Abstract
Nitric oxide (NO) is a simple molecule with a complex and pleitropic biological activity. NO or related species have been implicated in the regulation of many genes that participate in many diverse biological functions including programmed cell death or apoptosis. Apoptosis is a process that may potentially be disrupted in cancer cells conferring a survival advantage. In addition, malignant tumor cells can develop an intricate system of resistance to apoptotic stimuli. NO or related species have been shown to play a dual role in the regulation of apoptosis in malignant cells either promoting cell death or protecting cells from pro-apoptotic induction. However, the specific role of NO in the regulation of apoptosis/survival-related genes expression seems to tilt the balance toward the promotion of pro-apoptotic and the suppression of anti-apoptotic genes. Herein we have reviewed the most relevant aspects involving NO and/or reactive intermediates in the regulation of apoptosis-related genes –mainly– at the transcriptional level. We described the basic apoptotic molecules that potentially are affected by NO and how NO-mediated signaling gets transmitted to the transcriptional machinery that governs the expression of these genes. In addition, we discussed some of the fundamental functional consequences of the regulation of apoptosis-related genes by NO in cancer biology and its potential therapeutic implications.
Keywords: Nitric oxide, Reactive Nitrogen Species, Transcriptional regulation, Gene expression, Transcription factors, Apoptosis, S-nitrosylation, YY1, NF-κB, p53, FOXP3
Introduction
Nitric oxide (NO) is a diatomic molecule that plays important roles as the smallest pleiotropic signaling messenger in mammalian cells [1]. NO has an unpaired electron, it rapidly reacts with other molecules and easily diffuses through the plasma membranes to reach target proteins within the cell due to its lipophilic nature. NO is biologically synthesized by nitric oxide synthases (NOS). NOS catalyze the oxidation of L-arginine resulting in the formation of NO and L-citruline. NO is produced by three different NOS, two of which are generally constitutively expressed, primarily in neurons (nNOS or Type I) and endothelial cells (eNOS or Type III), respectively [2-4]. An inducible isoform (iNOS or Type II) can be upregulated considerably in immune cells and many other tissues [5, 6]. It has been shown that IFN-γ alone or in combination with TNF-α, interleukin 1β (IL-1β) and bacterial lipopolysaccharide (LPS) can induce the expression of iNOS in a wide variety of tissue organs and in some tumor cell lines [7, 8]. The inducible type of nitric oxide synthase (iNOS) is considered to be a central protein in the regulation of the immune response against tumor cells [9, 10].
The specific role of nitric oxide in tumor biology and cancer has remained elusive. A broad spectrum of activities has been assigned to either the physiology or the patho-physiology of nitric oxide in tumor cells (for a review, see ref. [11]). The first distinction we can make is related to the amount and sources of nitric oxide being generated. Low-output of nitric oxide has been correlated with increased blood flow and new blood vessels (angiogenesis) feeding the tumor area [12]. In addition, the generation of nitric oxide by tumor cells may inhibit the activation and proliferation or increase apoptosis of surrounding lymphocytes that can account for the immune suppression observed that accompanies tumor growth. Furthermore, high intratumoral-output of nitric oxide could inhibit the activation of caspases and therefore antagonizes the pro-apoptotic signals [13, 14]. However, the opposite effect also has been observed in many other systems whereby the generation of high-output of nitric oxide, either by iNOS induction or by the use of NO donors, inhibits tumor growth and metastasis [15]. Therefore, the final outcome of NO mediated effects will be determined by many factors including the local concentration and sources of nitric oxide in the tissue, and the presence of reactive molecules that might redirect the redox status in the cell.
Several lines of evidence support the hypothesis that NO regulates the expression of some genes that are implicated in the signal pathway involving regulatory cytokines that modify the cellular response to apoptotic stimuli [16-22]. However, the regulation of apoptosis-related genes by NO is not completely understood.
NO is known to interfere in the DNA binding activity of many zinc finger transcription factors via S-nitrosation (S-nitrosylation) of cysteine thiols groups and subsequent S-nitrosothiol formation [23, 24]. The formation of S-nitrosothiols, and subsequent oxidation of thiol proteins, might act as switches in signaling pathways [25-27]. Furthermore, S-nitrosylation may regulate many thiol-containing enzymes and regulatory proteins, such as the transcription factors nuclear factor kappa B (NF-κB) [28], AP-1 [29, 30] and CREB [31].
Herein we have reviewed the most relevant aspects involving NO and/or related species in the regulation of apoptosis-related genes –mainly– at the transcriptional level. We described the basic apoptotic molecules that potentially are affected by NO and how NO-mediated signaling gets transmitted to the transcriptional machinery that governs the expression of these genes. In addition, we discussed some of the fundamental functional consequences of the regulation of apoptosis-related genes by NO in cancer biology and its potential therapeutic implications.
Apoptosis/Survival-Related Genes: Main Players
Apoptosis is widely known as programmed cell death that is part of normal development, senescence, and other diverse biologic processes. Apoptosis is an evolutionary conserved process that contributes to development and maintenance of virtually all cell types. Accumulation of normal and abnormal tissue depends on the delicate balance between cell proliferation and apoptosis; as such, it is difficult to assess the importance of apoptosis without careful measurements of both proliferative and apoptotic components.
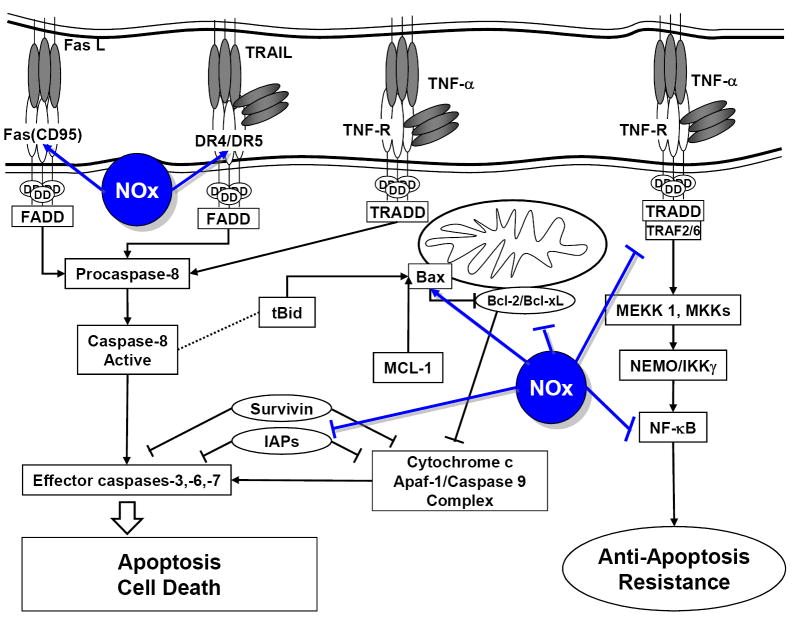
The intracellular signaling pathways that regulate apoptosis have been elucidated in many cell types. Signal transduction to transmit the apoptotic death signal is carefully regulated. There are basically two types of signaling pathways that regulate cellular apoptosis. First (Extrinsic), the binding of specific death ligands to their respective cell surface receptors, such as Fas, tumor necrosis factor (TNF) receptor (TNFR), and the DR3/4/5, activate downstream pathways through the recruitment of adapter molecules. These adapter molecules recruit an “initiator” cysteine protease (e.g. caspase 8), which, in turn, cleaves further caspases. The downstream “effector” caspases are responsible for the cleavage of various intracellular substrates and the activation of targets involved in DNA degradation. A parallel pathway that does not require the activation of cell surface death receptors is also present (Intrinsic). This second pathway is dependent on the release of mitochondrial cytochrome c and other pro-apoptotic molecules (e.g. Smac/DIABLO) into the cytoplasm. The association of cytochrome c with an adapter molecule, Apaf1, and caspase 9 in the cytoplasm activates the latter, which, in turn, activates downstream caspases. Whereas the intracellular triggers causing the loss of mitochondrial membrane integrity are varied and often difficult to identify, it is clear that the release of cytochrome c (and presumably other pro-apoptotic molecules) in response to these triggers is ultimately mediated by the relative expression or activity of members of the Bcl-2 family. Although the “death receptor” and “mitochondrial” pathways are distinct there is considerable crosstalk between them. For example, activated caspase 8 in response to TNF-α can cleave Bid (a Bcl-2 family member), thereby converting it into a pro-apoptotic molecule that can promote cytochrome c release. Nonetheless, this “mitochondrial amplification loop” is not always necessary because cytochrome c release is not always observed [32]. Moreover, it has been shown that nuclear factor kappa B (NF-κB) can prevent apoptosis induced by TNF-α through transcriptional regulation of antiapoptotic factors, such as TNFR-associated factor (TRAF) and inhibitor of apoptosis protein (IAP) (Figure 1).
Figure 1.
Schematic representation of the most relevant Apoptosis/Survival-related gene products and the effect of NO or related species (NOx) in the regulation of gene expression in cancer cells.
Apoptosis and Cancer
Apoptosis is a pathway that may potentially be disrupted in tumor cells conferring a survival advantage [33, 34]. Mutation affecting genes that are either inducers (i.e. c-myc, p53, bad, ICE and others) or repressors (i.e. bcl-2, bcl-xl, c-abl, ras and others) of apoptosis, may be a common genetic event during the development of malignancies (for review see [35] and references therein).
Resistance to apoptosis induction has been recently recognized as a common pathway to multiple drug resistance. Further, the development of resistance to either the immune system or chemo-immunotherapeutic strategies remains a disadvantage in the therapy of cancer, particularly in cases where recurrence and/or relapses occurred. Apoptosis has been recognized as a distinct pathological mechanism in tumors responding to anticancer therapies [36, 37]. Tumor cells, which are resistant to killing by the immune system or medical therapeutic treatment, can be sensitized to apoptosis-related cytotoxicity by combination treatments of subtoxic concentrations of chemotherapeutic drugs, toxins, cytokines, and antibodies (for review see [38] and references therein).
The survival of a tumor cell is dependent on the acquisition of resistance to cell death and escape from immune-surveillance [39]. In most cases, the machinery of death is intact within the cells and ready to be activated by signals that trigger the death process. It has been hypothesized that the resistance to cell death could arise from the deregulation in the expression of some genes that participate in the protective mechanism against cell death. Therefore, a logical approach to sensitize resistant tumor cells to be eliminated by the immune system or by therapeutic agents would be to increase the threshold of responsiveness by upregulating the expression of pro-apoptotic genes or by decreasing the expression of anti-apoptotic (survival) genes.
Nitric oxide/cGMP and Apoptosis
In general, the role of NO in apoptosis is controversial. Indeed, it has been shown that NO can have both pro- and anti-apoptotic properties. NO can prevent apoptosis in some cell lines, such as endothelial cells, lymphoma cells, ovarian follicles, cardiac myocytes, vascular smooth cells and hepatocytes. Inhibition of apoptosis by NO may be associated with the induction of heat shock protein 70 (Hsp 70) response, suppression of Bax expression, or guanylyl cyclase (GC) activation, induction of protective pathways through the induction of heme oxygenase and cyclo-oxygenase; may involve up-regulation of intracellular antioxidant systems, especially glutathione; may inhibit caspase 3-like enzymes via S-nitrosylation or through a cGMP-dependent mechanism, both leading to inactivation of caspases [40].
NO exerts most of its physiological effects by binding to its GC-coupled receptors. NO regulates a wide range of biological functions via post-translational modification of proteins [41]. The biological activities of NO can be divided into cyclic guanylate cyclase (cGMP)-dependent and cGMP-independent pathways. cGMP formation is considered to be the main physiological NO signaling pathway [42]. cGMP production leads to the activation of cGMP-dependent protein kinases and the suppression of caspase activity. High doses of NO may inhibit apoptosis through both cGMP-dependent and -independent mechanisms [43]. Significant portion of the function of the NO/cGMP-mediated signaling pathways involve cGMP-induced changes in gene expression. cGMP’s effects on apoptosis appear to be mediated, at least in part, through regulation of Bcl-2-related genes. Some effects of cGMP on gene expression involve cross-talk with other signaling pathways, such as MAP kinase, calcineurin, and RhoA pathways; other effects of cGMP may be directly attributed to PKG phosphorylation of specific transcription factors, such as the cAMP response element (CRE)-binding protein (CREB) and TFII-I (Reviewed in [44]).
NO regulates the expression of Apoptosis-related receptors: Role of the transcription repressor YY1
The best-characterized death receptors are Fas (also called CD95 or APO-1), death receptor 5 (DR5 or tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor 5) and TNFR1 (also called p55 or CD120a) [45-49]. The signal from Fas seems to be restricted to apoptosis, whereas the TNFR1 activates both apoptosis and the anti-apoptosis-related transcription factor NF-κB [50].
Initial evidences suggesting the potential role of NO in the regulation of Fas expression in cancer came from the observation of NO-mediated sensitization of ovarian tumor cells to Fas-induced apoptosis. It has been shown that the sensitization to Fas-mediated apoptosis observed in ovarian carcinoma cell treated with IFN-γ is due in part to the generation of NO, or its reaction products, by the induction of iNOS by the tumor cells. NOS inhibitors blocked this sensitization and the use of NO donors mimicked the IFN-γ-mediated sensitization to a Fas-agonist antibody. Furthermore, it was observed a concurrent upregulation of Fas receptor, either upon induction of iNOS by IFN-γ or by the treatment of the ovarian cancer cells with NO donors. Moreover, the observed upregulation of Fas receptor was abrogated by the use of NOS inhibitors suggesting a strong correlation that might account for the sensitization to Fas-induced apoptosis [51].
Previous work demonstrated the influence of IFN-γ in the regulation of Fas receptor expression on the tumor cell surface [52]. Studies searching for the role of NO in the vascular smooth muscle cell apoptosis showed that NO induces upregulation of Fas antigen expression via a cGMP-independent mechanism [53], and also that NO primes pancreatic β cells for Fas-induced apoptosis by increasing the surface CD95 receptor expression [54]. However, it was not clear how NO could interact with the transcriptional machinery in order to regulate Fas gene expression.
Characterization of the human Fas gene promoter has revealed three major regions within the approximately 2,000 bp 5’-flanking region. Functional analysis identified a silencer activity residing between nucleotide position -1,781 and -1,007 and a strong enhancer region between -1,007 and -425 in the human Fas gene. The region between -425 and -1 retained a basal promoter activity [55]. A subsequent study, aiming to identify the specific mechanisms by which NO could regulate the expression of Fas gene, demonstrated the direct effect of NO on the inactivation of a negative regulatory trans-acting signals on the Fas promoter. It was established that the mechanism by which NO upregulates the expression of the Fas receptor on different tumor cells is due to the specific inactivation of the transcription repressor Yin-Yang 1 (YY1) DNA-binding activity to the silencer region of the Fas promoter [56]. Similarly, it has been suggested the specific role of NO in the regulation of TRAIL receptor (DR5) gene expression via disruption of the repressor activity of YY1 [57].
Further, it was determined that the mechanism of NO-mediated inhibition of YY1 DNA-binding activity was due to S-nitrosylation of critical cysteines residues coordinated by Zn2+ residing at its four zinc fingers. This resulted in inhibition of the transcriptional repressive activity of YY1 and increasing the expression of Fas and DR5 and subsequent tumor cell sensitization to Fas- and TRAIL-induced apoptosis [58]. YY1 is a ubiquitous and multifunctional zinc-finger transcription factor (also known as delta factor, NF-E1, UCRBP and CF1) member of the Polycomb Group protein family, a group of homeobox gene receptors that can act as an activator or a repressor of transcriptional activity. YY1 interacts with many elements involved in cell cycle with an overall outcome of regulation of positive signals promoting cell proliferation (i.e., p53, MDM2, Cyclin D, etc.). In addition, YY1 has been implicated in the regulation of the activity and expression of apoptosis related molecules (i.e., NF-κB, Fas, DR5 (TRAIL receptor, etc.). It would not be surprising that deregulated YY1 activity might serve as central molecule causing dysfunctional cell proliferation and increased resistance to cell death, therefore promoting tumorigenesis (Reviewed in [59, 60]).
Although its diverse functions allow for the context-specific paradoxical effects of transcriptional initiation, activation, and repression, the overwhelming evidence of the role of YY1 in tumor biology would support the theory that YY1 functions to promote carcinogenesis and perhaps even confer cells with a mechanism for evading cell death in the face of cytotoxic stimuli including chemotherapy and/or immunotherapy. Primary mechanisms appear to include perturbations in cellular surveillance systems as well as modulation of key genes involved in cell cycle regulation and programmed cell death. Therefore, this NO sensitive transcriptional regulator might provide the molecular basis to target the expression of specific genes involved in the sensitivity of tumor cell to apoptosis.
NO regulates the expression of Apoptosis/Survival-related signaling molecules: Role of NF-κB, p53 and other transcription factors
NF-κB
The most relevant transcription factor participating in the regulation of genes involved in apoptosis is the nuclear factor kappa B (NF-κB) promoting the expression of anti-apoptotic genes, therefore conferring resistance to cell death stimuli [61-64].
NF-κB transcription factors are assembled through the dimerization of five subunits: RelA (p65), c-Rel, RelB, p50/NF-κB1 and p52/NF-κB2 [65]. In resting –unstimulated– state, most NF-κB dimmers are sequestered in the cytoplasm by binding to specific inhibitors IκBs. Cell stimulation activates the IκB kinase (IKK) complex. Activated IKK phosphorylates NF-κB-bound IκB proteins and targets them for polyubiquitination and rapid proteasome-mediated degradation [66]. Freed NF-κB dimers translocate to the nucleus where they control the transcriptional activation of several target genes in concert with other transcription factors [67-69].
NF-κB target genes include several antiapoptotic proteins including cIAP1, cIAP2, TRAF1, TRAF2, Bfl-1A1, Bcl-XL and FLIP [64, 70, 71]. These findings were initially made in the context of TNF-α signaling. Despite the presence of death domains (DD) in the cytoplasmic portion of TNF receptor (TNFR1), TNF-α does not trigger apoptosis unless is simultaneously used with inhibitors of RNA or protein synthesis. Moreover, the requirement for these inhibitors can be bypassed through specific inactivation of NF-κB by either deletion of its RelA subunit or expression of a degradation-resistant form of IκB [72]
NF-κB is an oxidative stress-responsive transcription factor that has been reported to be activated by reactive oxygen species (i.e. H2O2, , etc.) generated as part of the signaling cascade triggered by many molecules such as TNF-α [73, 74]. Reactive oxygen species (ROS) have been implicated in the signaling pathways initiated by TNF-α. Stimulation of mammalian cells with TNF-α triggers the generation of various ROS [75, 76]. Moreover, the use of antioxidants resulted in the inhibition of various TNF-α-related effects, such as the activation of transcription factors, gene expression, and cytotoxicity, and exogenous ROS mimic TNF-α biological activity [77]. In biological systems the most important ROS generated upon TNF-α stimulation are the result of enzymatic partial reduction of oxygen yielding superoxide ( ), which is immediately disproportionated by SOD to H2O2 and O2 or rapidly reacts with nitric oxide (NO) generating ONOO- [78-80].
It has been shown that nitric oxide (NO) sensitizes human ovarian carcinoma cells to TNF-α-mediated apoptosis through the specific disruption of the TNF-α-induced generation of hydrogen peroxide (H2O2) and the subsequent inhibition of the NF-κB-dependent expression of anti-apoptotic genes. In addition, these observations were also extended to other solid tumor cells such as human prostatic adenocarcinoma cells. The survival autocrine-paracrine loop involving the NF-κB-dependent expression of TNF-α could be interrupted by the inhibitory activity that nitric oxide exerts on the TNF-α-induced activation of NF-κB. Furthermore, in an in vivo situation, the exposure of tumor cells to pro-inflammatory cytokines such as IFN-γ will promote the generation of nitric oxide. Thus, either the endogenously or exogenously generated NO would scavenge the TNF-α-generated superoxide ( ) and decrease the H2O2-dependent activation of NF-κB [56]. Furthermore, treatment of cultured prostate cancer cells in the presence of NO resulted in sensitization of these tumor cells to TRAIL-mediated apoptosis by inhibiting the activity of the NF-κB and consequently the impaired expression of the anti-apoptotic gene product Bcl-XL facilitating the TRAIL-dependent activation of the apoptotic-signaling cascade [81].
In addition, NF-κB can be regulated by NO or related molecules via inhibition of its activation. It was originally suggested that NO stabilized the NF-κB inhibitor, IκBα, by preventing its degradation from NF-κB. NO also increased the mRNA expression of IκBα, but not NF-κB subunits, p65 or p50, suggesting specific transcriptional induction of IκBα by NO [82]. Also, NF-κB can be inhibited directly by NO through S-nitrosylation of the p50 subunit. This SNO modification of NF-kappa B has been shown to prevent binding to its target DNA site [83, 84].
p53
The tumor suppressor p53 is a transcription factor functioning as a critical regulator of downstream genes important in cell cycle progression, DNA repair and apoptosis. The important role that p53 plays in keeping a fine-tuned balance in mammalian cells is evident by the large number of tumors that bear mutations in this gene. Loss of p53 in many malignancies leads to genomic instability, impaired cell cycle regulation, and inhibition of apoptosis. Upon DNA damage, P53 restrains the cell at a checkpoint until DNA integrity is restored. If the damage persists and can’t be repaired by cellular mechanisms, apoptosis is triggered. However, the mechanisms by which p53 promotes apoptosis are still not fully understood [85, 86]. In unstressed cells, p53 exhibits an extreme short half-life and the protein amount is maintained at a low, often undetectable level. A group of cellular stressors such as DNA damage, oncogene activation of hypoxia stabilizes p53, predominantly by post-translational modification with a final consequence of nucleolar disruption [87].
As a transcription factor, p53 may stimulate transcription of cell cycle regulating genes such as p21WAF1/CIP1 or mdm2 but also apoptotic ones such as Bax or Fas [88-90]. NO or related molecules have been shown to promote the stabilization of p53 due to the necessity imposed by these reactive nitrogen species (RNS) to upregulate cell cycle modulators, proapoptotic proteins such as p21WAF1/CIP1 or Bax [91, 92]. Mechanisms by which NO or related molecules promote the stabilization of p53 remain largely unknown. Recent studies reported that NO or RNS neither use ataxia telangiectasia-mutated (ATM) nor the alternate reading frame (ARF) tumor suppressor protein to accumulate p53 [93].
Activation of various signaling pathways by NO to regulate gene expression has been shown by the screening of DNA microarrays [94]. Among different group of genes modulated by NO was a significant population of genes that specifically utilized p53, suggesting the notion that NO or related species stabilize and activate p53 and consequently modulate apoptotic/survival genes.
FOXP3
More recently, we have identified a potential transcription factor indirectly targeted by NO with implications on the resistance of tumor cells to apoptosis, FOXP3. FOXP3 is a member of the forkhead/winged-helix family of transcriptional regulators exhibiting both, activation and repression of transcription [95, 96]. FOXP3 is critically important for the development and function of T regulatory (Treg) cells and the consequent definition of immune tolerance [97-99]. It has been shown that expression of FOXP3 in tolerogenic environments correlates with increased resistance to apoptosis by the regulation of apoptosis-related genes such as Bcl-2 [100].
Although obviously important to the understanding of immunoregulation, the molecular events that govern the regulation of FOXP3 remain elusive. We have shown the role of the glucocorticoid (GR) and estrogen (ER) receptors in the expression of tumor-derived FOXP3. Previously, we have shown that nitric oxide (NO) is capable of modifying the structure of steroid receptors such as ER by S-nitrosylation at cysteine residues that coordinate Zn2+ within the two major DNA-binding Zn-finger domains of most steroid receptors (including the glucocorticoid receptor (GR)), resulting in selective inhibition of DNA-binding at specific steroid receptor responsive elements and subsequent blockade of steroid-dependent gene transcription [101]. We hypothesized that NO might suppress the expression of FOXP3 by interfering with the GR/ER-dependent transcriptional activation of FOXP3 promoter. We have examined the effect of NO in the regulation of FOXP3 in human melanoma and breast cancer cells, resulting in a suppression of FOXP3 mRNA expression. We also have determined the suppressive activity of NO in FOXP3 expression in dexamethasone- and estradiol-induced cells, resulting in complete abolition of the GR/ER-mediated expression of tumor-derived FOXP3. Collectively, these findings suggest the suppressive activity of NO on the transcriptional regulation of tumor-derived FOXP3 via inhibition of the GR/ER-dependent transcriptional activation of FOXP3. Therefore, NO-mediated disruption of FOXP3 expression resulted in increased sensitivity of tumor cells to apoptotic stimuli.
Final Remarks and Conclusions
The specific role of NO or related species in apoptosis remains controversial considering the dual action of NO in either promoting or impairing programmed cell death as reported by different research groups. This is not different when we analyzed the particular role of NO in the regulation of apoptosis/survival-related genes. However, analyzing the existing data focusing on the action of NO in the regulation of apoptosis/survival genes in cancer cells, the balance tilted predominantly towards promoting the expression of pro-apoptotic genes and the inhibition of expression of survival factors (Figure 1). Thus, as has been referred before, the biological activity of NO or related molecules depend on the source of nitric oxide generation, local concentration and the presence of other reactive molecules that can direct the function of NO.
NO can act directly or indirectly on the transcriptional machinery, orchestrating the expression of apoptosis/survival genes, either by affecting the signaling molecules that will activate or repress transcription factors or by directly modifying key transcription factors and their DNA binding activity. It can be also cGMP dependent or independent following the general principles of “small concentrations” of NO, in a tight cellular environment NO will tend to favor a cGMP dependent mechanism of regulation, whereas “high concentrations” of NO will trigger a cGMP independent set of actions.
Deregulation of the expression of genes involved in apoptosis has been shown to be a critical aspect in determining the development and progression of numerous cancer types. Therefore, understanding the molecular mechanism involved in the control of apoptosis-related gene expression might facilitate the development of targeted anti-tumor therapies.
The dynamic coordination of genetic factors plays a major role in the regulation of apoptosis-related gene expression under physiological or pathophysiological conditions. Uncontrolled activation of several transcription factors regulating the expression of genes involved in either pro-apoptotic or anti-apoptotic pathways have been identified as key players in the acquisition of the resistant phenotype of tumor cells. Among these transcription factors, we have examined the specific role of NO on the activity of the NF-κB as one of the most important regulators of anti-apoptotic gene expression, YY1 as a novel regulator (transcriptional repressor) of pro-apoptotic receptors, p53 as a key modulator of cell cycle and pro-apoptosis pathways and FOXP3 as a novel tolerogenic and apoptosis-resistance regulator in tumor cells. Thus, specific targeting of these genetic elements by NO or related species regulating the tumor cell sensitivity to apoptosis represents a plausible therapeutic alternative that can be used alone or in combination with already established anti-cancer therapies.
There is a define trend in the development and acquisition of knowledge pertinent to the role of NO and related species in the modulation of the sensitivity of cancerous cells to apoptotic stimuli. Specific identification of potential NO-sensitive targets involved in the regulation of apoptosis/survival gene expression in cancer cells will contribute to the development of more effective therapeutic alternatives.
Acknowledgments
We are indebted to Dr. Benjamin Bonavida, Dr. James S. Economou and Dr. Louis J. Ignarro for the academic and research support and Dr. Diana C. Márquez for her support and critical revisions of this manuscript. This work was supported in part by the Comprehensive Minority Biomedical Branch (CMBB) of the National Cancer Institute (H.J.G R01CA79976-8S1), the Jonsson Comprehensive Cancer Foundation (H.J.G.) and the USAMRMC (DOD) Breast Cancer Research Program (H.J.G. W81XWH-06-1-0612).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nathan C. Nitric oxide as a secretory product of mammalian cells. Faseb J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 2.Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 3.Mayer B, Hemmens B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem Sci. 1997;22:477–481. doi: 10.1016/s0968-0004(97)01147-x. published erratum appears in Trends Biochem Sci 1998 Feb;23(2):87. [DOI] [PubMed] [Google Scholar]
- 4.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie Q, Nathan C. The high-output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994;56:576–582. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt HH, Walter U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 7.Geller DA, Nussler AK, Di Silvio M, Lowenstein CJ, Shapiro RA, Wang SC, Simmons RL, Billiar TR. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993;90:522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherman PA, Laubach VE, Reep BR, Wood ER. Purification and cDNA sequence of an inducible nitric oxide synthase from a human tumor cell line. Biochemistry. 1993;32:11600–11605. doi: 10.1021/bi00094a017. [DOI] [PubMed] [Google Scholar]
- 9.Hokari A, Zeniya M, Esumi H. Cloning and functional expression of human inducible nitric oxide synthase (NOS) cDNA from a glioblastoma cell line A-172. J Biochem (Tokyo) 1994;116:575–581. doi: 10.1093/oxfordjournals.jbchem.a124563. [DOI] [PubMed] [Google Scholar]
- 10.Langrehr JM, Hoffman RA, Lancaster JR, Jr, Simmons RL. Nitric oxide--a new endogenous immunomodulator. Transplantation. 1993;55:1205–1212. doi: 10.1097/00007890-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 12.Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Stamler JS. NO: an inhibitor of cell death. Cell Death Differ. 1999;6:937–942. doi: 10.1038/sj.cdd.4400578. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Sidiropoulos P, Song G, Pagliari LJ, Birrer MJ, Stein B, Anrather J, Pope RM. TNF-alpha gene expression in macrophages: regulation by NF-kappa B is independent of c-Jun or C/EBP beta. J Immunol. 2000;164:4277–4285. doi: 10.4049/jimmunol.164.8.4277. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Lee JS, Galvin KM. Everything you have ever wanted to know about Yin Yang 1. Biochim Biophys Acta. 1997;1332:F49–66. doi: 10.1016/s0304-419x(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 16.Andrew PJ, Harant H, Lindley IJ. Nitric oxide regulates IL-8 expression in melanoma cells at the transcriptional level. Biochem Biophys Res Commun. 1995;214:949–956. doi: 10.1006/bbrc.1995.2378. [DOI] [PubMed] [Google Scholar]
- 17.Andrew PJ, Harant H, Lindley IJ. Up-regulation of interleukin-1beta-stimulated interleukin-8 in human keratinocytes by nitric oxide. Biochem Pharmacol. 1999;57:1423–1429. doi: 10.1016/s0006-2952(99)00052-0. [DOI] [PubMed] [Google Scholar]
- 18.Chang RH, Feng MH, Liu WH, Lai MZ. Nitric oxide increased interleukin-4 expression in T lymphocytes. Immunology. 1997;90:364–369. doi: 10.1111/j.1365-2567.1997.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowler AA, 3, Fisher BJ, Sweeney LB, Wallace TJ, Natarajan R, Ghosh SS, Ghosh S. Nitric oxide regulates interleukin-8 gene expression in activated endothelium by inhibiting NF-kappaB binding to DNA: effects on endothelial function. Biochem Cell Biol. 1999;77:201–208. [PubMed] [Google Scholar]
- 20.Frank S, Kampfer H, Podda M, Kaufmann R, Pfeilschifter J. Identification of copper/zinc superoxide dismutase as a nitric oxide-regulated gene in human (HaCaT) keratinocytes: implications for keratinocyte proliferation. Biochem J. 2000;346(Pt 3):719–728. [PMC free article] [PubMed] [Google Scholar]
- 21.Kallmann BA, Malzkorn R, Kolb H. Exogenous nitric oxide modulates cytokine production in human leukocytes. Life Sci. 1999;65:1787–1794. doi: 10.1016/s0024-3205(99)00431-2. [DOI] [PubMed] [Google Scholar]
- 22.Rothe H, Hartmann B, Geerlings P, Kolb H. Interleukin-12 gene-expression of macrophages is regulated by nitric oxide. Biochem Biophys Res Commun. 1996;224:159–163. doi: 10.1006/bbrc.1996.1000. [DOI] [PubMed] [Google Scholar]
- 23.Kroncke KD, Carlberg C. Inactivation of zinc finger transcription factors provides a mechanism for a gene regulatory role of nitric oxide. Faseb J. 2000;14:166–173. doi: 10.1096/fasebj.14.1.166. [DOI] [PubMed] [Google Scholar]
- 24.Shinyashiki M, Chiang KT, Switzer CH, Gralla EB, Valentine JS, Thiele DJ, Fukuto JM. The interaction of nitric oxide (NO) with the yeast transcription factor Ace1: A model system for NO-protein thiol interactions with implications to metal metabolism. Proc Natl Acad Sci U S A. 2000;97:2491–2496. doi: 10.1073/pnas.050586597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamler JS. S-nitrosothiols and the bioregulatory actions of nitrogen oxides through reactions with thiol groups. Curr Top Microbiol Immunol. 1995;196:19–36. doi: 10.1007/978-3-642-79130-7_4. [DOI] [PubMed] [Google Scholar]
- 26.Stamler JS, Hausladen A. Oxidative modifications in nitrosative stress. Nat Struct Biol. 1998;5:247–249. doi: 10.1038/nsb0498-247. news; comment. [DOI] [PubMed] [Google Scholar]
- 27.Gitler C, Zarmi B, Kalef E. General method to identify and enrich vicinal thiol proteins present in intact cells in the oxidized, disulfide state. Anal Biochem. 1997;252:48–55. doi: 10.1006/abio.1997.2294. [DOI] [PubMed] [Google Scholar]
- 28.Peng HB, Rajavashisth TB, Libby P, Liao JK. Nitric oxide inhibits macrophage-colony stimulating factor gene transcription in vascular endothelial cells. J Biol Chem. 1995;270:17050–17055. doi: 10.1074/jbc.270.28.17050. [DOI] [PubMed] [Google Scholar]
- 29.Tabuchi A, Sano K, Oh E, Tsuchiya T, Tsuda M. Modulation of AP-1 activity by nitric oxide (NO) in vitro: NO-mediated modulation of AP-1. FEBS Lett. 1994;351:123–127. doi: 10.1016/0014-5793(94)00839-6. [DOI] [PubMed] [Google Scholar]
- 30.Tabuchi A, Oh E, Taoka A, Sakurai H, Tsuchiya T, Tsuda M. Rapid attenuation of AP-1 transcriptional factors associated with nitric oxide (NO)-mediated neuronal cell death. J Biol Chem. 1996;271:31061–31067. doi: 10.1074/jbc.271.49.31061. [DOI] [PubMed] [Google Scholar]
- 31.Peunova N, Enikolopov G. Amplification of calcium-induced gene transcription by nitric oxide in neuronal cells. Nature. 1993;364:450–453. doi: 10.1038/364450a0. published erratum appears in Nature 1993 Sep 30;365 (6445):468. [DOI] [PubMed] [Google Scholar]
- 32.Kim HH, Kim K. Enhancement of TNF-alpha-mediated cell death in vascular smooth muscle cells through cytochrome c-independent pathway by the proteasome inhibitor. FEBS Lett. 2003;535:190–194. doi: 10.1016/s0014-5793(02)03894-2. [DOI] [PubMed] [Google Scholar]
- 33.Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 34.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 35.Martin SJ, Green DR. Apoptosis and cancer: the failure of controls on cell death and cell survival. Crit Rev Oncol Hematol. 1995;18:137–153. doi: 10.1016/1040-8428(94)00124-c. [DOI] [PubMed] [Google Scholar]
- 36.Dive C, Hickman JA. Drug-target interactions: only the first step in the commitment to a programmed cell death? Br J Cancer. 1991;64:192–196. doi: 10.1038/bjc.1991.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eastman A. Activation of programmed cell death by anticancer agents: cisplatin as a model system. Cancer Cells. 1990;2:275–280. [PubMed] [Google Scholar]
- 38.Frost P, Bonavida B. Circumvention of tumor cell escape following specific immunotherapy. Cancer Biother Radiopharm. 2000;15:141–152. doi: 10.1089/cbr.2000.15.141. [DOI] [PubMed] [Google Scholar]
- 39.Vaux DL, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci U S A. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blaise GA, Gauvin D, Gangal M, Authier S. Nitric oxide, cell signaling and cell death. Toxicology. 2005;208:177–192. doi: 10.1016/j.tox.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 41.Mannick JB, Schonhoff CM. NO means no and yes: regulation of cell signaling by protein nitrosylation. Free Radic Res. 2004;38:1–7. doi: 10.1080/10715760310001629065. [DOI] [PubMed] [Google Scholar]
- 42.Chung HT, Pae HO, Choi BM, Billiar TR, Kim YM. Nitric oxide as a bioregulator of apoptosis. Biochem Biophys Res Commun. 2001;282:1075–1079. doi: 10.1006/bbrc.2001.4670. [DOI] [PubMed] [Google Scholar]
- 43.Dash PR, Cartwright JE, Baker PN, Johnstone AP, Whitley GS. Nitric oxide protects human extravillous trophoblast cells from apoptosis by a cyclic GMP-dependent mechanism and independently of caspase 3 nitrosylation. Exp Cell Res. 2003;287:314–324. doi: 10.1016/s0014-4827(03)00156-3. [DOI] [PubMed] [Google Scholar]
- 44.Pilz RB, Casteel DE. Regulation of gene expression by cyclic GMP. Circ Res. 2003;93:1034–1046. doi: 10.1161/01.RES.0000103311.52853.48. [DOI] [PubMed] [Google Scholar]
- 45.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 46.Golstein P. Cell death: TRAIL and its receptors. Curr Biol. 1997;7:R750–753. doi: 10.1016/s0960-9822(06)90000-1. [DOI] [PubMed] [Google Scholar]
- 47.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 48.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 49.Gruss HJ, Dower SK. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1995;85:3378–3404. [PubMed] [Google Scholar]
- 50.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c- IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 51.Garban HJ, Bonavida B. Nitric oxide sensitizes ovarian tumor cells to Fas-induced apoptosis. Gynecol Oncol. 1999;73:257–264. doi: 10.1006/gyno.1999.5374. [DOI] [PubMed] [Google Scholar]
- 52.Morimoto H, Yonehara S, Bonavida B. Overcoming tumor necrosis factor and drug resistance of human tumor cell lines by combination treatment with anti-Fas antibody and drugs or toxins. Cancer Res. 1993;53:2591–2596. [PubMed] [Google Scholar]
- 53.Fukuo K, Hata S, Suhara T, Nakahashi T, Shinto Y, Tsujimoto Y, Morimoto S, Ogihara T. Nitric oxide induces upregulation of Fas and apoptosis in vascular smooth muscle. Hypertension. 1996;27:823–826. doi: 10.1161/01.hyp.27.3.823. [DOI] [PubMed] [Google Scholar]
- 54.Stassi G, De Maria R, Trucco G, Rudert W, Testi R, Galluzzo A, Giordano C, Trucco M. Nitric oxide primes pancreatic beta cells for Fas-mediated destruction in insulin-dependent diabetes mellitus. J Exp Med. 1997;186:1193–1200. doi: 10.1084/jem.186.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rudert F, Visser E, Forbes L, Lindridge E, Wang Y, Watson J. Identification of a silencer, enhancer, and basal promoter region in the human CD95 (Fas/APO-1) gene. DNA Cell Biol. 1995;14:931–937. doi: 10.1089/dna.1995.14.931. [DOI] [PubMed] [Google Scholar]
- 56.Garban HJ, Bonavida B. Nitric oxide inhibits the transcription repressor Yin-Yang 1 binding activity at the silencer region of the Fas promoter: a pivotal role for nitric oxide in the up-regulation of Fas gene expression in human tumor cells. J Immunol. 2001;167:75–81. doi: 10.4049/jimmunol.167.1.75. [DOI] [PubMed] [Google Scholar]
- 57.Lee JY, Huerta-Yepez S, Vega M, Baritaki S, Spandidos DA, Bonavida B. The NO TRAIL to YES TRAIL in cancer therapy (review) Int J Oncol. 2007;31:685–691. [PubMed] [Google Scholar]
- 58.Hongo F, Garban H, Huerta-Yepez S, Vega M, Jazirehi AR, Mizutani Y, Miki T, Bonavida B. Inhibition of the transcription factor Yin Yang 1 activity by S-nitrosation. Biochem Biophys Res Commun. 2005;336:692–701. doi: 10.1016/j.bbrc.2005.08.150. [DOI] [PubMed] [Google Scholar]
- 59.Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 60.Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25:1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- 61.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 62.Beg AA, Baltimore D. An Essential Role for NF-kB in Preventing TNF-alpha-Induced Cell Death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 63.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 64.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. see comments. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 66.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 67.Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
- 68.Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, Otsu K, Hoffmann A, Montminy M, Karin M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 69.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 70.Mayo MW, Baldwin AS. The transcription factor NF-kappaB: control of oncogenesis and cancer therapy resistance. Biochim Biophys Acta. 2000;1470:M55–62. doi: 10.1016/s0304-419x(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 71.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 72.Makris C, Godfrey VL, Krahn-Senftleben G, Takahashi T, Roberts JL, Schwarz T, Feng L, Johnson RS, Karin M. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell. 2000;5:969–979. doi: 10.1016/s1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- 73.Hong YH, Peng HB, La Fata V, Liao JK. Hydrogen peroxide-mediated transcriptional induction of macrophage colony-stimulating factor by TGF-beta1. J Immunol. 1997;159:2418–2423. [PubMed] [Google Scholar]
- 74.Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic Res Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 75.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 76.Hennet T, Richter C, Peterhans E. Tumour necrosis factor-alpha induces superoxide anion generation in mitochondria of L929 cells. Biochem J. 1993;289:587–592. doi: 10.1042/bj2890587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 78.Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 79.Fukuto JM, Wink DA. Nitric oxide (NO): formation and biological roles in mammalian systems. Met Ions Biol Syst. 1999;36:547–595. [PubMed] [Google Scholar]
- 80.Szabo C, Ohshima H. DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide. 1997;1:373–385. doi: 10.1006/niox.1997.0143. [DOI] [PubMed] [Google Scholar]
- 81.Huerta-Yepez S, Vega M, Jazirehi A, Garban H, Hongo F, Cheng G, Bonavida B. Nitric oxide sensitizes prostate carcinoma cell lines to TRAIL-mediated apoptosis via inactivation of NF-kappa B and inhibition of Bcl-xl expression. Oncogene. 2004;23:4993–5003. doi: 10.1038/sj.onc.1207655. [DOI] [PubMed] [Google Scholar]
- 82.Peng HB, Libby P, Liao JK. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem. 1995;270:14214–14219. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- 83.DelaTorre A, Schroeder RA, Kuo PC. Alteration of NF-kappa B p50 DNA binding kinetics by S-nitrosylation. Biochem Biophys Res Commun. 1997;238:703–706. doi: 10.1006/bbrc.1997.7279. [DOI] [PubMed] [Google Scholar]
- 84.Matthews JR, Botting CH, Panico M, Morris HR, Hay RT. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996;24:2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Benchimol S. p53-dependent pathways of apoptosis. Cell Death Differ. 2001;8:1049–1051. doi: 10.1038/sj.cdd.4400918. [DOI] [PubMed] [Google Scholar]
- 86.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rubbi CP, Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. Embo J. 2003;22:6068–6077. doi: 10.1093/emboj/cdg579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 89.Haupt S, Louria-Hayon I, Haupt Y. P53 licensed to kill? Operating the assassin. J Cell Biochem. 2003;88:76–82. doi: 10.1002/jcb.10311. [DOI] [PubMed] [Google Scholar]
- 90.Vousden KH. Switching from life to death: the Miz-ing link between Myc and p53. Cancer Cell. 2002;2:351–352. doi: 10.1016/s1535-6108(02)00186-1. [DOI] [PubMed] [Google Scholar]
- 91.Ishida A, Sasaguri T, Miwa Y, Kosaka C, Taba Y, Abumiya T. Tumor suppressor p53 but not cGMP mediates NO-induced expression of p21(Waf1/Cip1/Sdi1) in vascular smooth muscle cells. Mol Pharmacol. 1999;56:938–946. doi: 10.1124/mol.56.5.938. [DOI] [PubMed] [Google Scholar]
- 92.Tian B, Liu J, Bitterman PB, Bache RJ. Mechanisms of cytokine induced NO-mediated cardiac fibroblast apoptosis. Am J Physiol Heart Circ Physiol. 2002;283:H1958–1967. doi: 10.1152/ajpheart.01070.2001. [DOI] [PubMed] [Google Scholar]
- 93.Wang X, Michael D, de Murcia G, Oren M. p53 Activation by nitric oxide involves down-regulation of Mdm2. J Biol Chem. 2002;277:15697–15702. doi: 10.1074/jbc.M112068200. [DOI] [PubMed] [Google Scholar]
- 94.Hemish J, Nakaya N, Mittal V, Enikolopov G. Nitric oxide activates diverse signaling pathways to regulate gene expression. J Biol Chem. 2003;278:42321–42329. doi: 10.1074/jbc.M308192200. [DOI] [PubMed] [Google Scholar]
- 95.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 96.Kaufmann E, Knochel W. Five years on the wings of fork head. Mech Dev. 1996;57:3–20. doi: 10.1016/0925-4773(96)00539-4. [DOI] [PubMed] [Google Scholar]
- 97.Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 98.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 99.Ochs HD, Ziegler SF, Torgerson TR. FOXP3 acts as a rheostat of the immune response. Immunol Rev. 2005;203:156–164. doi: 10.1111/j.0105-2896.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 100.Hahn BH, Singh RP, La Cava A, Ebling FM. Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3-expressing, apoptosis-resistant, TGFbeta-secreting CD8+ T cell suppressors. J Immunol. 2005;175:7728–7737. doi: 10.4049/jimmunol.175.11.7728. [DOI] [PubMed] [Google Scholar]
- 101.Garban HJ, Marquez-Garban DC, Pietras RJ, Ignarro LJ. Rapid nitric oxide-mediated S-nitrosylation of estrogen receptor: regulation of estrogen-dependent gene transcription. Proc Natl Acad Sci U S A. 2005;102:2632–2636. doi: 10.1073/pnas.0409854102. [DOI] [PMC free article] [PubMed] [Google Scholar]