2006;95:709–729. doi: 10.1152/jn.00463.2005. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology. 1999;53:730–735. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex. Curr Opin Neurobiol. 2002;12:149–154. doi: 10.1016/s0959-4388(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Trompetto C, Abbruzzese G. Selective facilitation of responses to cortical stimulation of proximal and distal arm muscles by precision tasks in man. J Physiol. 1996;491:551–562. doi: 10.1113/jphysiol.1996.sp021239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci. 2004;24:1200–1211. doi: 10.1523/JNEUROSCI.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanne-Gariepy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res. 2002;145:91–103. doi: 10.1007/s00221-002-1078-9. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Nambu A. Organization of nonprimary motor cortical inputs on pyramidal and nonpyramidal tract neurons of primary motor cortex: An electrophysiological study in the macaque monkey. Cereb Cortex. 2000;10:58–68. doi: 10.1093/cercor/10.1.58. [DOI] [PubMed] [Google Scholar]
- Umilta MA, Brochier TG, Spinks RL, Lemon RN. Simultaneous recording of macaque premotor and primary motor cortex neuronal populations reveals different functional contributions to visuomotor grasp. J Neurophysiol. 2007;98:488–501. doi: 10.1152/jn.01094.2006. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]