Abstract
The atrioventricular (AV) valves of the heart develop from undifferentiated mesenchymal endocardial cushions, which later mature into stratified valves with diversified extracellular matrix (ECM). Because the mature valves express genes associated with osteogenesis and exhibit disease-associated calcification, we hypothesized the existence of shared regulatory pathways active in developing AV valves and in bone progenitor cells. To define gene regulatory programs of valvulogenesis relative to osteoblast progenitors, we undertook Affymetrix gene expression profiling analysis of murine embryonic day (E)12.5 AV endocardial cushions compared with E17.5 AV valves (mitral and tricuspid) and with preosteoblast MC3T3-E1 (subclone4) cells. Overall, MC3T3 cells were significantly more similar to E17.5 valves than to E12.5 cushions, supporting the hypothesis that valve maturation involves the expression of many genes also expressed in osteoblasts. Several transcription factors characteristic of mesenchymal and osteoblast precursor cells, including Twist1, are predominant in E12.5 cushion. Valve maturation is characterized by differential regulation of matrix metalloproteinases and their inhibitors as well as complex collagen gene expression. Among the most highly enriched genes during valvulogenesis were members of the small leucine-rich proteoglycan (SLRP) family including Asporin, a known negative regulator of osteoblast differentiation and mineralization. Together, these data support shared gene expression profiles of the developing valves and osteoblast bone precursor cells in normal valve development and homeostasis with potential functions in calcific valve disease.
Keywords: microarray, heart valve maturation, asporin, osteoglycin, Twist1, collagen
heart valve development is important for proper cardiovascular function, and congenitally malformed or degenerative valve disease is a significant cause of morbidity and mortality (15, 36, 48). Cardiac valve defects represent ∼20–30% of all congenital cardiovascular malformations, with an incidence rate of 1–2 infants per 1,000 live births in the US per year (28, 43). Studies of diseased valves in adult human patients have demonstrated defects associated with remodeling, including leaflet thickening, collagen fiber disorganization, variable cell distribution, and calcification of valve fibrosa matrix (34, 38). Moreover, recent reports also demonstrated the presence of shared pathways in valve pathogenesis and osteogenesis at the molecular level (1, 6, 40, 41). Importantly, calcific valve disease does not usually occur until late in adult life, supporting the existence of protective mechanisms in normal valves that prevent calcification. However, the molecular basis of these natural protective mechanisms is unknown.
The development of the heart valves initiates with the formation of endocardial cushions at atrioventricular (AV) junction and outflow tract of the primitive heart tube. The signaling cues from the surrounding myocardium result in the epithelial-to-mesenchymal transformation of activated endocardial cells, which later migrate into the cardiac jelly between the myocardial and endocardial layers to form the endocardial cushions (EC) (35). As development progresses, the highly proliferative migratory undifferentiated mesenchymal cells of the EC contribute to the differentiated valves with diversified extracellular matrix (ECM) (3, 25, 30). In the developing mouse embryo, mesenchymal undifferentiated EC that contain valve progenitor cells are apparent at embryonic day (E)12.5. These EC mature into elongated, differentiated heart valves with highly organized ECM by E17.5. Mature AV valve leaflets are stratified into three layers, the elastin-rich atrialis, the proteoglycan-rich spongiosa, and the highly organized collagen fiber-rich fibrosa (18, 27, 38). Some ECM components and remodeling enzymes have been identified, but a complete gene expression profile of heart valve maturation has not been established.
Recent research has demonstrated the existence of shared regulatory pathways between heart valve maturation and cartilage, tendon, and bone development (13, 23, 27). The mature valve leaflets and supporting structures are composed of different types of ECM with characteristics of diverse connective tissue types (18, 39). In previous work (24, 27), we demonstrated that regulatory pathways and transcription factors characteristic of cartilage and tendon development also are active in valve progenitor cells. Evidence for valve progenitors with characteristics of osteoblast precursors is the predominant expression of type I collagen fibers and other ECM proteins including osteonectin, periostin, and osteopontin in the valve fibrosa layer (18, 32). Importantly, normal heart valve function is dependent on ECM stratification, with the layers having properties of distinct types of connective tissue, whereas abnormal ECM distribution and composition is associated with congenital and adult valve disease (12, 38). In addition, aortic valve calcification is associated with increased expression of genes characteristic of osteogenesis and mineralization predominantly in the fibrosa layer (41). Therefore, we hypothesize that a significant number of genes differentially regulated during valve cell lineage maturation also are expressed in preosteoblasts. However, the molecular events that result in abnormal ECM gene expression and aberrant matrix organization characteristic of valve disease are poorly understood.
To compare gene expression profiles of developing heart valves and osteoblasts, we performed Affymetrix microarray gene expression analysis on mouse E12.5 EC, E17.5 AV valves, and preosteoblast MC3T3-E1 (subclone4) cells. The MC3T3-E1 cell line has been used previously for identification of osteoblast-related genes and can be induced to differentiate and mineralize under specific culture conditions (42). Therefore, genes expressed in both MC3T3-E1 cells and valve progenitors may be related to the normal structure and function of the valve fibrosa layer and could be involved in the potential of the valves to undergo mineralization under pathological conditions. Here, we demonstrate distinct gene expression profiles of early E12.5 EC and late E17.5 AV valves. In addition, we also demonstrate a significant percentage of shared genes between undifferentiated preosteoblast MC3T3-E1 cells and late AV valve compared with early EC. As expected, differentially expressed genes during AV valve development include several transcription factors, collagen genes, ECM remodeling enzymes, and genes associated with osteogenesis. Among the most differentially expressed genes are members of the small leucine-rich proteoglycan (SLRP) family, which exhibited high expression in the mature AV valves at E17.5 and in adults, but were not detected in AV cushion. Although not directly addressed in this report, the persistent expression of these known inhibitors of osteogenesis in adult AV valves has the potential to serve as a natural protection against valve disease pathogenesis.
MATERIALS AND METHODS
All experiments involving animals were carried out with experimental protocols and procedures reviewed and approved by the Cincinnati Children's Medical Center Biohazard Safety Committee and Institutional Animal Care and Use Committee.
RNA isolation.
Litters of embryos were generated from timed matings of wild-type FVBN mice, with evidence of a copulation plug considered E0.5. Embryos were collected at E12.5 for isolation of EC and at E17.5 for isolation of AV valves. Hearts were isolated and rinsed in phosphate-buffered saline (PBS), and EC or AV valves were carefully dissected out with tungsten needles with minimal myocardial contamination. Dissected cushions and valves were washed twice in 1× PBS and collected in 200 μl of TRIzol reagent (Invitrogen), with 10–12 EC or AV valves pooled as one biological sample; therefore 20–24 EC or AV valves altogether were used for biological replicates of each sample set. In addition, the mouse preosteoblast cell line MC3T3-E1 (subclone4) was maintained in α-minimum essential medium (GIBCO; supplemented with ribonucleosides, deoxyribonucleosides, 2 mM l-glutamine, and 1 mM sodium pyruvate) containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Two 100-mm tissue culture dishes of MC3T3-E1 (subclone4) cells at 80–90% confluence were used to isolate RNA. Total RNA was isolated with TRIzol reagent (Invitrogen) according to the manufacturer's standard guidelines, followed by an additional RNA purification with a RNeasy Mini Kit (Qiagen). The purified total RNA samples were submitted to the Cincinnati Children's Medical Center Affymetrix Microarray Core for further sample processing.
Microarray hybridization and gene expression analysis.
Total RNA quality was confirmed with a Agilent 2100 Bioanalyzer (Agilent Technologies) and a RNA 6000 Nano Assay. After quality control testing of the six submitted samples (E12.5 EC, E17.5 AV valve, and MC3T3-E1 cells in biological duplicate), a total of 400 ng of RNA per sample was used to generate double-stranded cDNA with the TargetAMP1-Round Aminoallyl-aRNA Amplification kit (Epicenter). The cDNA was used to synthesize biotin-labeled cRNA with the IVT Labeling Kit (Affymetrix). The biotin-labeled cRNA target was then chemically fragmented and hybridized to the Mouse Genome 430 2.0 Array (Affymetrix) with standard protocols. Arrays were washed and stained with a Fluidics Station 450 (Affymetrix) and scanned with a GeneChip scanner 3000 (Affymetrix). The scanned gene expression data were exported as “.CEL” files, which were loaded into GeneSpring Gx 7.3 software (Agilent Technologies) and subjected to robust multichip average (RMA) analysis for quantile normalization. Each gene's relative expression in each of the three sample types was referenced to the median value of its intensity to generate an overall expression value. With initial filtering of expression values (with raw intensity value >100), 27,195 probe sets were identified as expressed, representing the top 50% of measured probe sets. Statistical analysis (ANOVA) was performed with a parametric test assuming the variances were equal and a P value of 0.05 with Benjamini-Hochberg's false discovery rate of multiple testing corrections. Of these, 3,119 probe sets were identified as either up- or downregulated by at least twofold between E12.5 EC and E17.5 AV valves.
To compare genes differentially expressed during valve maturation with genes that are also expressed in MC3T3 cells, probe sets within the 3,119 differentially expressed genes with expression values >1.5 in MC3T3 cells were included in the heat map. Venn diagrams were generated to show the number of probe sets differentially expressed in E12.5 EC versus E17.5 AV valves that are also expressed in MC3T3-E1 cells. Similar results in relative shared gene expression were obtained with direct comparison of all genes with raw intensity value >100 in E12.5 EC, E17.5 AV valves, and MC3T3-E1 cells. The 3,119 base gene list was functionally categorized with the PANTHER (Protein Analysis Through Evolutionary Relationships) gene classification system (49, 50). The 3,119 gene list was placed in a table, and the complete data set can be accessed in the GEO database (http://www.ncbi.nlm.gov/geo/) with the accession number GSE 11040.
Real-time quantitative RT-PCR.
Forward and reverse primer sequences used for quantitative real time RT-PCR (qRT-PCR) are shown in Table 1A with optimal annealing temperature and expected product size. Total RNA was isolated from 10 E12.5 EC or 10 E17.5 AV valves for each experimental group collected in 200 μl of TRIzol reagent (Invitrogen), as described by the manufacturer's protocol. Total RNA was also isolated from E17.5 limbs and E13.5 whole embryos with 500 μl of TRIzol reagent as positive controls for qRT-PCR. cDNA was generated with oligo(dT) primers and the SuperScript first-strand synthesis kit (Invitrogen) from 1 μg of total RNA. One microliter of synthesized cDNA was used for analysis by qRT-PCR (MJ Research Opticon 2). Gene expression levels determined by qRT-PCR were calculated as previously reported (24, 37, 45, 46). A standard curve was generated for each experimental primer set with either E17.5 limbs or E13.5 whole embryo cDNA, and all the values were normalized to ribosomal protein L7 expression (17). qRT-PCR results represent three independent experiments (biological 3×) performed in triplicate (technical 3×). Expression is represented as arbitrary units of fluorescence intensity for data generated with equivalent RNA input and normalized to L7 expression. Expression was calculated as a fold increase in intensity values of highly expressed genes over low-level expression at E12.5 or E17.5. Statistical significance of observed differences was determined by Student's t-test.
Table 1.
Primers
| Gene | Annealing Temperature, °C | Product Size, bp | Primer Sequences | |||
|---|---|---|---|---|---|---|
| A. qRT-PCR | ||||||
| Twist1 | 55 | 157 | 5′-CGGACAAGCTGAGCAAGATT-3′ | |||
| 5′-ATCCTCCAGACGGAGAAGG-3′ | ||||||
| Osteonectin | 55 | 160 | 5′-TCCCATTGGCGAGTTTGAGAAGG-3′ | |||
| 5′-CAGGCAGGGGGCGATGTATTTG-3′ | ||||||
| Col1a1 | 55 | 211 | 5′-TCCTGACGCATGGCCAAGAAGACA-3′ | |||
| 5′-TCCGGGCAGAAAGCACAGCACTC-3′ | ||||||
| Col9a3 | 55 | 151 | 5′-TGTACCTGGCATCAGCAAAG-3′ | |||
| 5′-ACCTCCCCCTAACACAGCTC-3′ | ||||||
| Col14a1 | 55 | 184 | 5′-AGCCCAAAGTCAAGGTTGTG-3′ | |||
| 5′-AACGCTGTGACCAGGTTTTC-3′ | ||||||
| Asporin | 56.4 | 212 | 5′-CTGGGCCTAGGAAACAACAA-3′ | |||
| 5′-TTCATCTTTGGCACTGTTGG-3′ | ||||||
| Osteoglycin | 56.4 | 218 | 5′-TGCTTTGTGGTCACATGGAT-3′ | |||
| 5′-GAAGCTGCACACAGCACAAT-3′ | ||||||
| Matrilin2 | 56.4 | 214 | 5′-CCCAGCGCTTAGAAGAAATG-3′ | |||
| 5′-TGTCCAGCAGTCAAGCAATC-3′ | ||||||
| B. Generation of antisense RNA probes | ||||||
| Osteonectin | 616 | 5′-AGGGCCTGGATCTTCTTTCTC-3′ | ||||
| 5′-CCTCCAGGCGCTTCTCATTC-3′ | ||||||
| Col1a1 | 828 | 5′-TGGTCTTCCTGGCCCCTCTGGTG-3′ | ||||
| 5′-TCGGGGCTGCGGATGTTCTCAAT-3′ | ||||||
| Col9a3 | 420 | 5′-CTGGAAAACCCGGCAAGGATGG-3′ | ||||
| 5′-GCGGGCAGATGGCGGGACAC-3′ | ||||||
| Col14a1 | 870 | 5′-GAAGTTCCCGCCCAGCAATAC-3′ | ||||
| 5′-CACGAGGCCAGTCAGAGCATCAC-3′ | ||||||
| Asporin | 953 | 5′-AGGACACGTTCAAGGGAATG-3′ | ||||
| 5′-TGCTGTTCTTGCCAGTTTTG-3′ | ||||||
| Osteoglycin | 950 | 5′-TGCAAGGCTAATGACACTCG-3′ | ||||
| 5′-GAAGCTGCACACAGCACAAT-3′ | ||||||
| Matrilin2 | 814 | 5′-AGGACCATGTCTTCCTGGTG-3′ | ||||
| 5′-CTGGAGCAGGTCTTGAGGTC-3′ | ||||||
qRT-PCR, quantitative RT-PCR.
Probe generation and in situ hybridization.
The forward and reverse primer sequences used to generate mouse antisense RNA probes for in situ hybridization (ISH) are shown in Table 1B with the expected product size. The Asporin sequence (GenBank accession no. NM_025711) was amplified from E18.5 limb cDNA. Osteoglycin (GenBank accession no. NM_008760) and Matrilin2 (GenBank accession no. NM_016762) sequences were amplified from E12.5 limb cDNA. The Osteonectin sequence (GenBank accession no. NM_009242.2) was amplified from E14.5 heart cDNA. The Col1a1 sequence (GenBank accession no. NM_007742.3) was amplified from neonatal limb cDNA. The Col9a3 sequence (GenBank accession no. NM_009936.2) was amplified from E13.5 whole embryo cDNA. The Col14a1 sequence (GenBank accession no. NM_181277.2) was amplified from neonatal limb cDNA. All sequences were amplified by RT-PCR with a temperature gradient of 53–65°C, subcloned into pGEM T-vector (Promega), and confirmed by sequencing. The plasmid for generation of the Twist1 probe for ISH was a generous gift from Dr. James Martin (University of Texas Institute of Biotechnology at Houston) (29).
Antisense RNA probes for ISH were generated as previously reported (8) with the following modifications. The Asporin, Osteonectin, Col1a1, and Col14a1 probes were synthesized with SP6 polymerase from plasmids linearized with NcoI. The Osteoglycin and Col9a3 probes were synthesized with T7 polymerase from plasmids linearized with NotI. The Matrilin2 probe was synthesized with SP6 polymerase from a plasmid linearized with SacI. The Twist1 probe was synthesized with T3 polymerase from a plasmid linearized with XbaI. ISH of tissue sections was performed as previously described (45, 47) with the following modifications. Tissue sections were treated with 20 μg/ml proteinase K-PBS for 8–10 min for embryos and 15 min for adult hearts at 37°C. Hybridizations using 170 μl of 0.05 μg/ml DIG-labeled riboprobe were carried out in Coverwell Incubation Chambers (Grace Biolabs). Color reactions using nitro blue tetrazolium-5-bromo-4-chloro-3-indolyl phosphate (NBT-BCIP; Roche) were allowed to develop for 1–16 h. For each in situ probe, color reactions were stopped at the same time in E12.5 and E17.5 tissue sections.
RESULTS
Heat map and Venn diagram analysis of genes expressed in valve development and ECM remodeling relative to preosteoblast MC3T3 cells.
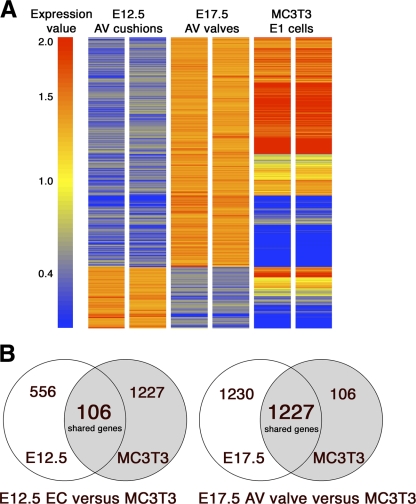
Differential gene expression of E12.5 EC versus E17.5 AV valve compared with MC3T3 cells was assessed in order to determine the distinct genetic profile of early and late stages of AV valve development relative to preosteoblast MC3T3 cells. Gene expression data of E12.5 EC, E17.5 AV, and MC3T3 cells were analyzed after filtering and data normalization (see materials and methods) and subjected to hierarchical clustering to form a heat map (Fig. 1A). A total of 3,119 genes were identified that are differentially expressed by at least twofold between E12.5 EC and E17.5 AV valves (Supplemental Table S1).1 Relative expression of these differentially expressed genes in preosteoblast MC3T3 cells is also indicated in the heat map. Genes with increased expression in either E12.5 EC or E17.5 AV valve and also expressed in preosteoblast MC3T3 cells are indicated with corresponding Venn diagrams (Fig. 1B). Of 3,119 differentially expressed genes during valvulogenesis, 2,457 genes are more strongly expressed in E17.5 AV valve cells and 662 genes are predominant in E12.5 EC cells (Fig. 1B).
Fig. 1.
Differential gene expression in atrioventricular (AV) valve development and similarity with a preosteoblast cell line. A: heat map representing Affymetrix gene expression profiling analysis of murine embryonic day (E)12.5 AV endocardial cushions (EC), E17.5 AV valves, and preosteoblast MC3T3-E1 (subclone 4) cells. A total of 3,119 genes with differential expression (>2.0-fold) in E17.5 valve compared with E12.5 EC are shown, and expression in mouse preosteoblast MC3T3 cells is also indicated. Arbitrary gene expression level based on raw intensity values is indicated by color-coded expression value bar. Red indicates increased, blue indicates decreased, and yellow shows unchanged expression. B: Venn diagram showing significant similarity in differentially expressed genes enriched in E17.5 valves and preosteoblast MC3T3 cells. Genes common to E12.5 EC and preosteoblast MC3T3 or E17.5 AV valve and preosteoblast MC3T3 are indicated in Venn diagrams. In E12.5 AV cushion, a total of 662 genes are more strongly expressed, whereas 2,457 genes are predominant in E17.5 AV valve. Importantly, in E12.5 AV cushion, of 662 increased genes, only 106 genes (16%) are shared with MC3T3 cells. In contrast, in E17.5 AV valve, of total 2,457 increased genes, 1,227 genes (49.9%) are shared with MC3T3 cells.
To compare the differentially expressed genes in E17.5 AV valve versus E12.5 EC with mouse preosteoblast MC3T3 cells, an initial filtering based on expression values of ≥1.5 was done to identify 1,333 probe sets that are top ranked for expression in MC3T3 cells. Remarkably, of these 1,333 MC3T3 highly expressed genes, 1,227 probe sets overlap with E17.5 AV valve, but only 106 probe sets overlap with E12.5 EC expressed genes. Thus, in E17.5 AV valves, of a total of 2,457 probe sets, 1,227 probe sets (49.9%) are shared with preosteoblast MC3T3 cells. Conversely, in E12.5 EC only 106 probe sets of a total of 662 increased probe sets (16%) are also highly expressed in preosteoblast MC3T3 cells (Fig. 1B). The gene expression profile of E17.5 AV valves includes nearly 50% shared genes in preosteoblasts, which is significantly increased over E12.5 EC. Similar results were obtained when the genes differentially expressed during valve development were compared with osteoblast enriched genes (GEO database GSE11339; Supplemental Table S2, Supplemental Methods). In this analysis, 75% of the shared osteoblast-enriched probe sets were increased in E17.5 AV valves compared with 25% shared probe sets with increased expression in E12.5 AV cushions. Therefore the osteoblast lineage enhanced gene expression profile is more similar to the E17.5 AV valves than to the immature valve progenitor cells in the E12.5 AV cushion.
Differential expression of valve maturation and ECM remodeling genes in E12.5 EC and E17.5 AV valves.
The base list of 3,119 genes differentially expressed during valvulogenesis was categorized by gene function with the PANTHER classification system (49, 50). The entire PANTHER-classified list of 3,119 genes was used to generate a selected list of 46 genes likely to be related to EC development and valve maturation. The list of 46 genes includes transcription factors, ECM structural proteins, matrix metalloproteases, and tissue inhibitors of matrix metalloproteases, as well as genes associated with bone and cartilage development (Table 2). While many of these genes are shared with osteoblast progenitor cells, genes characteristic of all three ECM layers of the E17.5 AV valves also are included in Table 2, B–D, as molecular indicators of the valve maturation process.
Table 2.
Differentially expressed genes in E17.5 atrioventricular valve versus E12.5 atrioventricular endocardial cushion
| Gene Symbol | Gene Title | GenBank Accession No. | Fold Change | |||
|---|---|---|---|---|---|---|
| A. Transcription factors | ||||||
| Twist1* | Twist gene homolog 1 | NM_011658 | −8.2 | |||
| Prrx2* | Paired related homeo box 2 | NM_009116 | −4.4 | |||
| Msx2 | Homeo box, msh-like 2 | NM_013601 | −3.8 | |||
| Msx1 | Homeo box, msh-like 1 | NM_010835 | −3.2 | |||
| Sox11* | SRY-box containing gene 11 | NM_009234 | −2.5 | |||
| Sox9* | SRY-box containing gene 9 | NM_011448 | −2.4 | |||
| Id2 | Inhibitor of DNA binding 2 | NM_010496 | −2.3 | |||
| Id1 | Inhibitor of DNA binding 1 | NM_010495 | −2.0 | |||
| B. ECM structural proteins | ||||||
| Col14a1 | Procollagen, type XIV, α1 | NM_181277 | 6.4 | |||
| Tnxb | Tenascin Xb | NM_031176 | 4.8 | |||
| Col4a4 | Procollagen, type IV, α4 | NM_007735 | 4.5 | |||
| Col6a3* | Procollagen, type VI, α3 | XM_897036 | 3.9 | |||
| Col15a1 | Procollagen, type XVI, α1 | NM_009928 | 3.3 | |||
| Eln | Elastin | NM_007925 | 3.1 | |||
| Col1a1* | Procollagen, type I, α1 | NM_007742 | 2.7 | |||
| Col4a1 | Procollagen, type IV, α1 | NM_009931 | 2.5 | |||
| Col1a2* | Procollagen, type I, α2 | NM_007743 | 2.4 | |||
| Col23a1 | Procollagen, type XXIII, α1 | NM_153393 | 2.4 | |||
| Col6a1* | Procollagen, type VI, α1 | NM_009933 | 2.3 | |||
| Col4a3bp | Procollagen, type IV, α3 binding protein | NM_023420 | 2.2 | |||
| Tnc* | Tenascin C | NM_011607 | 2.2 | |||
| Col4a5 | Procollagen, type IV, α5 | NM_007736 | 2.1 | |||
| Col3a1 | Procollagen, type III, α1 | NM_009930 | 2.1 | |||
| Col2a1* | Procollagen, type II, α1 | NM_031163 | −7.0 | |||
| Col9a1 | Procollagen, type IX, α1 | NM_007740 | −6.5 | |||
| Col9a3 | Procollagen, type IX, α3 | NM_009936 | −5.9 | |||
| C. Matrix metalloproteases and tissue inhibitors of matrix metalloproteases | ||||||
| Adamts12* | A disintegrin and metallopeptidase with thrombospondin type 1 motif (Adamts), 12 | NM_175501 | 5.0 | |||
| Adamts2 | Adamts 2 | NM_175643 | 3.9 | |||
| Adamts8 | Adamts 8 | NM_013906 | 3.7 | |||
| Adam9 | Adam domain 9 | NM_007404 | 2.7 | |||
| Mmp16 | Matrix metallopeptidase 16 | NM_019724 | 2.5 | |||
| Timp3* | Tissue inhibitor of metallopeptidase 3 | NM_011595 | 2.1 | |||
| Adamts15 | Adamts 15 | NM_001024139 | −3.4 | |||
| Mmp11* | Matrix metallopeptidase 11 | NM_008606 | −2.5 | |||
| Adamtsl1 | Adamtslike 1 | NM_029967 | −2.1 | |||
| D. ECM genes associated with bone and cartilage development | ||||||
| Aspn* | Asporin | NM_025711 | 44.2 | |||
| Ogn* | Osteoglycin | NM_008760 | 17.1 | |||
| Ecm2 | Extracellular matrix protein 2 | NM_001012324 | 5.4 | |||
| Sparcl1 | SPARC-like 1(mast9, hevin) | NM_010097 | 5.0 | |||
| Obscn | Obscurin | NM_001003914 | 4.3 | |||
| Matn2 | Matrilin2 | NM_016762 | 3.5 | |||
| Dcn | Decorin | NM_007833 | 2.6 | |||
| Postn | Periostin | NM_015784 | 2.6 | |||
| Comp | Cartilage oligomeric matrix protein | NM_016685 | 2.2 | |||
| Mgp* | Matrix gla protein | NM_008597 | 2.2 | |||
| Hapln1* | Hyaluronan and proteoglycan link protein 1 | NM_013500 | −6.7 | |||
E, embryonic day; ECM, extracellular matrix.
Genes that are also expressed in preosteoblast MC3T3-E1 (subclone4) cells.
Several transcription factor genes are expressed more highly in E12.5 EC than in E17.5 AV valves. Among these, the basic helix-loop-helix (bHLH) transcription factor Twist1 is expressed 8.2-fold higher in E12.5 EC than in E17.5 AV valve (Table 2A). Additional genes expressed in progenitor and mesenchyme populations, including Id1, Id2, Msx1, and Msx2, are relatively increased in E12.5 cushion relative to E17.5 valve. Paired related homeobox 2 (Prrx2) and SRY-box containing genes Sox9 and Sox11 are also increased in early cushions compared with late AV valves (Table 2A). In addition, Twist1, Prrx2, Sox9, and Sox11 transcription factor genes also are expressed in preosteoblast MC3T3 cells, as indicated by asterisks in Table 2. High expression of progenitor and mesenchyme transcription factors is observed in early (E12.5) cushion cells relative to more mature (E17.5) AV valves. Interestingly, several of these genes also are expressed very early in osteoblast progenitor cells before differentiation.
Valve maturation is characterized by increased expression and organization of ECM proteins (18). Among the most highly regulated ECM proteins in valve maturation are collagens, several of which were identified as differentially expressed in the microarray gene expression analysis of mouse AV valve development. Procollagen type XIV α1 (Col14a1) was identified as one of the most increased (6.4-fold) genes in late E17.5 AV valves, whereas Col2a1 was identified as one of the most decreased (−7.0-fold) genes in late E17.5 AV valves (Table 2B). Likewise, several other collagens including procollagen types I, III, IV, VI, XV, and XXIII are increased in E17.5 valves, and type IX is increased in E12.5 EC (Table 2B). The most abundant types of fibrillar collagens such as types I and III are expressed in late AV valves, whereas collagen type II is predominant in early AV cushion (Table 2B). In addition, fibril-associated collagens with interrupted triple helices (FACIT) collagen type IX is highly expressed in early AV cushion, whereas collagen type XIV is detected in late AV valves (Table 2B). Moreover, nonfibrillar and network-forming collagens including multiple type IV collagen or basement membrane type VI collagen are also increased in late AV valves (Table 2B). Other ECM proteins, including elastin, tenascins C and Xb, and Col4a3 binding protein, are also increased in late E17.5 AV valves (Table 2B). In addition, several of these ECM genes, including Col6a3, Col1a1, Col1a2, Col6a1, Tnc, and Col2a1, are also expressed in preosteoblast MC3T3 cells (Table 2B). Therefore, these changes are indicative of differential collagen gene expression and increased ECM complexity during mouse AV valve development.
Consistent with important functions in valve maturation, several ECM remodeling enzymes and tissue inhibitor of matrix metalloproteases (TIMPs) are differentially expressed during mouse AV valve development (Table 2C). Among these, Adamts12 is one of the most increased genes in E17.5 AV valves, followed by Adamts2, Adamts8, Adam9, Mmp16, and Timp3, whereas Adamts15, Mmp11, and Adamtsl1 are decreased in late AV valves. These remodeling enzymes have not previously been associated with valve development, but Adamts12, Adamts2, Mmp16, and Timp3 are increased, while Adamtsl1 and Adamts15 are decreased, in hip cartilage from patients with osteoarthritis (21). Therefore, a similar gene expression profile in the developing valves is suggestive of the existence of an active ECM remodeling program related to cartilage homeostasis in the E17.5 AV valves. In addition, Adamts12, Timp3, and Mmp11 are also expressed in preosteoblast MC3T3 cells (Table 2C). Overall, we detect a distinct ECM remodeling profile in E17.5 AV valve versus E12.5 AV cushion during mouse AV valve development.
Several ECM genes associated with bone and cartilage development (Table 2D) are also differentially expressed in mature valves versus EC. Among the most increased in E17.5 AV valves is a family of SLRPs. Asporin (Aspn), Extra-cellular matrix protein 2 (Ecm2), Decorin (Dcn), and Osteoglycin (Ogn) were identified as significantly increased genes in late E17.5 AV valves. Aspn is the most increased gene (44.2-fold), followed by Ogn (17.1-fold), Ecm2 (5.4-fold), and Dcn (2.6-fold) (Table 2D). Matrilin2 (Matn2), a large oligomeric matrix protein, is also increased in late AV valves. Beside SLRPs and Matn2, other ECM genes associated with bone and cartilage development and overall matrix organization (Table 2D) are also increased in late E17.5 AV valves compared with E12.5 EC. In contrast, hyaluronan and proteoglycan link protein 1 (Hapln1) gene is expressed more highly in E12.5 EC than in late E17.5 AV valve. Aspn, Ogn, Mgp, and Hapln1 are also expressed in preosteoblast MC3T3 cells, consistent with shared regulatory programs in this cell type with developing heart valves (Table 2D). Together these analyses show that a family of SLRPs and other ECM genes associated with osteoblast lineage development are predominantly expressed in E17.5 mature AV valves relative to E12.5 AV cushions.
Validation of differential gene expression by qRT-PCR and in situ hybridization.
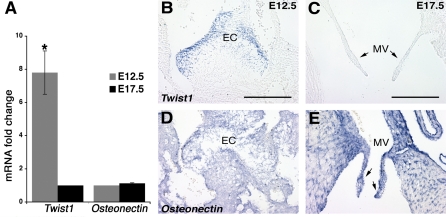
Differential gene expression detected by Affymetrix microarray was validated with quantitative real-time PCR (qRT-PCR) and in vivo expression localized by ISH. Gene expression of the transcription factor Twist1 was examined as the most differentially expressed gene enriched in early E12.5 EC over E17.5 valve leaflets (Fig. 2). Osteonectin expression was also determined as a control gene that did not change in the microarray analysis (data not shown). qRT-PCR revealed a 7.8-fold higher expression of Twist1 mRNA in early E12.5 EC (Fig. 2A) than in E17.5 AV valve, whereas the Osteonectin mRNA levels did not change significantly (Fig. 2A). In the developing heart, Twist1 mRNA is expressed predominantly in the EC cells (Fig. 2B) but is not detected in E17.5 MV leaflets (Fig. 2C). Moreover, Osteonectin is similarly expressed in both E12.5 EC cells (Fig. 2D) and E17.5 MV leaflets (Fig. 2E).
Fig. 2.
Mouse Twist1 is highly expressed in E12.5 AV cushion but is downregulated during AV valve maturation. A: quantitative RT-PCR (qRT-PCR) validation of microarray results for Twist1 in EC at E12.5 and AV valves at E17.5. Normalized Twist1 expression in E17.5 AV valves is set to 1, and then the fold change is shown for E12.5 EC. Osteonectin expression is also shown as a control gene that did not change significantly in E12.5 EC and E17.5 AV valves. The average fold increase is depicted, where n = biological 3×; error bars represent SE. Statistical significance was determined by Student's t-test; *P < 0.05. B–E: in situ hybridization (ISH) is shown on 14-μm paraffin sections of E12.5 and E17.5 mouse hearts with probes specific for mouse Twist1 (B and C) and Osteonectin (D and E). Twist1 is strongly expressed in E12.5 EC cells (B) in contrast to E17.5 mitral valve (MV) leaflets (arrows in C). Osteonectin ISH is shown as control for similar gene expression in both EC (D) and late remodeled MV leaflets (arrows in E). Scale bars, 200 nm.
Differential expression of collagens associated with specific types of connective tissue detected in the microarray analysis was assessed in the developing valves (Fig. 3). Collagens examined include type I Col1a1 associated with fibrous connective tissue and bone (10), cartilage-associated type IX Col9a3 (9,) and tendon-associated type XIV Col14a1 (53). By qRT-PCR, we detected a 10.1-fold increase in Col9a3 mRNA in early E12.5 EC relative to E17.5 AV valve, compared with a 2.2-fold increase in Col1a1 mRNA and a 5.1-fold increase in Col14a1 mRNA in late E17.5 AV valve relative to E12.5 EC (Fig. 3A). Consistent increased expression of Col9a3 was observed in early E12.5 EC cells relative to E17.5 MV leaflets (Fig. 3, B and C). In contrast, relatively less Col1a1 is expressed in the E12.5 EC, but Col1a1 expression is predominant in late E17.5 MV leaflets (Fig. 3, D and E). Likewise, increased expression of Col14a1 was observed in late E17.5 MV leaflets (Fig. 3G) compared with no detectable expression in E12.5 EC (Fig. 3F). The same pattern of differential regulation of Col9a3, Col1a1, and Col14al expression was observed in the tricuspid as well as both semilunar valves (data not shown). Therefore, these data demonstrate differential expression of collagen genes associated with different types of connective tissue during mouse AV valve development.
Fig. 3.
Differential expression of collagen genes during mouse embryonic AV valve development. A: qRT-PCR validation of microarray result for Col9a3, Col1a1, and Col14a1 was performed as in Fig. 2A. Col9a3 expression in E17.5 AV valves is normalized to 1, and then the fold changes are calculated for E12.5 EC. In contrast, Col1a1 and Col14a1 expression in E12.5 EC is normalized to 1, and then the fold changes are calculated for E17.5 AV valve. Statistical significance was determined by Student's t-test; *P < 0.05. B–G: ISH with probes specific for mouse Col9a3 (B and C), Col1a1 (D and E), and Col14a1 (F and G). Strong expression of Col9a3 is detected in E12.5 EC cells (B) compared with its expression in E17.5 MV leaflets (arrows in C). In contrast, strong expression of Col1a1 (E) and Col14a1 (G) is detected in E17.5 MV leaflets compared with their expression in E12.5 EC (D and F, respectively). Scale bars, 200 nm.
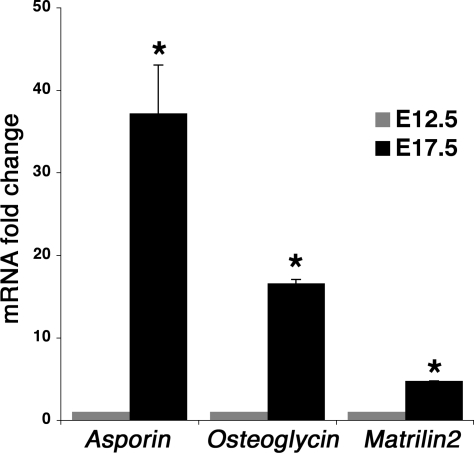
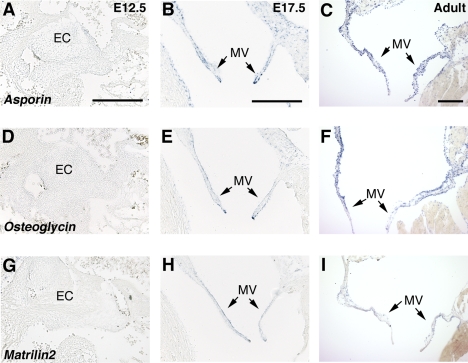
The most highly upregulated family of genes detected in the E17.5 AV valves compared with E12.5 EC were SLRPs. Expression of Aspn and Ogn and the large oligomeric matrix gene Matrilin2 (Matn2) was examined in developing and adult heart by qRT-PCR (Fig. 4) and ISH (Fig. 5). Consistent with the microarray gene expression analysis, Aspn expression is increased by 37.2-fold, Ogn by 16.6-fold, and Matn2 by 4.8-fold in E17.5 AV valves compared with E12.5 EC (Fig. 4) at the mRNA level. Likewise, strong expression of Aspn, Ogn, and Matn2 was apparent in the E17.5 MV leaflets (Fig. 5, B, E, H) and also in the tricuspid and semilunar valves (Supplemental Fig. S1 and data not shown). In contrast, no Aspn, Ogn, or Matn2 expression was detected in the EC at E12.5 (Fig. 5, A, D, G). In adult hearts, strong expression of Aspn (Fig. 5C) and Ogn (Fig. 5F) in MV leaflets was observed. Aspn and Ogn also are predominantly expressed in adult aortic valve leaflets (Supplemental Fig. S1) as well as in undifferentiated MC3T3-E1 (subclone 4) cells (data not shown). In the developing limbs, both Aspn and Ogn are expressed in osteoblast progenitor cells, but not mineralized bone, and both are also expressed in articular cartilage at E17.5 (Supplemental Fig. S2). Expression of Matn2 is also detected in the adult MV leaflets (Fig. 5I), although at a lower level compared with Aspn and Ogn. Increased expression and localization of SLRP gene expression (Asporin and Osteoglycin) in late embryonic E17.5 and adult mitral and aortic valves is consistent with functions in valve maturation and adult valve homeostasis.
Fig. 4.
qRT-PCR validation of microarray results for mouse Asporin (Aspn), Osteoglycin (Ogn), and Matrilin2 (Matn2). qRT-PCR was performed with specific primer sets for Aspn, Ogn, and Matn2 as described in Table 1A. The normalized gene expression in E12.5 cushions is set to 1, and then the fold changes are calculated for E17.5 AV valves. Statistical significance was determined by Student's t-test; *P < 0.05.
Fig. 5.
Expression of mouse Asporin, Osteoglycin, and Matrilin2 in embryonic and adult AV valves: ISH on 14-μm paraffin sections of E12.5, E17.5, and adult mouse hearts with probes specific for Aspn (A–C), Ogn (D–F), and Matn2 (G–I). Strong expression of Aspn (B and C), Ogn (E and F), and Matn2 (H and I) are demonstrated in late embryonic (E17.5) and adult MV leaflets (arrows in B, C, E, F, H, and I), but not in E12.5 EC (A, D, and G). Scale bars, 200 nm.
DISCUSSION
The AV valves of the heart develop from undifferentiated mesenchymal EC that later mature into stratified valves with diversified ECM. However, the genetic profile of the transition from EC to valve leaflets is not completely defined. Previous studies have demonstrated the existence of shared regulatory mechanisms in heart valve cell lineages related to cartilage, tendon, and bone development (23, 24). Therefore, we hypothesize that the genetic profile of valve cell lineage maturation shares molecular components of preosteoblasts. To compare genetic profiles of AV valve maturation with gene expression of known osteoprogenitor cells, we performed an Affymetrix microarray analysis of RNA derived from mouse E12.5 AV cushions, E17.5 AV valves, and undifferentiated MC3T3-E1 preosteoblasts. This gene expression analysis strategy is designed to reveal the genetic profile of heart valve maturation as well as shared gene expression patterns with undifferentiated bone progenitor cells.
Among the genes with increased expression in the early E12.5 EC compared with the E17.5 AV valves were several transcription factors associated with mesenchymal cell types and undifferentiated progenitor populations. Twist1, a bHLH transcription factor, was the most highly enriched gene in E12.5 cushion cells. In comparatively staged chicken embryo valve progenitor cells, Twist1 is expressed in the EC mesenchyme and promotes mesenchymal cell proliferation and migration while inhibiting differentiation (46). Twist1 also is expressed early in osteoblast lineage development, where it inhibits differentiation (5). Likewise, enhanced gene expression of Id1 and Id2, antagonists of bHLH proteins, in early E12.5 EC could be modulating Twist1 function in these cells, as has been demonstrated in differentiating osteoblasts (16). Msx1 and Msx2, members of the highly conserved NK family of homeodomain transcription factors, are expressed in mesenchymal cell lineages throughout the embryo including limb bud, neural crest, and heart (7). In the periodontal ligament, Msx2 can inhibit ossification, and it is also expressed at the initial stages of cardiovascular calcification (44, 52). However, the roles of Msx1 and Msx2 in valve development and pathogenesis have not yet been fully determined. Expression of the paired-related homeobox gene Prrx2, increased in early E12.5 EC, also is associated with mesenchymal cell development and has been implicated in the development of cardiovascular connective tissues (4). Together, the increased expression of E12.5 EC enhanced transcription factor genes is consistent with a mesenchymal progenitor cell population in the EC with similarities to early-stage undifferentiated osteoblast precursors.
Major ECM constituents of tendon, cartilage, and bone as well as multiple matrix metalloproteinases and their inhibitors are differentially expressed during heart valve development. The mature valve leaflets are stratified into diverse ECM layers with structural components characteristic of different types of connective tissue (18, 27). Therefore the enhanced expression at E17.5 of elastin, tenascins, matricellular proteins, and multiple collagen genes apparent in the microarray analysis is indicative of the increased complexity and higher order structure of the stratified valve ECM. Several of the differentially expressed ECM proteins including type I collagen (col1a1), periostin (Postn), cartilage oligomeric matrix protein (Comp), and matrix gla protein (Mgp), as well as previously reported osteonectin and osteopontin, are also highly expressed in bone progenitors and other connective tissues with the potential to mineralize (18, 32). Maturation and homeostasis of the valve ECM also involves the expression and activity of several matrix metalloproteinases and their inhibitors, as evidenced by the differential expression of multiple types of remodeling enzymes observed in the microarray analysis of valve development. Expression of several members of the disintegrin and metallopeptidase with thrombospondin type 1 motif (ADAM-TS) family were increased at E17.5. These genes have not previously been associated with valve development but have been implicated in cartilage homeostasis and osteoarthritis (21). Overall, the microarray analysis provides a genetic profile underlying the increased complexity and organization of the ECM during AV valve maturation that could be related to common connective tissue homeostasis and disease mechanisms.
Differential expression of genes encoding specific types of collagen is an important aspect of heart valve maturation. The most abundant ECM protein in the mature valves is type I fibrillar collagen, and type II, III, V, VI, and XI collagens also have been reported in the developing and immature valve leaflets (18, 19, 25, 26). Increased expression of multiple collagens, including fibrillar, FACIT, and basement membrane-associated collagen types, was observed in microarray analysis of E17.5 AV valves versus E12.5 EC. Type XIV collagen associated with tendon and type I collagen characteristic of bone and skin are expressed preferentially in the mature AV valves. Interestingly, type II and IX collagens characteristic of cartilage were expressed more highly at E12.5 than at E17.5. Deficiencies in specific collagens are associated with cardiac as well as connective tissue diseases (26, 27). Overall, the developmental and disease implications of highly regulated or dysregulated expression of multiple collagen types in the developing heart valves have not yet been fully examined.
The most highly enriched genes with increased expression in the late E17.5 valves were members of the SLRP family, which have not previously been associated with heart valve development or homeostasis. The SLRPs are characterized by a small core protein containing a varying number of leucine-rich repeats, and several of the family members function to regulate collagen fibrillogenesis (20). In addition, mutations in SLRP family member genes are associated with a wide spectrum of connective tissue diseases, including osteoporosis, osteoarthritis, muscular dystrophy, Ehlers-Danlos syndrome, and corneal diseases (2, 20). Increased expression of Asporin and Osteoglycin was observed in E17.5 and adult mitral and aortic valve leaflets. Asporin, also called periodontal ligament-associated protein (PLAP), is a negative regulator of osteoblast mineralization and calcification in periodontal ligament cells (51), and mutations in human Aspn are associated with osteoarthritis (22). Osteoglycin can regulate collagen fibrillogenesis and is dynamically regulated during atherosclerotic progression (11, 14). The abilities of these proteins to prevent osteoblast mineralization and affect collagen fibril formation likely contribute to normal valve structure and function. Furthermore, they may also serve as a cardioprotective mechanism against valve mineralization associated with disease, but this has not yet been demonstrated.
In human calcified aortic valve disease, the characteristic deposition of mineralized calcium can lead to progressive valve stiffening and stenosis, necessitating replacement in many cases (31, 41). Valve calcification has been described as an active osteogenic process because many genes characteristic of bone, including the transcription factor Runx2 (Cbfa-1), osteopontin, osteocalcin, and alkaline phosphatase, are upregulated in human valve disease as well in mouse and rabbit models of valve calcification (1, 6, 33, 41). The microarray gene expression analysis reported here demonstrates significant shared gene expression profiles in heart valve maturation and osteoblast progenitor cells. Neither of these cell types is mineralized, but the shared gene expression profile underscores the potential of both to undergo this process. In addition, several genes known to inhibit the differentiation or mineralization of osteoblasts are expressed in the developing valves. The functions of these genes in heart valve development, function, and disease have not yet been established.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-082716 to K. E. Yutzey.
Supplementary Material
Acknowledgments
We thank Michelle Combs and Christina Alfieri for technical assistance and scientific discussions. We also thank Shawn Smith and the Cincinnati Children's Medical Center Affymetrix Microarray Core for assisting with the generation of the microarray data.
Address for reprint requests and other correspondence: K. E. Yutzey, Div. of Molecular Cardiovascular Biology, Cincinnati Children's Medical Center ML 7020, 240 Albert Sabin Way, Cincinnati, OH 45229 (e-mail: katherine.yutzey@cchmc.org).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation 115: 377–386, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology 12: 107R–116R, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res 95: 459–470, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergwerff M, Gittenberger-de Groot AC, Wisse LJ, DeRuiter MC, Wessels A, Martin JF, Olson EN, Kern MJ. Loss of function of the Prx1 and Prx2 homeobox genes alters architecture of the great elastic arteries and ductus arteriosus. Virchows Arch 436: 12–19, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ, Karsenty G. A twist code determines the onset of osteoblast differentiation. Dev Cell 6: 423–435, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol 47: 1707–1712, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson D The function and evolution of Msx genes: pointers and paradoxes. Trends Genet 11: 405–411, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Ehrman LA, Yutzey KE. Lack of regulation in the heart forming region of avian embryos. Dev Biol 207: 163–175, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Fassler R, Schnegelsberg PN, Dausman J, Shinya T, Muragaki Y, McCarthy MT, Olsen BR, Jaenisch R. Mice lacking alpha 1 (IX) collagen develop noninflammatory degenerative joint disease. Proc Natl Acad Sci USA 91: 5070–5074, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feres-Filho EJ, Menassa GB, Trackman PC. Regulation of lysyl oxidase by basic fibroblast growth factor in osteoblastic MC3T3-E1 cells. J Biol Chem 271: 6411–6416, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez B, Kampmann A, Pipp F, Zimmermann R, Schaper W. Osteoglycin expression and localization in rabbit tissues and atherosclerotic plaques. Mol Cell Biochem 246: 3–11, 2003. [PubMed] [Google Scholar]
- 12.Fornes P, Heudes D, Fuzellier JF, Tixier D, Bruneval P, Carpentier A. Correlation between clinical and histologic patterns of degenerative mitral valve insufficiency: a histomorphometric study of 130 excised segments. Cardiovasc Pathol 8: 81–92, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature 437: 270–274, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Ge G, Seo NS, Liang X, Hopkins DR, Hook M, Greenspan DS. Bone morphogenetic protein-1/tolloid-related metalloproteinases process osteoglycin and enhance its ability to regulate collagen fibrillogenesis. J Biol Chem 279: 41626–41633, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Gruber PJ, Epstein JA. Development gone awry: congenital heart disease. Circ Res 94: 273–283, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi M, Nimura K, Kashiwagi K, Harada T, Takaoka K, Kato H, Tamai K, Kaneda Y. Comparative roles of Twist-1 and Id1 in transcriptional regulation by BMP signaling. J Cell Sci 120: 1350–1357, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Hemmerich P, von Mikecz A, Neumann F, Sozeri O, Wolff-Vorbeck G, Zoebelein R, Krawinkel U. Structural and functional properties of ribosomal protein L7 from humans and rodents. Nucleic Acids Res 21: 223–231, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinton RB, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res 98: 1431–1438, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Icardo JM, Colvee E. Atrioventricular valves of the mouse. III. Collagenous skeleton and myotendinous junction. Anat Rec 243: 367–375, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Iozzo RV The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem 274: 18843–18846, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Kevorkian L, Young DA, Darrah C, Donell ST, Shepstone L, Porter S, Brockbank SM, Edwards DR, Parker AE, Clark IM. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum 50: 131–141, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Kizawa H, Kou I, Iida A, Sudo A, Miyamoto Y, Fukuda A, Mabuchi A, Kotani A, Kawakami A, Yamamoto S, Uchida A, Nakamura K, Notoya K, Nakamura Y, Ikegawa S. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet 37: 138–144, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Lange AW, Yutzey KE. NFATc1 expression in the developing heart valves is responsive to the RANKL pathway and is required for endocardial expression of cathepsin K. Dev Biol 292: 407–417, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Lincoln J, Alfieri CM, Yutzey KE. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev Biol 292: 292–302, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn 230: 239–250, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Lincoln J, Florer JB, Deutsch GH, Wenstrup RJ, Yutzey KE. ColVa1 and ColXIa1 are required for myocardial morphogenesis and heart valve development. Dev Dyn 235: 3295–3305, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Lincoln J, Lange AW, Yutzey KE. Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol 294: 292–302, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Loffredo CA Epidemiology of cardiovascular malformations: prevalence and risk factors. Am J Med Genet 97: 319–325, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 132: 5601–5611, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Manasek FJ Macromolecules of the extracellular compartment of embryonic and mature hearts. Circ Res 38: 331–337, 1976. [DOI] [PubMed] [Google Scholar]
- 31.Mohler ER, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation 103: 1522–1528, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev 19: 1093–1104, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation 114: I547–I552, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of “degenerative” valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 90: 844–853, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol 243: 287–335, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Pierpont ME, Basson CT, Benson DW Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young; endorsed by the American Academy of Pediatrics. Circulation 115: 3015–3038, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Plageman TF, Yutzey KE. Microarray analysis of Tbx5-induced genes expressed in the developing heart. Dev Dyn 235: 2868–2880, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 104: 2525–2532, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Rabkin-Aikawa E, Mayer JE Jr, Schoen FJ. Heart valve regeneration. Adv Biochem Eng Biotechnol 94: 141–179, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation 112: I229–I234, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 107: 2181–2184, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raouf A, Seth A. Discovery of osteoblast-associated genes using cDNA microarrays. Bone 30: 463–471, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Rubin JD, Ferencz C, Loffredo C. Use of prescription and non-prescription drugs in pregnancy. The Baltimore-Washington Infant Study Group. J Clin Epidemiol 46: 581–589, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest 115: 1210–1220, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shelton EL, Yutzey KE. Tbx20 regulation of endocardial cushion cell proliferation and extracellular matrix gene expression. Dev Biol 302: 376–388, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shelton EL, Yutzey KE. Twist1 function in endocardial cushion cell proliferation, migration, and differentiation during heart valve development. Dev Biol 317: 282–295, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somi S, Buffing AA, Moorman AF, Van Den Hoff MJ. Dynamic patterns of expression of BMP isoforms 2, 4, 5, 6, and 7 during chicken heart development. Anat Rec A Discov Mol Cell Evol Biol 279: 636–651, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 113: e85–e151, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res 13: 2129–2141, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas PD, Kejariwal A, Guo N, Mi H, Campbell MJ, Muruganujan A, Lazareva-Ulitsky B. Applications for protein sequence-function evolution data: mRNA/protein expression analysis and coding SNP scoring tools. Nucleic Acids Res 34: W645–W650, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada S, Tomoeda M, Ozawa Y, Yoneda S, Terashima Y, Ikezawa K, Ikegawa S, Saito M, Toyosawa S, Murakami S. PLAP-1/asporin, a novel negative regulator of periodontal ligament mineralization. J Biol Chem 282: 23070–23080, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Yoshizawa T, Takizawa F, Iizawa F, Ishibashi O, Kawashima H, Matsuda A, Endo N. Homeobox protein MSX2 acts as a molecular defense mechanism for preventing ossification in ligament fibroblasts. Mol Cell Biol 24: 3460–3472, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young BB, Gordon MK, Birk DE. Expression of type XIV collagen in developing chicken tendons: association with assembly and growth of collagen fibrils. Dev Dyn 217: 430–439, 2000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.