Abstract
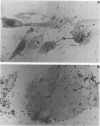
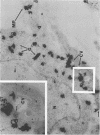
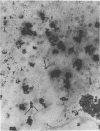
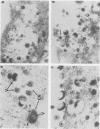
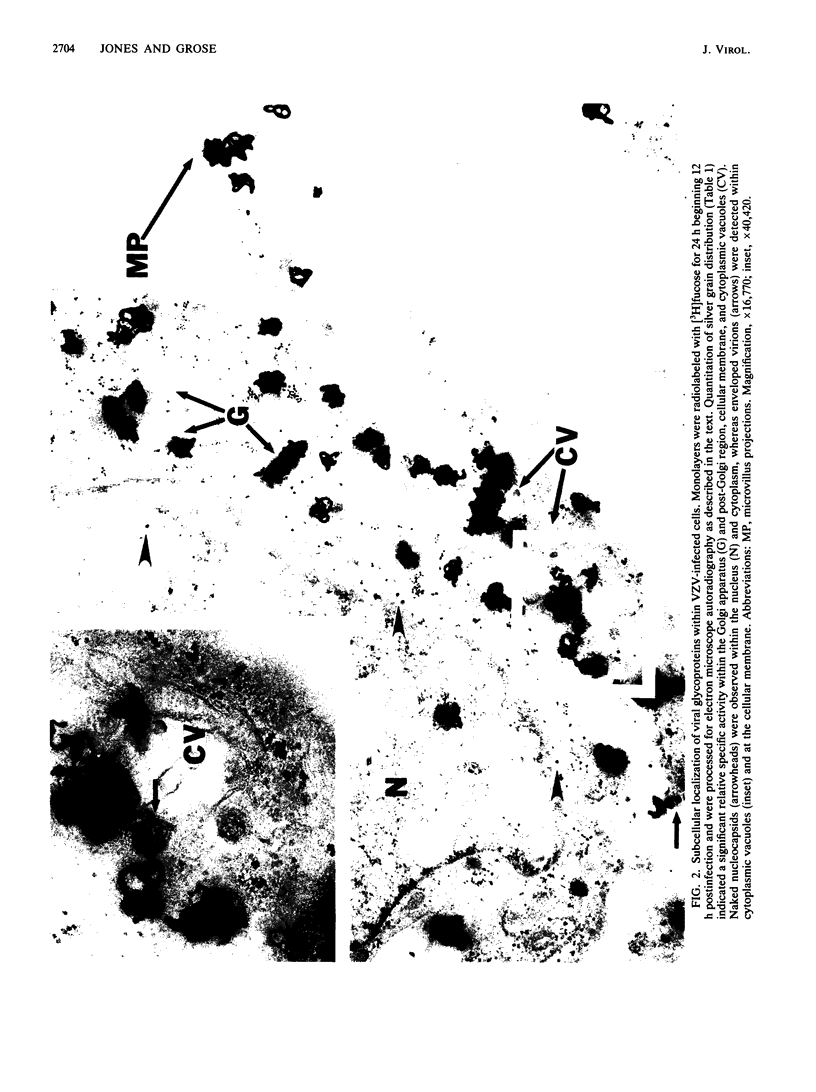
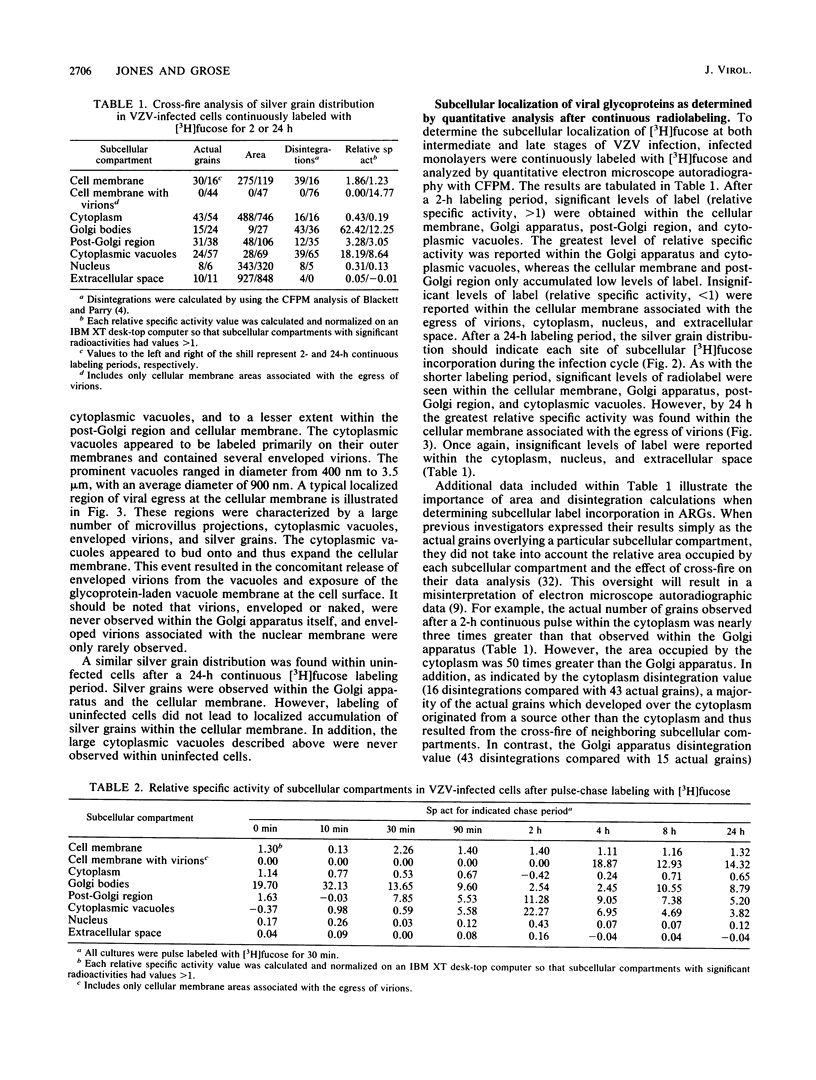
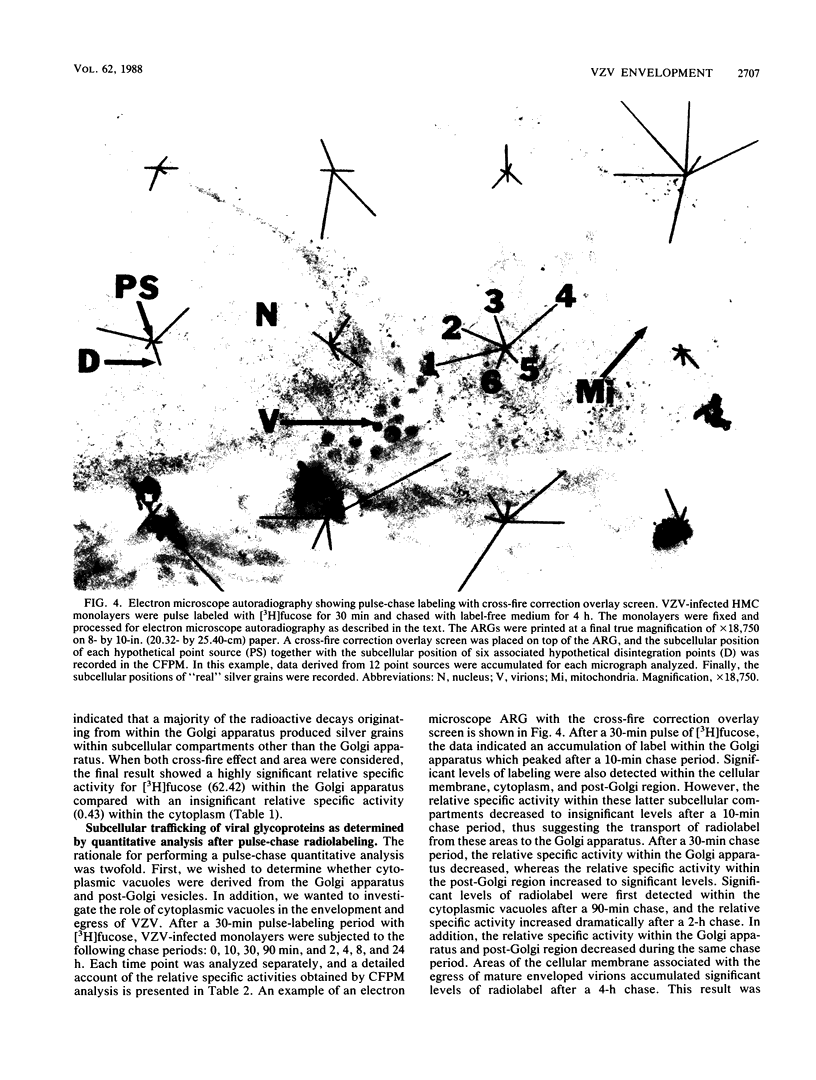
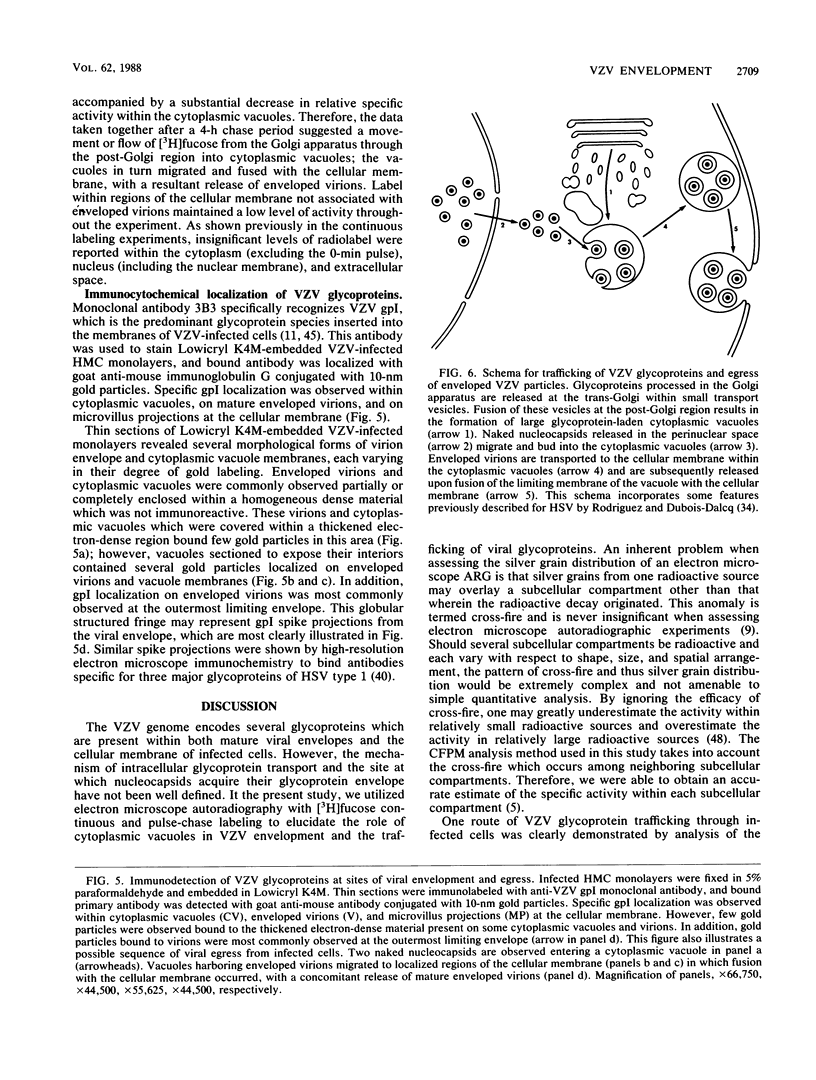
Varicella-zoster virus (VZV) encodes several glycoproteins which are present on both mature viral envelopes and the surfaces of infected cell membranes. Mechanisms of VZV glycoprotein transport and virion envelopment were investigated by both continuous radiolabeling and pulse-chase analyses with tritiated fucose in VZV-infected cells. We studied in detail the large cytoplasmic vacuoles which were present in infected cells but absent from uninfected cells. The specific activity in each subcellular compartment was defined by quantitative electron microscope autoradiography, using a cross-fire probability matrix analysis to more accurately assess the individual compartment demarcated by the silver grains. By these techniques, we documented a progression of activity originating in the Golgi apparatus and traveling through the post-Golgi region into virus-induced cytoplasmic vacuoles and finally to areas of the cellular membrane associated with the egress of viral particles. Significant amounts of radiolabel were not observed in the nucleus, and only low levels of radiolabel were associated with the cellular membrane not involved with the egress of viral particles. In addition, immunolabeling of Lowicryl-embedded VZV-infected cells demonstrated the presence of VZV glycoproteins within cytoplasmic vacuole membranes as well as on virion envelopes. These observations suggested that cytoplasmic vacuoles harbored VZV-specified glycoproteins and were also the predominant site of VZV virion envelopment within the infected cell. Neither enveloped nor unenveloped viral particles were observed within the Golgi apparatus itself.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso F. V., Compans R. W. Differential effect of monensin on enveloped viruses that form at distinct plasma membrane domains. J Cell Biol. 1981 Jun;89(3):700–705. doi: 10.1083/jcb.89.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. A., Barr S., Timbury M. C. The fine structure of cells infected with temperature-sensitive mutants of herpes simplex virus type 2. J Gen Virol. 1978 Jul;40(1):103–119. doi: 10.1099/0022-1317-40-1-103. [DOI] [PubMed] [Google Scholar]
- Bennett G., Leblond C. P., Haddad A. Migration of glycoprotein from the Golgi apparatus to the surface of various cell types as shown by radioautography after labelled fucose injection into rats. J Cell Biol. 1974 Jan;60(1):258–284. doi: 10.1083/jcb.60.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackett N. M., Parry D. M. A new method for analyzing electron microscope autoradiographs using hypothetical grain distributions. J Cell Biol. 1973 Apr;57(1):9–15. doi: 10.1083/jcb.57.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackett N. M., Parry D. M. A simplified method of "hypothetical grain" analysis of electron microscope autoradiographs. J Histochem Cytochem. 1977 Mar;25(3):206–214. doi: 10.1177/25.3.839062. [DOI] [PubMed] [Google Scholar]
- Cok M. L., Stevens J. G. Replication of varicella-zoste virus in cell culture: an ultrastructural study. J Ultrastruct Res. 1970 Aug;32(3):334–350. doi: 10.1016/s0022-5320(70)80014-4. [DOI] [PubMed] [Google Scholar]
- Compton T., Courtney R. J. Virus-specific glycoproteins associated with the nuclear fraction of herpes simplex virus type 1-infected cells. J Virol. 1984 Feb;49(2):594–597. doi: 10.1128/jvi.49.2.594-597.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs A., Williams M. A. An iterative approach to the analysis of EM autoradiographs. I. The method. J Microsc. 1978 Nov;114(2):143–156. doi: 10.1111/j.1365-2818.1978.tb00126.x. [DOI] [PubMed] [Google Scholar]
- Grose C., Perrotta D. M., Brunell P. A., Smith G. C. Cell-free varicella-zoster virus in cultured human melanoma cells. J Gen Virol. 1979 Apr;43(1):15–27. doi: 10.1099/0022-1317-43-1-15. [DOI] [PubMed] [Google Scholar]
- Heine U., Ablashi D. V., Armstrong G. R. Morphological studies on Herpesvirus saimiri in subhuman and human cell cultures. Cancer Res. 1971 Jul;31(7):1019–1029. [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumoto T., Hirano A., Kurimura T., Takagi A. In situ electron microscopical observation of cells infected with herpes simplex virus. J Gen Virol. 1981 Feb;52(Pt 2):267–278. doi: 10.1099/0022-1317-52-2-267. [DOI] [PubMed] [Google Scholar]
- Kopriwa B. A comparison of various procedures for fine grain development in electron microscopic radioautography. Histochemistry. 1975 Aug 28;44(3):201–224. doi: 10.1007/BF00491492. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Ledger P. W., Uchida N., Tanzer M. L. Immunocytochemical localization of procollagen and fibronectin in human fibroblasts: effects of the monovalent ionophore, monensin. J Cell Biol. 1980 Dec;87(3 Pt 1):663–671. doi: 10.1083/jcb.87.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo E. A., Parmley R. T., Grose C. Structural analysis of the varicella-zoster virus gp98-gp62 complex: posttranslational addition of N-linked and O-linked oligosaccharide moieties. J Virol. 1985 Mar;53(3):761–770. doi: 10.1128/jvi.53.3.761-770.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo E. A., Parmley R. T., Grose C. Varicella-zoster viral glycoprotein envelopment: ultrastructural cytochemical localization. J Histochem Cytochem. 1986 Feb;34(2):281–284. doi: 10.1177/34.2.3003184. [DOI] [PubMed] [Google Scholar]
- Nii S. Electron microscopic observations on FL cells infected with herpes simplex virus. I. Viral forms. Biken J. 1971 Jun;14(2):177–190. [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M., Hsu K. C. Electron microscopy of herpes simplex virus. IV. Studies with ferritin-conjugated antibodies. J Virol. 1968 Oct;2(10):1172–1184. doi: 10.1128/jvi.2.10.1172-1184.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D. J., Randall C. C. Molecular anatomy of herpesviruses: recent studies. Prog Med Virol. 1976;22:152–210. [PubMed] [Google Scholar]
- Orci L., Glick B. S., Rothman J. E. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport within the Golgi stack. Cell. 1986 Jul 18;46(2):171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Poliquin L., Levine G., Shore G. C. Involvement of Golgi apparatus and a restructured nuclear envelope during biogenesis and transport of herpes simplex virus glycoproteins. J Histochem Cytochem. 1985 Sep;33(9):875–883. doi: 10.1177/33.9.2991363. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., Dubois-Dalcq M. Intramembrane changes occurring during maturation of herpes simplex virus type 1: freeze-fracture study. J Virol. 1978 May;26(2):435–447. doi: 10.1128/jvi.26.2.435-447.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Fine R. E. Coated vesicles transport newly synthesized membrane glycoproteins from endoplasmic reticulum to plasma membrane in two successive stages. Proc Natl Acad Sci U S A. 1980 Feb;77(2):780–784. doi: 10.1073/pnas.77.2.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Bachmann L., Salpeter E. E. Resolution in electron microscope radioautography. J Cell Biol. 1969 Apr;41(1):1–32. doi: 10.1083/jcb.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard L. M., Fuller A. O., Spear P. G. Herpes simplex virus glycoproteins associated with different morphological entities projecting from the virion envelope. J Gen Virol. 1987 Mar;68(Pt 3):715–725. doi: 10.1099/0022-1317-68-3-715. [DOI] [PubMed] [Google Scholar]
- Sturgess J. M., Minaker E., Mitranic M. M., Moscarello M. A. The incorporation of L-fucose into glycoproteins in the Golgi apparatus of rat liver and in serum. Biochim Biophys Acta. 1973 Aug 17;320(1):123–132. doi: 10.1016/0304-4165(73)90172-4. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P., Détraz M. Comparative studies of intracellular transport of secretory proteins. J Cell Biol. 1978 Dec;79(3):694–707. doi: 10.1083/jcb.79.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N., Smilowitz H., Tanzer M. L. Monovalent ionophores inhibit secretion of procollagen and fibronectin from cultured human fibroblasts. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1868–1872. doi: 10.1073/pnas.76.4.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle K. A., Grose C. Common expression of varicella-zoster viral glycoprotein antigens in vitro and in chickenpox and zoster vesicles. J Infect Dis. 1983 Oct;148(4):630–638. doi: 10.1093/infdis/148.4.630. [DOI] [PubMed] [Google Scholar]
- Williams M. A. Autoradiography: its methodology at the present time. J Microsc. 1982 Oct;128(Pt 1):79–94. doi: 10.1111/j.1365-2818.1982.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Williams M. A., Downs A. An iterative approach to the analysis of EM autoradiographs. II. Estimates of sample sizes and confidence limits. J Microsc. 1978 Nov;114(2):157–178. doi: 10.1111/j.1365-2818.1978.tb00127.x. [DOI] [PubMed] [Google Scholar]