Abstract
In the presence of oxygen, organic compounds can be oxidized by zero-valent iron or dissolved Fe(II). However, this process is not a very effective means of degrading contaminants because the yields of oxidants are usually low (i.e., typically less than 5% of the iron added is converted into oxidants capable of transforming organic compounds). The addition of polyoxometalate (POM) greatly increases the yield of oxidants in both systems. The mechanism of POM enhancement depends on solution pH. Under acidic conditions, POM-mediates the electron transfer from nanoparticulate zero-valent iron (nZVI) or Fe(II) to oxygen, increasing the production of hydrogen peroxide, which is subsequently converted to hydroxyl radical through the Fenton reaction. At neutral pH values, iron forms a complex with POM, preventing iron precipitation on the nZVI surface and in bulk solution. At pH 7, the yield of oxidant approaches the theoretical maximum in the nZVI/O2 and the Fe(II)/O2 systems when POM is present, suggesting that coordination of iron by POM alters the mechanism of the Fenton reaction by converting the active oxidant from ferryl ion to hydroxyl radical. Comparable enhancements in oxidant yields are also observed when nZVI or Fe(II) are exposed to oxygen in the presence of silica-immobilized POM.
Introduction
Several investigators have recently reported the oxidation of pesticides, aromatic compounds, and chelating agents when granular or nanoparticulate zero-valent iron (nZVI) is exposed to oxygen (1–7). The rapid oxidation of recalcitrant compounds during iron oxidation provides a new approach for remediating contaminated soil and groundwater. Under acidic conditions, the two-electron oxidation of ZVI by oxygen produces hydrogen peroxide (H2O2), which is subsequently converted to hydroxyl radical (•OH) or ferryl ion (Fe[IV]) through reaction with ferrous ion (Fe[II]) (3, 7). In the neutral pH range, the oxidation of Fe(II), the primary product of ZVI oxidation by oxygen, also produces H2O2 with superoxide radical (O2•−) serving as an intermediate (8–10). The subsequent oxidation of Fe(II) by oxygen is responsible for the most of the oxidants produced by nZVI at neutral pH values (7). Although the oxidation of Fe(II) and ZVI by oxygen could provide a means of producing strong oxidants without the need for unstable reagents, like H2O2 or ozone, this approach is unlikely to be competitive with existing advanced oxidation processes because less than 5% of the iron added is converted into oxidants species capable of degrading contaminants (7). Possible explanations for the low efficiency of the reaction include loss of oxidants through competing reactions and precipitation of iron oxides and hydroxides (7). Furthermore, the main oxidant produced at neutral pH values, which is believed to be Fe(IV), is unable to oxidize aromatic compounds and other recalcitrant contaminants.
The efficiency of the oxidative ZVI system appears to improve in the presence of ethylenediaminetetraacetate (EDTA) (1, 5). However, EDTA consumes oxidants produced in the oxidative ZVI system, and must be added continuously with iron. A recent study indicates that polyoxometalate (POM), a metal-oxygen cluster anion, also enhances the rate for oxidation of several recalcitrant organic compounds in the granular ZVI/O2 system under acidic conditions (6). POM is known to resist oxidation and to catalyze redox reactions by serving as an electron shuttle (11).
The objective of the present study was to assess the potential for POM to enhance oxidant yields in the nZVI/O2 and the Fe(II)/O2 systems under conditions that might be used in treatment systems. For this purpose, a series of experiments was carried out with compounds that are known to react with •OH or Fe(IV). To evaluate the potential for retaining the catalyst in a treatment system, silica-immobilized POM, capable of mediating the transformation of contaminants at neutral pH values using Fe(II) and oxygen, was synthesized and evaluated.
Materials and Methods
Reagents
All chemicals were of reagent grade and were used without further purification except for 2,4-dinitrophenyl hydrazine (DNPH), which was recrystallized three times from acetonitrile. All chemicals were obtained from Fisher Scientific Inc. except for DNPH, ferrous sulfate, and benzoic acid, para-hydroxybenzoic acid, and phenol which were obtained from Sigma-Aldrich Co.. All solutions were prepared using 18 MΩ Milli-Q water from a Millipore system. Nanoparticulate zero-valent iron (nZVI) was synthesized by aqueous-phase reduction of ferrous sulfate as described previously (7, 12). The nZVI stock solution was prepared daily. The total iron concentration in the nZVI stock solution was determined to be 52 ± 2.6 mM by analyzing an acidified aliquot. Fe(II) stock solution (50 mM) was prepared by dissolving ferrous sulfate in 0.1 mM HCl solution. The POM selected in this study was sodium polyoxotungstate (Na3PW12O40), which exhibited higher yields for oxidation of organic compounds relative to other POMs in the granular ZVI/O2 system (6). Silica-immobilized POM (SiO2-POM) was prepared by a sol-gel hydrothermal method (13, 14). The SiO2-POM catalyst prepared by this method had a POM content of 20 wt% and a BET surface area of 326 m2/g (13).
Measurement of Oxidant Yields Using Probe Compounds
Methanol, 2-propanol, and benzoic acid (pKa = 4.2) were used as probe compounds for detecting hydroxyl radical or ferryl ion. Details regarding the selection of probe compounds and their reactivity with •OH and Fe(IV) are described elsewhere (7). An excess of probe compound (i.e., 100 mM methanol and 2-propanol, 10 mM benzoic acid) was employed to ensure that all of the oxidants were scavenged by the probe compound. In all three cases, the major oxidized product of the probe compound was quantified by high-performance liquid chromatography (HPLC). With the exception of several experiments conducted in the absence of oxygen, the concentration of nZVI or Fe(II) (initial concentration of 0.1 mM) did not result in depletion of oxygen during the experiments which used solutions that were air-saturated ([O2]0 = 0.25 mM) at the start of the expeirments.
Experimental Setup and Procedure
All experiments on the oxidation of probe compounds were performed in 60-mL Pyrex vials at room temperature (20 ± 2°C). A pH buffer was not used for reactions at pH 2 – 4. No significant change in pH was observed at pH 2 – 3. At pH 4, the pH varied by less than 0.5 units during the reaction. The pH of neutral and alkaline solutions was buffered with 1.5 mM piperazine-N,N′-bis(ethanesulfonic acid) (PIPES) for pH 6 – 7 or 2 mM borate for pH 8.5 – 10. PIPES buffer was selected because it does not form complexes with dissolved iron (15). The solution pH was adjusted using 1 N HCl or 1 N NaOH solutions. The experiments were initiated by adding an aliquot of freshly prepared nZVI stock suspension or Fe(II) stock solution to an air-saturated, pH-adjusted solution containing the probe compound. Samples were withdrawn at predetermined timed intervals using a 5-mL glass syringe and filtered immediately through a 0.22-μm nylon syringe filter. 1,10–Phenanthroline was used to prevent the possible reactions of Fe(II) with oxygen or H2O2 that could have occurred after filtration. For experiments conducted in the absence of oxygen, ultra-pure argon gas was bubbled with a needle-type diffuser for 15 min prior to the initiation of the reaction and during the entire experiment. Most of experiments were carried out in triplicate, and average values and the standard deviations are presented.
Analytical Methods
The concentration of HCHO and acetone (i.e., the oxidized products of methanol and 2-propanol, respectively) were determined using DNPH derivatization followed by HPLC and UV absorbance detection at 350 nm (16). Benzoic acid, para-hydroxybenzoic acid (p-HBA), and phenol were analyzed using HPLC with UV detection at both 255 and 270 nm. Separation was performed on a Waters Symmetry C18 column (150 mm × 4.6 mm, 5 μm), using water with 10 mM formic acid and acetonitrile as the eluent, at a flow rate of 1.0 mL min−1. Analysis of Fe(II) was carried out using 1,10–phenanthroline (17). Total iron concentration was determined by analyzing Fe(II) after Fe(III) was reduced with hydroxylamine hydrochloride. UV/visible absorption spectra of POM and iron solutions were obtained using a Perkin Elmer Lambda 14 spectrophotometer with a quartz cell with a 1 cm optical path length.
Results
Enhanced Oxidation of Methanol in the Presence of POM
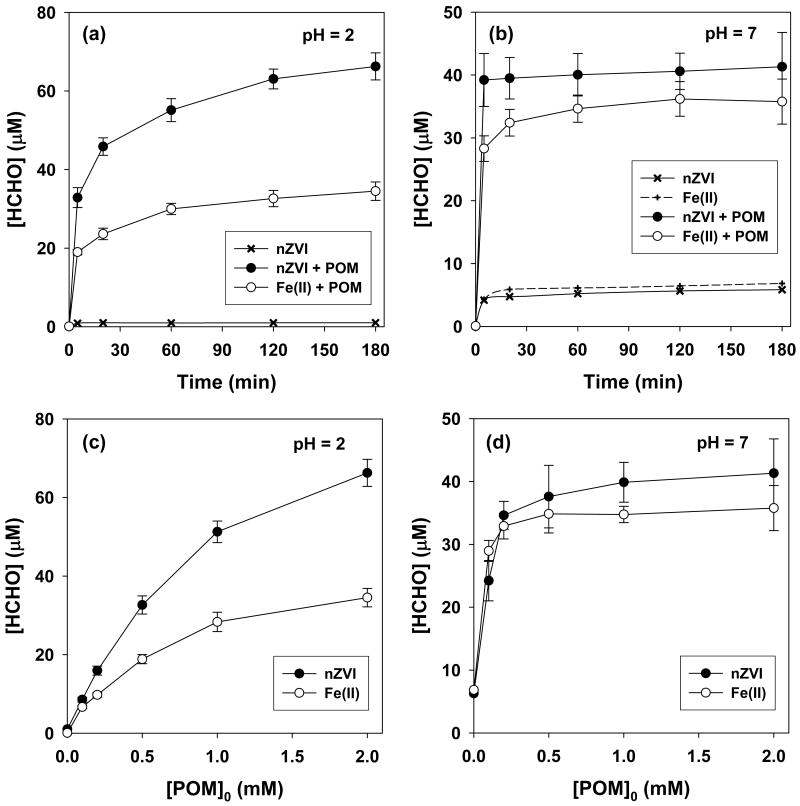
The oxidation of methanol (100 mM) to HCHO in the nZVI/O2 or the Fe(II)/O2 systems was investigated in the absence and presence of POM. In the presence of 2 mM POM, HCHO production was enhanced by approximately an order of magnitude both at pH 2 and 7 (Figure 1a & 1b). At pH 2, POM increased the HCHO production from 1 to 67 μM, and from 0.1 to 36 μM after 180 min in the nZVI/O2 and Fe(II)/O2 systems, respectively (Figure 1a). Significant enhancements of HCHO yields were also observed at pH 7 in these two systems (from 6 to 36–40 μM, Figure 1b). The concentrations of HCHO slowly increased over 180 min at pH 2, whereas HCHO production at pH 7 was almost instantaneous. Likewise, HCHO production after 180 min slowly increased as the concentration of POM increased at pH 2, whereas the yields plateaued after the addition of 0.5 mM POM at pH 7 (Figs. 1c & 1d). These different trends of HCHO formation at pH 2 and 7 suggest that the mechanism of the POM enhancement depends on solution pH.
Figure 1.
Effect of POM on HCHO production from nZVI and Fe(II) in air-saturated solution as a function of reaction time (a) & (b) and POM concentration (c) & (d): [POM]0 = 2 mM for (a) & (b); reaction time = 180 min for (c) & (d); [Fe0]0 = [Fe(II)]0 = 100 μM; [methanol]0 = 100 mM.
Effects of Solution pH on Oxidation of Probe Compounds
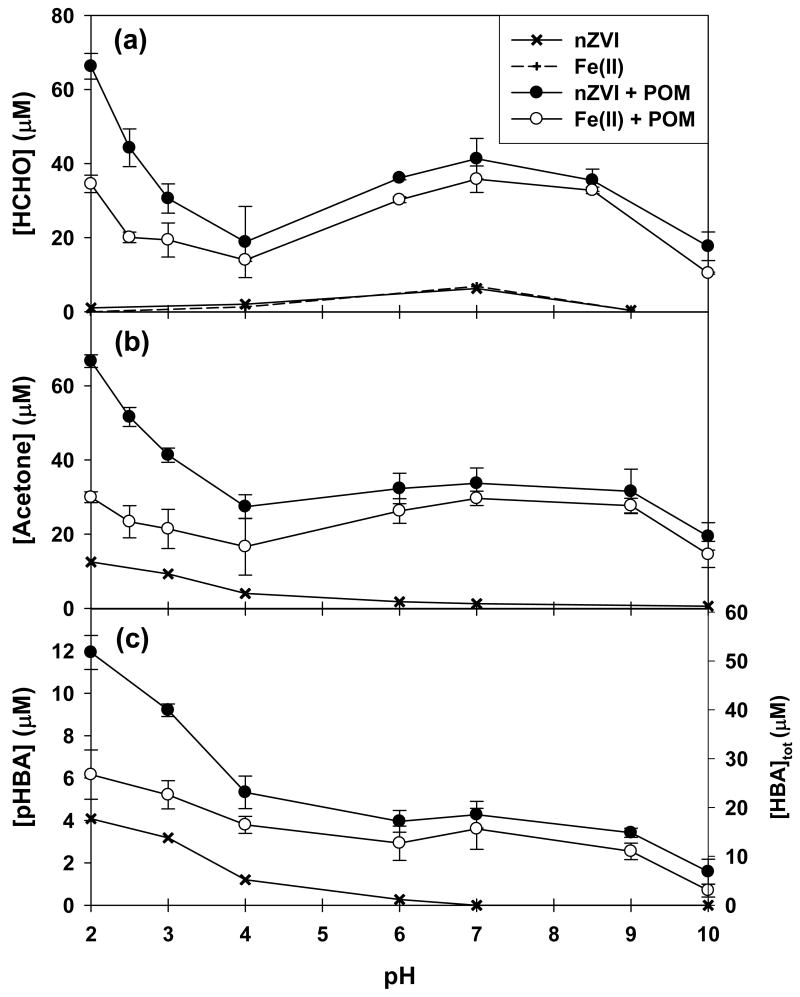
The addition of 2 mM POM enhanced the oxidation of all three probe compounds in the nZVI/O2 and the Fe(II)/O2 systems from pH 2–10 (Figure 2). The highest yields for HCHO formation were observed at around pH 2 and 7, which supports the hypothesis that different reaction mechanisms are involved in the POM enhancement under acidic and neutral pH conditions.
Figure 2.
HCHO, acetone, and pHBA production as a function of pH: [Fe0]0 = [Fe(II)]0 = 100 μM; [POM]0 = 2 mM; [methanol]0 for (a) = [2-propanol]0 for (b) = 100 mM; [benzoic acid]0 for (c) = 10 mM; reaction time = 180 min; [HBA]tot in (c) indicates the calculated values from the yields of pHBA.
The trends in pH dependence for production of acetone (from oxidation of 2-propanol) and p-HBA (from oxidation of benzoic acid) were similar to those observed for HCHO (Figure 2). The low concentration of p-HBA relative to those of HCHO and acetone is due to the formation of the ortho and meta isomers of HBA, which were not quantified due to their co-elution in the HPLC.
Total HBA yields were calculated based on the reported product ratio from the reaction of benzoic acid with •OH (i.e., o-HBA:m-HBA:p-HBA = 1.7:2.3:1.2; 18). It has been reported that HCHO, acetone, and HBAs account for more than 90% of the primary products from oxidation of methanol, 2-propanol, and benzoic acid by •OH (18–21).
Spectrophotometric Measurements of POM− and Iron-POM Complexes
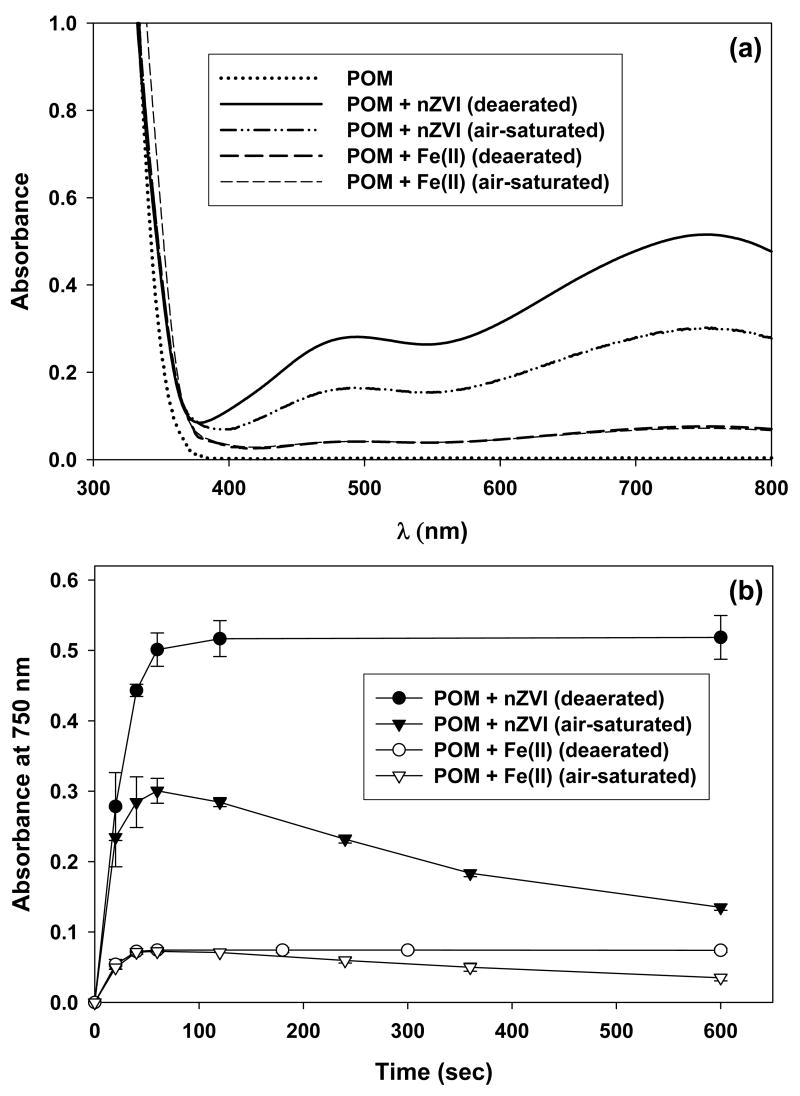
At pH 2, under both air-saturated and deaerated conditions, the colorless solution of POM rapidly turned blue-black upon the addition of nZVI or Fe(II), with broad visible absorption bands centered at around 490 and 750 nm (Figure 3). These visible absorption bands were consistent with the reported spectrum of reduced POM, POM− (22). The increase of the visible absorbance occurred rapidly, and was complete in approximately 60 sec under all conditions. However, the maximum values of the absorbance were different, depending on the form of iron (i.e., nZVI or Fe[II]) and the aeration condition.
Figure 3.
(a) UV-vis absorption spectra of POM solutions with nZVI and Fe(II) under air-saturated and deaerated conditions (reaction time = 60 sec) and (b) time-dependent variations in the absorbance at 750 nm: [Fe0]0 = [Fe(II)]0 = 100 μM; [POM]0 = 1 mM; pH = 2.
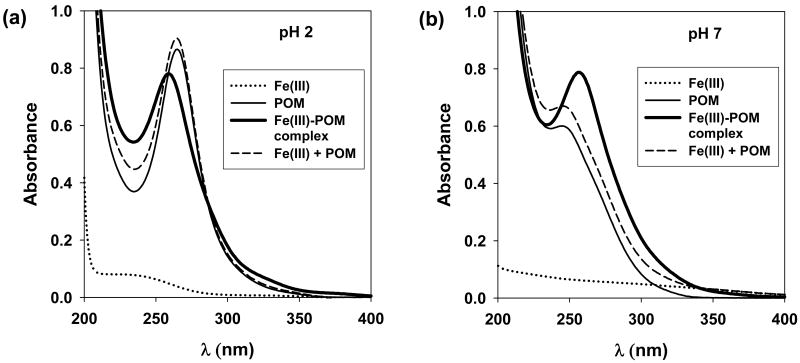
A solution containing POM and Fe(III) exhibited a UV absorption spectrum at both pH 2 & 7 (Figure 4), that was different from the physical summation of the individual spectra of POM and Fe(III) (compare dashed to heavy solid lines), indicating that POM formed complexes with Fe(III).
Figure 4.
UV-vis absorption spectra of the solutions of POM and Fe(III) at pH 2 & 7: [Fe(III)]0 = [POM]0 = 20 μM.
Silica-Immobilized POM
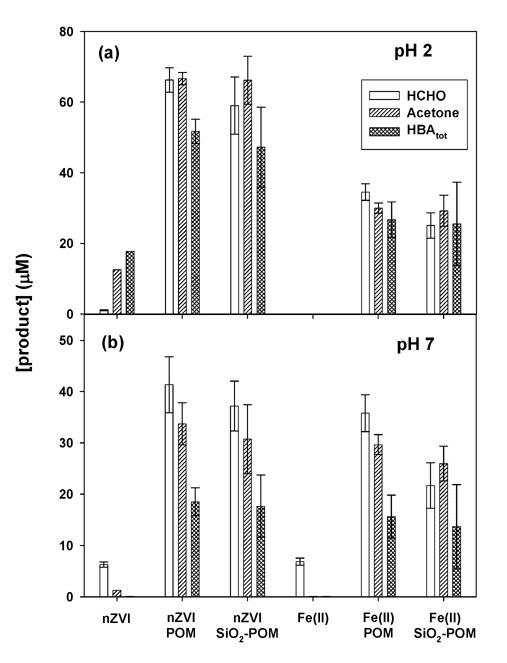
POM immobilized into a silica matrix (SiO2-POM) was insoluble in water, and was readily separable by filtration or settling. At pH 2 & 7, SiO2-POM also enhanced the oxidation of probe compounds in both the nZVI/O2 and the Fe(II)/O2 systems (Figure 5). Attaching the POM to silica resulted in a slightly lower yield of the oxidized products (i.e., SiO2-POM showed approximately 60–90% of the yields observed in the unsupported systems).
Figure 5.
HCHO, acetone, and HBA production with silica-immobilized POM: [Fe0]0 = [Fe(II)]0 = 100 μM; [POM]0 = 2 mM; [methanol]0 = [2-propanol]0 = 100 mM; [benzoic acid]0 = 10 mM; reaction time = 180 min; [HBA]tot indicates the calculated values from the yields of pHBA.
Discussion
Oxidant Production by Reactions of nZVI and Fe(II) with Oxygen
ZVI oxidation by dissolved oxygen produces H2O2 via a two-electron transfer from the particle surface to oxygen (reaction 1; 23, 24). The H2O2 produced by reaction 1 is either reduced to water by another two-electron transfer from ZVI (four-electron transfer; reactions 1 & 2), or converted to •OH or Fe(IV) (e.g., FeO2+) by reaction with Fe(II) (Fenton reaction; reactions 3a & 3b). Our previous study showed that the nZVI oxidation by oxygen occurs mainly through the four-electron mechanism, and depending on the solution pH, only 1–5% of the nZVI participates in the Fenton reaction to produce oxidants (7).
| (1) |
| (2) |
| (3a) |
| (3b) |
Under neutral pH conditions, the oxidation of Fe(II) by oxygen also produces H2O2 via a series of one-electron transfers (reactions 4 & 5), which subsequently yields oxidants through the Fenton reaction (8–10). The oxidation of Fe(II), produced as the primary product of ZVI oxidation (reaction 1), accounts for the most of the oxidants produced by nZVI at neutral pH values (7). Previous studies have suggested that Fe(IV) rather than •OH is the predominant oxidant produced by the Fenton reaction at neutral pH (7,25,26). According to reactions 3–5, the theoretical yield of Fe(IV) should be 33% with respect to Fe(II) added, because three moles of Fe(II) are oxidized to produce one mole of Fe(IV). However, the precipitation of iron oxides and hydroxides on the nZVI surface, and the co-precipitation of Fe(II) and Fe(III) in bulk solution may limit the iron availability at neutral pH, leading to the low oxidant yields observed in both nZVI and Fe(II) systems.
| (4) |
| (5) |
POM as an Electron Shuttle in nZVI and Fe(II) Systems at Acidic pH
POM (PW12O403− in this study) mediates the transfer of electrons from ZVI to oxygen to increase H2O2 production (6). When POM reacts with ZVI, it is reduced to POM− (PW12O404−) (reaction 6). POM−, which is a stable blue-black species, slowly reduces oxygen via a series of one-electron transfers to produce H2O2 (reactions 7 & 8; 27). Some investigators have also suggested that the reduction of oxygen to H2O2 by POM− occurs via an inner-sphere complexation mechanism without producing O2•− as an intermediate (6, 28). In either case, the electron shuttle process mediated by POM separates H2O2 from ZVI surfaces, and decreases the loss of H2O2 by reaction 2. As a result, the oxidant yields at pH 2 (with respect to nZVI added) approached the theoretical maximum (i.e., one •OH for each Fe0; Figure 1a & 1c).
| (6) |
| (7) |
| (8) |
POM also mediates the transfer of electrons from Fe(II) to oxygen. The oxidation of Fe(II) by oxygen is negligible at acidic pH due to its slow reaction rate (10); no production of HCHO was observed in the Fe(II)/O2 system without POM at pH 2 (Figures 1a & 1c). However, significant production of HCHO was observed in the presence of POM, indicating that the electron transfer from Fe2+ to POM (reaction 9) followed by oxidant production (reactions 7, 8, & 3) occurred under acidic conditions. The occurrence of reaction 9 was also supported by observing the blue-black color (due to the production of POM−) during the reaction of Fe2+ with POM at pH 2. The HCHO yield obtained in the Fe(II)/O2 system with POM at pH 2 (Figure 2a) approached the theoretical yield of oxidants from reactions 3, 7–9 (one •OH for every three Fe(II) added).
| (9) |
Reaction 9 can be reversible. According to the redox potentials of Fe3+/Fe2+ (EH0 = +0.771 V; 29) and POM/POM− (EH0[PW12O403−/PW12O404−] = +0.218 V; 27), the transfer of electrons from POM− to Fe3+ (the reverse reaction) is thermodynamically favored.
Spectrophotometric measurements of POM− provide a better understanding of the mechanism by which the redox reactions of POM occur (Figure 3). Under air-saturated conditions, the initial rapid increase of absorbance was followed by a slow decrease due to the reaction of POM− with oxygen (reaction 7). In the reaction of nZVI with POM, the lower absorbance observed in the presence of oxygen is attributable to the competitive reaction of nZVI with oxygen (reaction 1). The reactions of Fe(II) with POM showed nearly identical absorbance in the initial stage of reaction in the presence and absence of oxygen, which is consistent with the extremely slow reaction of Fe(II) with oxygen (reaction 4) at acidic pH.
The concentration of POM− produced under each condition can be calculated from the reported molar absorption coefficient of POM− (ε750nm = 2000 M−1 cm−1; 22). In the absence of oxygen, 0.25 ± 0.015 mM POM− was produced from the reaction of 0.1 mM nZVI with 1 mM POM, whereas only 0.037 ± 0.0014 mM POM− was produced from the reaction of 0.1 mM Fe(II) with 1 mM POM. POM− produced from Fe(II) is attributed to reaction 9, and the POM− produced from nZVI is by reactions 6 & 9. The low yield of POM− (3.7%) from Fe(II) suggests that the reverse reaction of reaction 9 is faster than the forward reaction, which is consistent with the half-cell potentials. Based on the stoichiometry of reaction 9, the initial concentrations of Fe(II) and POM, and the equilibrium concentration of POM− (i.e., 0.1, 1, and 0.037 mM, respectively), the equilibrium constant for reaction 9 is calculated to be (0.037)2 / (0.063 × 0.963) = 2.26 × 10-2. This value is much higher than that estimated from the redox potentials of free iron ions, Fe3+/Fe2+ and POM/POM− (i.e., KEstimated = 3.06 × 10-10). However, the coordination of iron by POM, an electron-withdrawing ligand (described in the following section) may lower the redox potential of the Fe3+/Fe2+ couple. For example, complexation of iron by oxalate lowers the redox potential of the Fe3+/Fe2+ couple to +0.035 V (28). Based on the equilibrium constant determined above, the redox potential of Fe(III)-POM/Fe(II)-POM couple is estimated to be +0.314 V (for details, see Supporting Information, S1). The trend of HCHO production at pH 2 can be explained by the reactions described above. The production of HCHO with time (Figure 1a) appears to be controlled by reaction 7 (k7 = 7.5 M−1 s−1 at pH 2; 27). Assuming [O2]0 = 0.25 mM, the half life of POM− is estimated to be approximately 6.2 min, which is in reasonable agreement with the rates of HCHO production (Figure 1a). The increase in HCHO yield with increasing concentrations of POM (Figure 1c) results from the competitive reactions of nZVI with oxygen and POM (reactions 1 & 6) for the nZVI/O2 system, and the reversible reactions of Fe2+ and POM (reaction 9) for the Fe(II)/O2 system.
Hydrolysis of POM and Formation of Iron-POM complexes at Neutral pH
In neutral and alkaline solutions, POM undergoes hydrolysis, with increasing hydrolysis rates as pH increases (30, 31). At pH 7, POM (PW12O403−) is hydrolyzed to PW9O349− (denoted as POMH) in less than 10 seconds, which subsequently decomposes over several hours to yield PO43− and WO42− (31). At pH 7, the measured spectrum of POM was consistent with that of POMH (31). When the pH was lowered to 2, the UV absorption spectrum changed into that of POM, indicating that the primary hydrolysis of POM to POMH is reversible (reaction 10; Supporting Information, S2).
| (10) |
No significant visible absorption band of POM− or any other species was observed at pH 7 in the solution containing POM and nZVI (or Fe[II]), indicating that the hydrolysis of POM to POMH limits its role as an electron shuttle (i.e., reactions 6 – 9 are unimportant at pH 7).
Spectrophotometric measurements of the solutions of POM and Fe(III) at pH 2 & 7 indicated that both POM and POMH formed complexes with Fe(III) (Figure 4). Although the exact structure of the Fe(III)-POM complexes is unknown, the spectrum of the complex shifted with pH. It was also verified that Fe(II) forms complexes with POMH at pH 7 in the absence of oxygen (Supporting Information, S2).
The formation of Fe(III)-POM complexes prevents the precipitation of Fe(III) and Fe(II) oxides and hydroxides on the nZVI surface and in bulk solution at neutral pH, which increases the availability of nZVI and Fe(II), and subsequently increases oxidant yields. The addition of EDTA, a well-known iron-chelating agent also enhances methanol oxidation in the nZVI/O2 system, indirectly supporting this explanation (Supporting Information, S3). In the Fe(II)/O2 system with POM at pH 7, the HCHO yields (Figure 1) are close to the maximum theoretical yield of oxidants (33% with respect to Fe(II) added) from the Fe(II) oxidation by oxygen (reactions 3–5), indicating that no significant loss of Fe(II) occurred through iron precipitation. The higher yields of HCHO in the nZVI/O2 system is probably due to the reaction of nZVI with oxygen (reactions 1–3). The slight difference of HCHO yield between the Fe(II)/O2 and the nZVI/O2 systems at neutral pH is in agreement with the previous observation that the oxidation of Fe(II) by oxygen is mainly responsible for the oxidant production by nZVI (7).
Experiments conducted with different probe compounds (methanol, 2-propanol, and benzoic acid; Figure 2) suggest that POM coordination to Fe(II) may alter the mechanism of Fenton reaction at neutral pH, converting the active oxidant from Fe(IV) to •OH. •OH reacts with all of the probe compounds at nearly diffusion controlled rates (32). In contrast, Fe(IV) is more selective than •OH, exhibiting low reactivity toward 2-propanol and benzoic acid (e.g., kFe(IV),benzoic acid = 8.0 × 101 M−1 s−1) (25, 26, 33, 34). In the absence of POM, the maximum yield of HCHO from methanol was observed at pH 7, whereas benzoic acid and 2-propanol oxidation was observed only under acidic conditions, supporting the presumption that the Fenton reaction produces Fe(IV) rather than •OH at neutral pH in the absence of Fe(II)-complexing ligands (25, 26). However, in the presence of POM, significant oxidation of benzoic acid and 2-propanol was observed at neutral pH, with the formation of 20 – 30 μM of oxidation products at pH 7. The similar trends in pH dependence regardless of the probe compound suggest that, for Fe(II)-POM complexes, the Fenton reaction predominantly produces •OH over a wide pH range. Previous studies have shown that the mechanism of the Fenton reaction depends on the coordination of Fe(II) (33, 35, 36), but additional research is needed to assess the role of ligands on the oxidant yield in the nZVI/O2 and the Fe(II)/O2 systems.
Implications for Contaminant Oxidation
Both the nZVI/O2 and Fe(II)/O2 systems combined with POM could be useful approaches for the oxidative treatment of wastewater, with iron providing an electron source for converting oxygen to •OH. However, the Fe(II)/O2 system is probably more cost-effective method than the nZVI/O2 system because the synthesis of nZVI requires sodium borohydride to reduce Fe(II). In the absence of POM, the low yields of oxidants produced by both systems require high doses of iron for significant degradation of contaminants. For example, the nZVI/O2 system required approximately 5 – 20 mM ZVI for 60 – 80% degradation of 100 ppb molinate (0.55 μM) at the optimum pH (pH 4) (2). The addition of POM can reduce the iron dose and eliminate the need for acidification in both the nZVI/O2 and the Fe(II)/O2 systems by increasing oxidant yields and shifting the oxidant from Fe(IV) to •OH. In experiments with 1 mM nZVI or Fe(II) with 10 μM phenol or benzoic acid at pH 2 & 7, less than 10% of the contaminants were transformed in the absence of POM. When 1 mM of POM was added, 50 – 90% of the contaminants were transformed (Supporting Information, S4).
POM and its hydrolyzed products (PO43− and WO42−) are relatively nontoxic (37), and therefore their potential release to the aquatic environment is unlikely to be a serious concern. However, POM is expensive and the reuse of POM would be necessary for the cost-effective application of these systems. A combined process with nanofiltration to retain POM or a process using a supported catalyst such as SiO2-POM (Figure 5) may provide a means of recovering and recycling POM. In addition, SiO2-POM is insoluble (11) and likely resistant to hydrolysis at neutral pH. Additional research is needed to assess the feasibility of these processes under conditions likely to be encountered in treatment systems.
Supplementary Material
Detailed calculation of the equilibrium constant for reaction 9 and the redox potential of Fe(III)-POM/Fe(II)-POM couple (S1), additional UV-vis absorption spectra of POM and iron-POM complexes (S2), and figures showing the effect of EDTA on methanol oxidation (S3), and the loss of phenol or benzoic acid in the presence and absence of POM (S4): This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This research was supported by the US National Institute for Environmental Health Sciences (Grant P42 ES004705-19).
Footnotes
One sentence synopsis: Polyoxometalate greatly increases the yield of oxidants from nanoparticulate zero-valent iron and ferrous ion by serving as an electron shuttle and an iron-chelating agent.
Literature Cited
- 1.Noradoun C, Engelmann M, McLauglin M, Hutcheson R, Breen K, Paszczynski A, Cheng IF. Destruction of chlorinated phenols by dioxygen activation under aqueous room temperature and pressure conditions. Ind Eng Chem Res. 2003;42:5024–5030. [Google Scholar]
- 2.Joo SH, Feitz AJ, Waite TD. Oxidative degradation of the carbothiolate herbicide, molinate, using nanoscale zero-valent iron. Environ Sci Technol. 2004;38:2242–2247. doi: 10.1021/es035157g. [DOI] [PubMed] [Google Scholar]
- 3.Joo SH, Feitz AJ, Sedlak DL, Waite TD. Quantification of the oxidizing capacity of nanoparticulate zero-valent iron. Environ Sci Technol. 2005;39:1263–1268. doi: 10.1021/es048983d. [DOI] [PubMed] [Google Scholar]
- 4.Feitz AJ, Joo SH, Guan J, Sun Q, Sedlak DL, Waite TD. Oxidative transformation of contaminants using colloidal zero-valent iron. Colloid Surf A-Physicochem Eng Asp. 2005;265:88–94. [Google Scholar]
- 5.Englehardt J, Meeroof D, Echegoyen L, Deng Y, Raymo F, Shibata T. Oxidation of aqueous EDTA and associated organics and coprecipitation of inorganics by ambient iron-mediated aeration. Environ Sci Technol. 2007;41:270–276. doi: 10.1021/es061605j. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Kim J, Choi W. Oxidation on zerovalent iron promoted by polyoxometalate as an electron shuttle. Environ Sci Technol. 2007;41:3335–3340. doi: 10.1021/es062430g. [DOI] [PubMed] [Google Scholar]
- 7.Keenan CR, Sedlak DL. Factors affecting the yields of oxidants from the reaction of nanoparticulate zero-valent iron and oxygen. Environ Sci Technol. 2008;42:1262–1267. doi: 10.1021/es7025664. [DOI] [PubMed] [Google Scholar]
- 8.Stumm W, Lee GF. Oxygenation of ferrous iron. Ind Eng Chem. 1961;53:143–146. [Google Scholar]
- 9.Millero FJ, Izauirre M. Effect of ionic strength and ionic interactions on the oxidation of Fe(II) J Sol Chem. 1989;18:585–599. [Google Scholar]
- 10.King DW, Lounsbury HA, Millero FJ. Rates and mechanism of Fe(II) oxidation at nanomolar total iron concentrations. Environ Sci Technol. 1995;29:818–824. doi: 10.1021/es00003a033. [DOI] [PubMed] [Google Scholar]
- 11.Kozhevnikov IV. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem Rev. 1998;98:171–198. doi: 10.1021/cr960400y. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Fan MH, Brown RC, Van Leeuwen JH, Wang JJ, Wang WH, Song YH, Zhang PY. Synthesis, properties, and environmental applications of nanoscale iron-based materials: A review. Crit Rev Environ Sci Technol. 2006;36:405–431. [Google Scholar]
- 13.Yue B, Zhou Y, Xu J, Wu Z, Zhang X, Zou Y, Jin S. Photocatalytic degradation of aqueous 4-chlorophenol by silica-immobilized polyoxometalates. Environ Sci Technol. 2002;36:1325–1329. doi: 10.1021/es011038u. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Park H, Choi W. Comparative study of homogeneous and heterogeneous photocatalytic redox reactions: PW12O403- vs TiO2. J Phys Chem B. 2004;108:6402–6411. doi: 10.1021/jp049789g. [DOI] [PubMed] [Google Scholar]
- 15.Yu Q, Kandegedara A, Xu Y, Rorabacher DB. Avoiding interferences from Good's buffers: A contiguous series of noncomplexing tertiary amine buffers covering the entire range of pH 3-11. Analytical Biochem. 1997;253:50–56. doi: 10.1006/abio.1997.2349. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Mopper K. Determination of photochemically produced hydroxyl radicals in seawater and freshwater. Mar Chem. 1990;30:71–88. [Google Scholar]
- 17.Tamura H, Goto K, Yotsuyanagi T, Nagayama M. Spectrophotometric determination of iron(II) with 1,10phenanthroline in the presence of large amounts of iron(III) Talanta. 1974;21:314–318. doi: 10.1016/0039-9140(74)80012-3. [DOI] [PubMed] [Google Scholar]
- 18.Klein GW, Bhatla K, Madhavan V, Schuler RH. Reaction of hydroxyl radicals with benzoic acid: Isomer distribution in the radical intermediates. J Phys Chem. 1975;79:1767–1774. [Google Scholar]
- 19.Asmus KD, Mockel H, Henglein A. Pulse radiolytic study of the site of OH radical attach on aliphatic alcohols in aqueous solution. J Phys Chem. 1973;77:1218–1221. [Google Scholar]
- 20.Hess WP, Tully FP. Hydrogen-atom abstraction from methanol by OH. J Phys Chem. 1989;93:1944–1947. [Google Scholar]
- 21.Walling C, Kato S. The oxidation of alcohols by Fenton's reagent. The effect of copper ion. J Am Chem Soc. 1971;93:4275–4281. [Google Scholar]
- 22.Papaconstantinou E. Photochemistry of polyoxometalates of molybdenum and tungsten and/or vanadium. Chem Soc Rev. 1989;18:1–31. [Google Scholar]
- 23.Zecevic S, Drazic DM, Gojkovic S. Oxygen reduction on iron. Part III. An analysis of the rotating disk-ring electrode measurements in near neutral solutions. J Electroanal Chem. 1989;265:179–193. [Google Scholar]
- 24.Zecevic S, Drazic DM, Gojkovic S. Oxygen reduction on iron. Part IV. The reduction of hydrogen peroxide as the intermediate in oxygen reduction reaction in alkaline solutions. Electrochimica Acta. 1991;36:5–14. [Google Scholar]
- 25.Hug SJ, Canonica L, Wegelin M, Gechter D, von Gunten U. Solar oxidation and recmoval of arsenic at circumneutral pH in iron containing waters. Environ Sci Technol. 2001;35:2114–2121. doi: 10.1021/es001551s. [DOI] [PubMed] [Google Scholar]
- 26.Hug SJ, Leupin O. Iron-catalyzed oxidation of arsenic(III) by oxygen and by hydrogen peroxide: pH-dependent formation of oxidant in the Fenton reaction. Environ Sci Technol. 2003;37:2734–2742. doi: 10.1021/es026208x. [DOI] [PubMed] [Google Scholar]
- 27.Weinstock IA. Homogeneous-phase electron-transfer reactions of polyoxometalates. Chem Rev. 1998;98:113–170. doi: 10.1021/cr9703414. [DOI] [PubMed] [Google Scholar]
- 28.Ducan DC, Hill CL. Mechanism of reaction of reduced polyoxometalates with O2 evaluated by 17O NMR. J Am Chem Soc. 1997;119:243–244. [Google Scholar]
- 29.Bard AJ, Parsons R, Jordan J. Standard potentials in aqueous solution. Marcel Dekker, Inc.; New York, Basel: 1985. [Google Scholar]
- 30.Jürgensen A, Moffat JB. The stability of 12-molybdosilicic, 12-tungstosilicic, 12-molybdophosphoric and 12-tungstophosphoric acids in aqueous solution at various pH. Catal Lett. 1995;34:237–244. [Google Scholar]
- 31.Kyle JH. Kinetics of the base decomposition of dodecatungstophosphate(3−) in weakly alkaline solutions. J Chem Soc Dalton Trans. 1983:2609–2612. [Google Scholar]
- 32.Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals in aqueous solution. J Phys Chem Ref Data. 1988;17:513–886. [Google Scholar]
- 33.Rush JD, Koppenol WH. Oxidizing intermediates in the reaction of ferrous EDTA with hydrogen peroxide. J Bio Chem. 1986;261:6730–6733. [PubMed] [Google Scholar]
- 34.Jacobsen F, Holcman J, Sehested K. Reactions of the ferryl ion with some compounds found in cloud water. Int J Chem Kinetics. 1998;30:215–221. [Google Scholar]
- 35.Rahhal S, Richter HW. Reduction of hydrogen peroxide by the ferrous iron chelate of diethylenetriamine-N,N,N′,N″,N″-pentaacetate. J Am Chem Soc. 1988;110:3126–3133. [Google Scholar]
- 36.Yamazaki I, Piette LH. EPR Spin-trapping study on the oxidizing species formed in the reaction of the ferrous ion with hydrogen peroxide. J Am Chem Soc. 1991;113:7588–7593. [Google Scholar]
- 37.Rhule JT, Hill CL, Judd DA, Schinazi RF. Polyoxometalates in medicine. Chem Rev. 1998;98:327–357. doi: 10.1021/cr960396q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed calculation of the equilibrium constant for reaction 9 and the redox potential of Fe(III)-POM/Fe(II)-POM couple (S1), additional UV-vis absorption spectra of POM and iron-POM complexes (S2), and figures showing the effect of EDTA on methanol oxidation (S3), and the loss of phenol or benzoic acid in the presence and absence of POM (S4): This material is available free of charge via the Internet at http://pubs.acs.org.