Abstract
We molecularly cloned the tandem direct reiteration (R1) present in open reading frame (ORF) 11 from three independent strains of varicella-zoster virus. Comparison of the R1 sequences among varicella-zoster virus strains revealed that, although the portion of R1 near the 5' terminus of ORF 11 was conserved among strains, the 3'-terminal portion varied remarkably. This variation was due to the different arrangement of two elements (A and B) and a segment produced by fusion of A and B and to a single-base change in the A element. Since the difference in the size of R1 among strains was a multiple of 3 base pairs, the variation in R1 caused no frame shift in ORF 11.
Full text
PDF
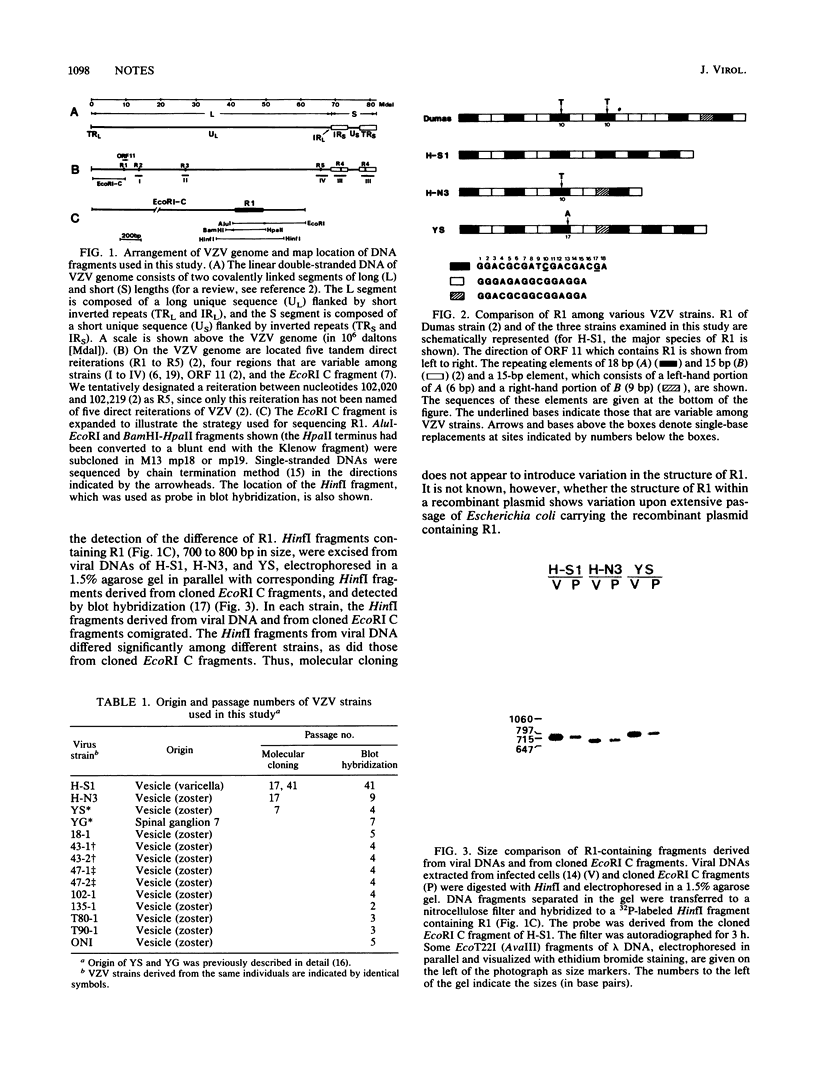


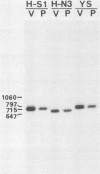
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casey T. A., Ruyechan W. T., Flora M. N., Reinhold W., Straus S. E., Hay J. Fine mapping and sequencing of a variable segment in the inverted repeat region of varicella-zoster virus DNA. J Virol. 1985 May;54(2):639–642. doi: 10.1128/jvi.54.2.639-642.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classification and nomenclature of viruses. Fourth report of the International Committee on Taxonomy of Viruses. Intervirology. 1982;17(1-3):1–199. doi: 10.1159/000149278. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y., Torigoe S., Shiraki K., Yamanishi K., Takahashi M. Biologic and biophysical markers of a live varicella vaccine strain (Oka): identification of clinical isolates from vaccine recipients. J Infect Dis. 1984 Jun;149(6):956–963. doi: 10.1093/infdis/149.6.956. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y., Yamamoto T., Yamanishi K., Takahashi M. Analysis of varicella-zoster virus DNAs of clinical isolates by endonuclease HpaI. J Gen Virol. 1986 Sep;67(Pt 9):1817–1829. doi: 10.1099/0022-1317-67-9-1817. [DOI] [PubMed] [Google Scholar]
- Hondo R., Yogo Y., Kurata T., Aoyama Y. Genome variation among varicella-zoster virus isolates derived from different individuals and from the same individuals. Arch Virol. 1987;93(1-2):1–12. doi: 10.1007/BF01313890. [DOI] [PubMed] [Google Scholar]
- Kinchington P. R., Reinhold W. C., Casey T. A., Straus S. E., Hay J., Ruyechan W. T. Inversion and circularization of the varicella-zoster virus genome. J Virol. 1985 Oct;56(1):194–200. doi: 10.1128/jvi.56.1.194-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. H., Dohner D. E., Wellinghoff W. J., Gelb L. D. Restriction endonuclease analysis of varicella-zoster vaccine virus and wild-type DNAs. J Med Virol. 1982;9(1):69–76. doi: 10.1002/jmv.1890090110. [DOI] [PubMed] [Google Scholar]
- McKnight J. L., Pellett P. E., Jenkins F. J., Roizman B. Characterization and nucleotide sequence of two herpes simplex virus 1 genes whose products modulate alpha-trans-inducing factor-dependent activation of alpha genes. J Virol. 1987 Apr;61(4):992–1001. doi: 10.1128/jvi.61.4.992-1001.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Iltis J. P., Hyman R. W., Rapp F. Analysis by restriction enzyme cleavage of human varicella-zoster virus DNAs. Virology. 1977 Oct 15;82(2):353–361. doi: 10.1016/0042-6822(77)90010-1. [DOI] [PubMed] [Google Scholar]
- Richards J. C., Hyman R. W., Rapp F. Analysis of the DNAs from seven varicella-zoster virus isolates. J Virol. 1979 Dec;32(3):812–821. doi: 10.1128/jvi.32.3.812-821.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L. J., Crutchfield D. B., Panitz P. J., Clanton D. J. Isolation of human cytomegalovirus DNA from infected cells. Intervirology. 1983;19(2):113–120. doi: 10.1159/000149345. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuta H., Ishikawa T., Hondo R., Aoyama Y., Kurata K., Matumoto M. Varicella virus isolation from spinal ganglion. Arch Gesamte Virusforsch. 1974;45(4):382–385. doi: 10.1007/BF01242884. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Aulakh H. S., Ruyechan W. T., Hay J., Casey T. A., Vande Woude G. F., Owens J., Smith H. A. Structure of varicella-zoster virus DNA. J Virol. 1981 Nov;40(2):516–525. doi: 10.1128/jvi.40.2.516-525.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Hay J., Smith H., Owens J. Genome differences among varicella-zoster virus isolates. J Gen Virol. 1983 May;64(Pt 5):1031–1041. doi: 10.1099/0022-1317-64-5-1031. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Reinhold W., Smith H. A., Ruyechan W. T., Henderson D. K., Blaese R. M., Hay J. Endonuclease analysis of viral DNA from varicella and subsequent zoster infections in the same patient. N Engl J Med. 1984 Nov 22;311(21):1362–1364. doi: 10.1056/NEJM198411223112107. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Morton D. H., Stanton L. W., Neff B. J. Restriction endonuclease analysis of the DNA from varicella-zoster virus: stability of the DNA after passage in vitro. J Gen Virol. 1981 Jul;55(Pt 1):207–211. doi: 10.1099/0022-1317-55-1-207. [DOI] [PubMed] [Google Scholar]