Abstract
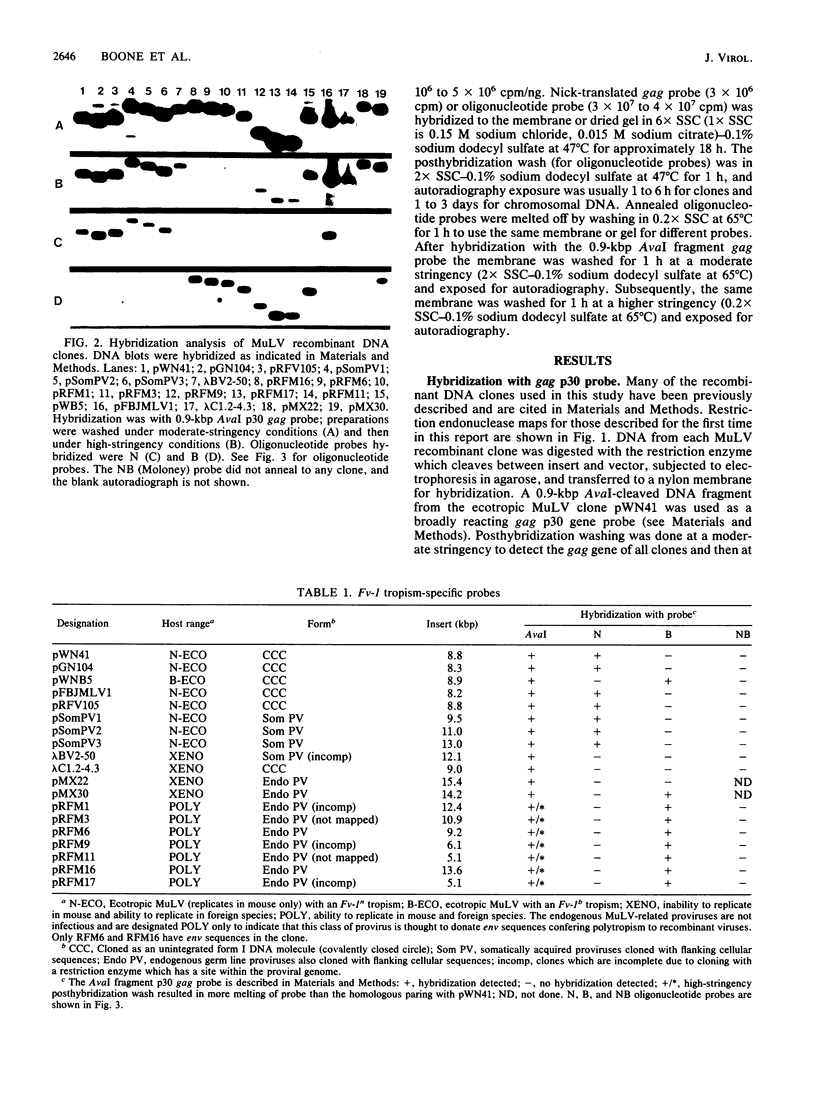
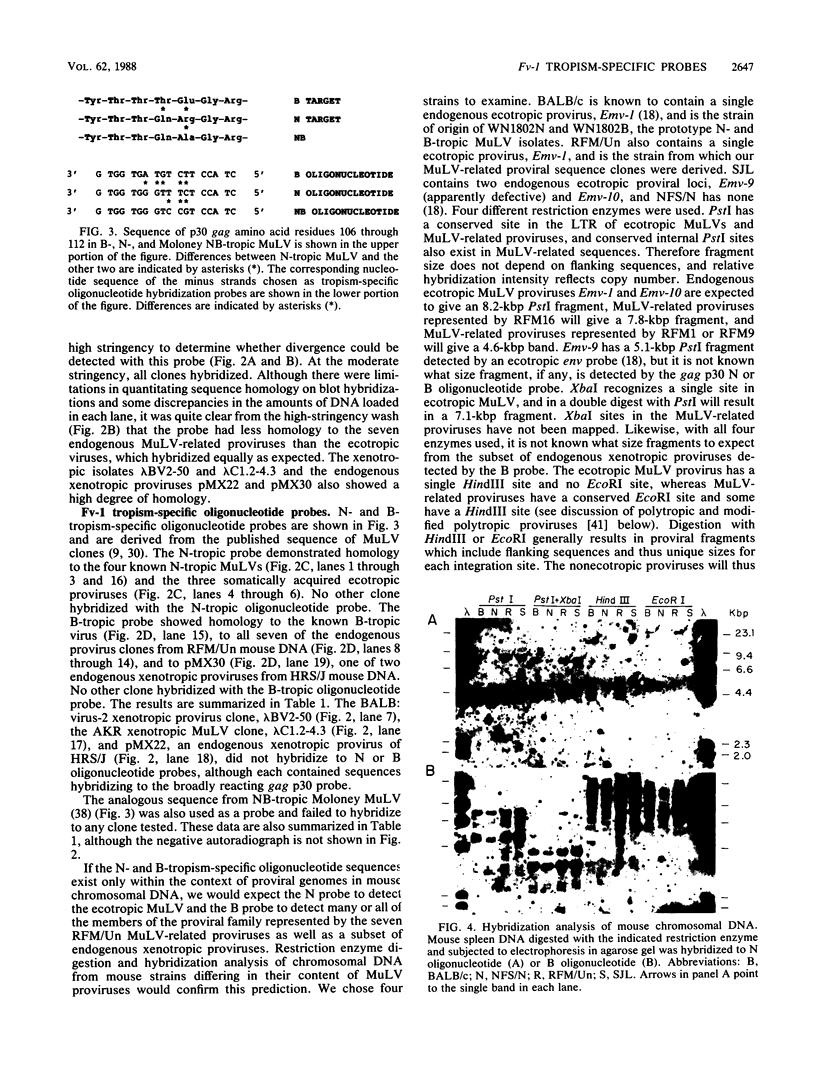
Oligonucleotide probes specific for the Fv-1 N- and B-tropic host range determinants of the gag p30-coding sequence were used to analyze DNA clones of various murine leukemia virus (MuLV) and endogenous MuLV-related proviral genomes and chromosomal DNA from four mouse strains. The group of DNA clones consisted of ecotropic MuLVs of known Fv-1 host range, somatically acquired ecotropic MuLV proviruses, xenotropic MuLV isolates, and endogenous nonecotropic MuLV-related proviral sequences from mouse chromosomal DNA. As expected, the prototype N-tropism determinant is carried by N-tropic viruses of several different origins. All seven endogenous nonecotropic MuLV-related proviral sequence clones derived from RFM/Un mouse chromosomal DNA, although not recognized by the N probe, showed positive hybridization with the prototype B-tropism-specific probe. The two xenotropic MuLV clones derived from infectious virus (one of BALB:virus-2 and one of AKR xenotropic virus) failed to hybridize with the N- and B-tropic oligonucleotide probes tested and with one probe specific for NB-tropic Moloney MuLV. One of two endogenous xenotropic class proviruses derived from HRS/J mouse chromosomal DNA (J. P. Stoye and J. M. Coffin, J. Virol. 61:2659-2669, 1987) also failed to hybridize to the N- and B-tropic probes, whereas the other hybridized to the B-tropic probe. In addition, analysis of mouse chromosomal DNA from four strains indicates that hybridization with the N-tropic probe correlates with the presence or absence of endogenous ecotropic MuLV provirus, whereas the B-tropic probe detects abundant copies of endogenous nonecotropic MuLV-related proviral sequences. These results suggest that the B-tropism determinant in B-tropic ecotropic MuLV may arise from recombination between N-tropic ecotropic MuLV and members of the abundant endogenous nonecotropic MuLV-related classes including a subset of endogenous xenotropic proviruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Barbacid M. Viral genes involved in leukemogenesis. I. Generation of recombinants between oncogenic and nononcogenic mouse type-C viruses in tissue culture. J Exp Med. 1980 Feb 1;151(2):467–480. doi: 10.1084/jem.151.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Stephenson J. R. Independent segregation of loci for activation of biologically distinguishable RNA C-type viruses in mouse cells. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2055–2058. doi: 10.1073/pnas.70.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benade L. E., Ihle J. N., Declève A. Serological characterization of B-tropic viruses of C57BL mice: possible origin by recombination of endogenous N-tropic and xenotropic viruses. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4553–4557. doi: 10.1073/pnas.75.9.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone L. R., Boone G. S., Innes C. L., Yang W. K., Tennant R. W. Hematopoietic neoplasias of the RFM/Un mouse contain somatic re-integration of the restriction endogenous ecotropic provirus. Carcinogenesis. 1986 Apr;7(4):529–534. doi: 10.1093/carcin/7.4.529. [DOI] [PubMed] [Google Scholar]
- Boone L. R., Myer F. E., Yang D. M., Ou C. Y., Koh C. K., Roberson L. E., Tennant R. W., Yang W. K. Reversal of Fv-1 host range by in vitro restriction endonuclease fragment exchange between molecular clones of N-tropic and B-tropic murine leukemia virus genomes. J Virol. 1983 Oct;48(1):110–119. doi: 10.1128/jvi.48.1.110-119.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Gupta S., Rands E., Lowy D. R. Origin of mink cytopathic focus-forming (MCF) viruses:comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology. 1981 Sep;113(2):465–483. doi: 10.1016/0042-6822(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Curran T., MacConnell W. P., van Straaten F., Verma I. M. Structure of the FBJ murine osteosarcoma virus genome: molecular cloning of its associated helper virus and the cellular homolog of the v-fos gene from mouse and human cells. Mol Cell Biol. 1983 May;3(5):914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J Virol. 1983 Dec;48(3):685–696. doi: 10.1128/jvi.48.3.685-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautsch J. W., Elder J. H., Jensen F. C., Lerner R. A. In vitro construction of a B-tropic virus by recombination: B-tropism is a cryptic phenotype of xenotropic murine retroviruses. Proc Natl Acad Sci U S A. 1980 May;77(5):2989–2993. doi: 10.1073/pnas.77.5.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautsch J. W., Elder J. H., Schindler J., Jensen F. C., Lerner R. A. Structural markers on core protein p30 of murine leukemia virus: functional correlation with Fv-1 tropism. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4170–4174. doi: 10.1073/pnas.75.9.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Isolation of naturally occurring viruses of the murine leukemia virus group in tissue culture. J Virol. 1969 Feb;3(2):126–132. doi: 10.1128/jvi.3.2.126-132.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Risser R. Molecular and biological characterization of the endogenous ecotropic provirus of BALB/c mice. J Virol. 1985 Dec;56(3):798–806. doi: 10.1128/jvi.56.3.798-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Fischinger P., Bolognesi D., Elder J., Gautsch J. W. B-MuX: a unique murine C-type virus containing the "env" gene of xenotropic viruses and the "gag" gene of the ecotropic virus. Virology. 1978 Oct 15;90(2):255–264. doi: 10.1016/0042-6822(78)90309-4. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Joseph D. R. Genetic analysis of the endogenous C3H murine leukemia virus genome: evidence for one locus unlinked to the endogenous murine leukemia virus genome of C57BL/6 mice. Virology. 1978 Jun 15;87(2):298–306. doi: 10.1016/0042-6822(78)90135-6. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph D. R. Molecular cloning of AKR xenotropic murine leukemia virus unintegrated proviral DNA. Gene. 1982 Mar;17(3):341–344. doi: 10.1016/0378-1119(82)90151-2. [DOI] [PubMed] [Google Scholar]
- Khan A. S., Martin M. A. Endogenous murine leukemia proviral long terminal repeats contain a unique 190-base-pair insert. Proc Natl Acad Sci U S A. 1983 May;80(9):2699–2703. doi: 10.1073/pnas.80.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S. Nucleotide sequence analysis establishes the role of endogenous murine leukemia virus DNA segments in formation of recombinant mink cell focus-forming murine leukemia viruses. J Virol. 1984 Jun;50(3):864–871. doi: 10.1128/jvi.50.3.864-871.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Rowe W. P., Martin M. A. Cloning of endogenous murine leukemia virus-related sequences from chromosomal DNA of BALB/c and AKR/J mice: identification of an env progenitor of AKR-247 mink cell focus-forming proviral DNA. J Virol. 1982 Nov;44(2):625–636. doi: 10.1128/jvi.44.2.625-636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of the ecotropic murine leukemia virus-inducing locus of BALB/c mouse to chromosome 5. Science. 1979 Apr 6;204(4388):69–71. doi: 10.1126/science.219475. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of xenotropic murine leukemia virus-inducing loci in five mouse strains. J Exp Med. 1980 Jul 1;152(1):219–228. doi: 10.1084/jem.152.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll B., Hartley J. W., Rowe W. P. Induction of B-tropic and N-tropic murine leukemia virus from B10.BR/SgLi mouse embryo cell lines by 5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1979 Jul;63(1):213–217. [PubMed] [Google Scholar]
- Nikbakht K. N., Boone L. R., Glover P. L., Myer F. E., Yang W. K. Characterization of a molecular clone of RFM/Un mouse chromosomal DNA that contains a full-length endogenous murine leukaemia virus-related proviral genome. J Gen Virol. 1987 Mar;68(Pt 3):683–693. doi: 10.1099/0022-1317-68-3-683. [DOI] [PubMed] [Google Scholar]
- Nikbakht K. N., Ou C. Y., Boone L. R., Glover P. L., Yang W. K. Nucleotide sequence analysis of endogenous murine leukemia virus-related proviral clones reveals primer-binding sites for glutamine tRNA. J Virol. 1985 Jun;54(3):889–893. doi: 10.1128/jvi.54.3.889-893.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. R., Khan A. S., Hoggan M. D., Hartley J. W., Martin M. A., Repaske R. Specific hybridization probes demonstrate fewer xenotropic than mink cell focus-forming murine leukemia virus env-related sequences in DNAs from inbred laboratory mice. J Virol. 1986 May;58(2):359–366. doi: 10.1128/jvi.58.2.359-366.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Boone L. R., Koh C. K., Tennant R. W., Yang W. K. Nucleotide sequences of gag-pol regions that determine the Fv-1 host range property of BALB/c N-tropic and B-tropic murine leukemia viruses. J Virol. 1983 Dec;48(3):779–784. doi: 10.1128/jvi.48.3.779-784.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Boone L. R., Yang W. K. A novel sequence segment and other nucleotide structural features in the long terminal repeat of a BALB/c mouse genomic leukemia virus-related DNA clone. Nucleic Acids Res. 1983 Aug 25;11(16):5603–5620. doi: 10.1093/nar/11.16.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. L., Spahn G. J., Rabstein L. S., Kelloff G. J., Huebner R. J. Murine C-type RNA virus from spontaneous neoplasms: in vitro host range and oncogenic potential. Science. 1973 Aug 17;181(4100):665–667. doi: 10.1126/science.181.4100.665. [DOI] [PubMed] [Google Scholar]
- Rassart E., Sankar-Mistry P., Lemay G., DesGroseillers L., Jolicoeur P. New class of leukemogenic ecotropic recombinant murine leukemia virus isolated from radiation-induced thymomas of C57BL/6 mice. J Virol. 1983 Feb;45(2):565–575. doi: 10.1128/jvi.45.2.565-575.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins K. C., Cabradilla C. D., Stephenson J. R., Aaronson S. A. Segregation of genetic information for a B-tropic leukemia virus with the structural locus for BALB:virus-1. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2953–2957. doi: 10.1073/pnas.74.7.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin R., Young J. M., Mural R. J., Bell T. E., Ihle J. N. Molecular cloning and characterization of murine leukemia virus-related DNA sequences from C3H/HeN mouse DNA. J Virol. 1982 Jul;43(1):113–126. doi: 10.1128/jvi.43.1.113-126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Narita H., Adachi A., Tsuruta S., Yorifuji T., Ishimoto A. Fv-1 determinants in xenotropic murine leukemia viruses studied with biological assay systems: Isolation of xenotropic virus with N-tropic Fv-1 activity in the cryptic form. J Virol. 1982 Apr;42(1):331–336. doi: 10.1128/jvi.42.1.331-336.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Stephension J. R., Reynolds R. K., Tronick S. R., Aaronson S. A. Distribution of three classes of endogenous type-C RNA viruses among inbred strains of mice. Virology. 1975 Oct;67(2):404–414. doi: 10.1016/0042-6822(75)90442-0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. A genetic locus for inducibility of C-type in BALB-c cells: the effect of a nonlinked regulatory gene on detection of virus after chemical activation. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2798–2801. doi: 10.1073/pnas.69.10.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. Polymorphism of murine endogenous proviruses revealed by using virus class-specific oligonucleotide probes. J Virol. 1988 Jan;62(1):168–175. doi: 10.1128/jvi.62.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. The four classes of endogenous murine leukemia virus: structural relationships and potential for recombination. J Virol. 1987 Sep;61(9):2659–2669. doi: 10.1128/jvi.61.9.2659-2669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]