Abstract
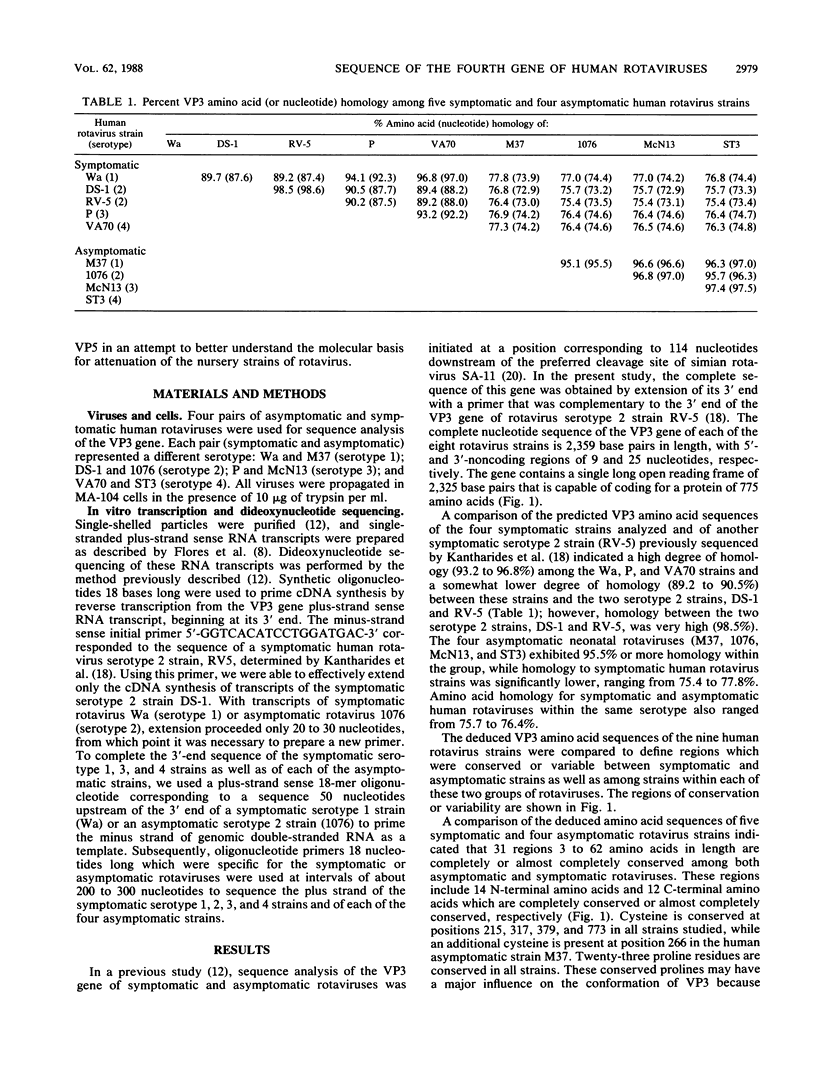
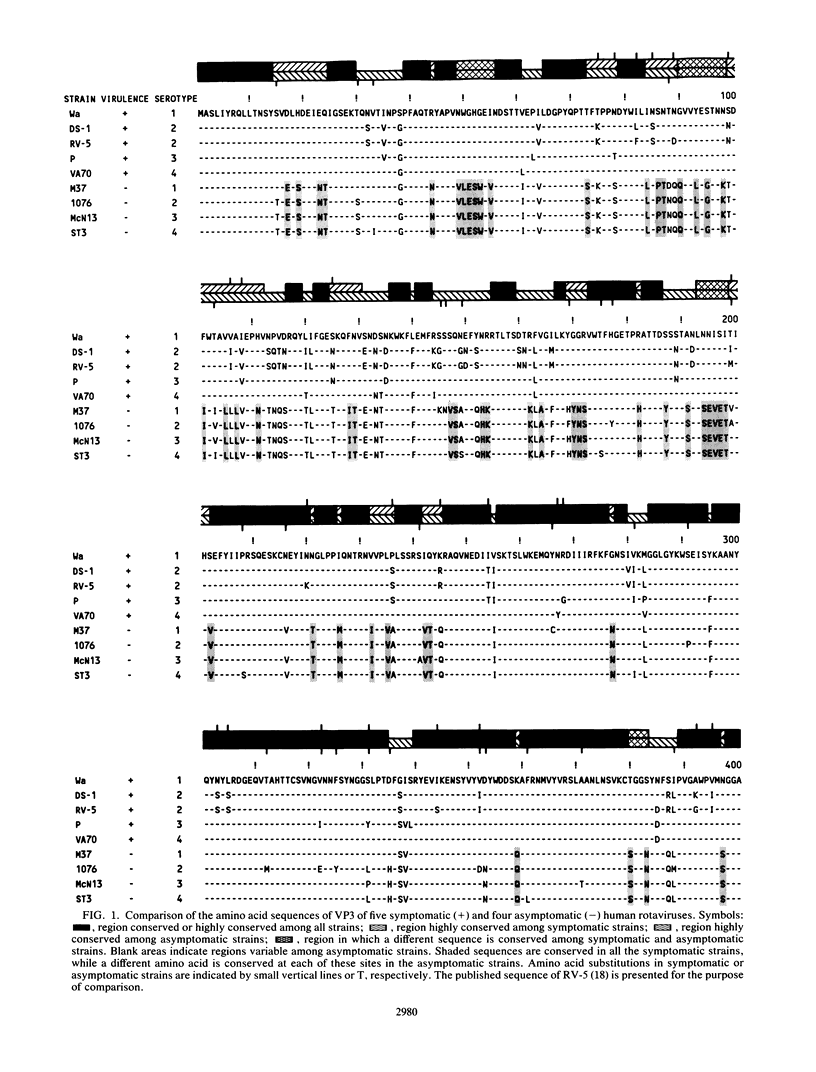
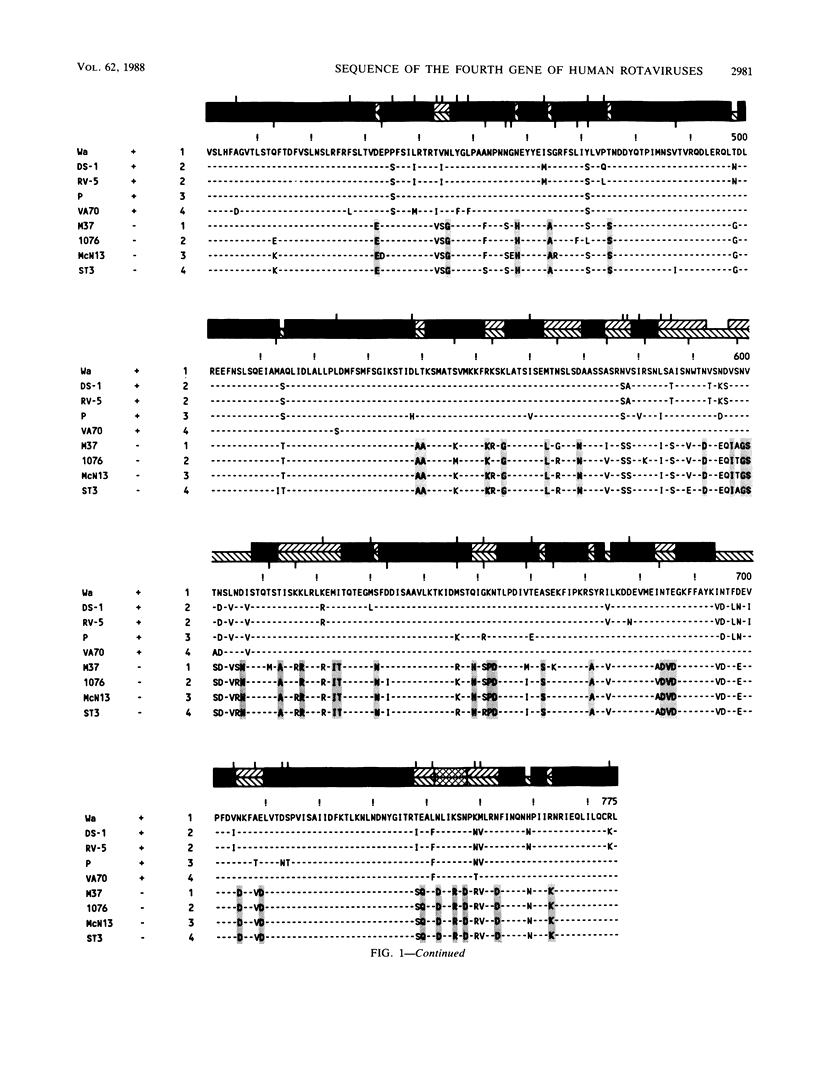
The complete nucleotide sequence of the fourth gene of symptomatic (Wa, DS-1, P, and VA70) and asymptomatic (M37, 1076, McN13, and ST3) rotaviruses of serotype 1, 2, 3, or 4 was determined by the dideoxy chain termination method. In each strain, the fourth gene, which encodes the outer capsid protein VP3, is 2,359 base pairs in length and has 5'- and 3'-noncoding regions of 9 and 25 nucleotides, respectively. The gene has a single long open reading frame of 2,325 base pairs that is capable of coding for a protein of 775 amino acids. A total of 14 N-terminal and 12 C-terminal amino acids are completely conserved or almost completely conserved, respectively, among nine human rotavirus VP3 genes that have been sequenced. In addition, there is conservation of arginine at the two trypsin cleavage sites as well as conservation of clusters of amino acids in different regions of the two VP3 cleavage products, VP8 and VP5. Three distinct forms of VP3 were identified among the nine human rotavirus strains analyzed. Three symptomatic rotaviruses (serotypes 1, 3, and 4) possess highly related VP3 genes (92.2 to 97% nucleotide identity). Two symptomatic serotype 2 rotaviruses possess VP3 genes which are even more closely related to each other (98.6% nucleotide identity) and only moderately related to the aforementioned VP3 genes of serotypes 1, 3, and 4 (87.4 to 88.2% nucleotide identity). The four asymptomatic rotaviruses, which constitute the third group, possess highly related VP3 genes (95.5 to 97.5% nucleotide identity) which are distinct from those of the virulent rotaviruses (73 to 74.8% nucleotide identity). At 91 positions in the protein sequence of VP3, an amino acid is conserved among the asymptomatic rotaviruses, while a different amino acid is conserved among the symptomatic rotaviruses. Notably, five regions are conserved among the symptomatic rotaviruses, while a different set of sequences are conserved among the asymptomatic rotaviruses. It is possible that some or all of these regions of sequence dimorphism may be responsible for the difference in virulence of these two groups of human rotaviruses. There are 13 regions in the VP3 protein sequence which exhibit the greatest variability; the majority of these variable regions are observed between amino acids 106 to 192. These regions may represent potential antigenic sites related to heterotypic rotavirus neutralization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Unicomb L. E., Barnes G. L., Bishop R. F. Cultivation and characterization of rotavirus strains infecting newborn babies in Melbourne, Australia, from 1975 to 1979. J Clin Microbiol. 1987 Sep;25(9):1635–1640. doi: 10.1128/jcm.25.9.1635-1640.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R. F., Barnes G. L., Cipriani E., Lund J. S. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983 Jul 14;309(2):72–76. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- Bridger J. C., Pocock D. H. Variation in virulence of bovine rotaviruses. J Hyg (Lond) 1986 Apr;96(2):257–264. doi: 10.1017/s0022172400066031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrystie I. L., Totterdell B., Baker M. J., Scopes J. W., Banatvala J. E. Letter: Rotavirus infections in a maternity unit. Lancet. 1975 Jul 12;2(7924):79–79. doi: 10.1016/s0140-6736(75)90525-5. [DOI] [PubMed] [Google Scholar]
- Davidson G. P., Bishop R. F., Townley R. R., Holmes I. H. Importance of a new virus in acute sporadic enteritis in children. Lancet. 1975 Feb 1;1(7901):242–246. doi: 10.1016/s0140-6736(75)91140-x. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., López S., Arias C. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. J Virol. 1981 Jan;37(1):156–160. doi: 10.1128/jvi.37.1.156-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Mason B. B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981 Sep;39(3):879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Greenberg H. B., Myslinski J., Kalica A. R., Wyatt R. G., Kapikian A. Z., Chanock R. M. Use of transcription probes for genotyping rotavirus reassortants. Virology. 1982 Sep;121(2):288–295. doi: 10.1016/0042-6822(82)90168-4. [DOI] [PubMed] [Google Scholar]
- Flores J., Midthun K., Hoshino Y., Green K., Gorziglia M., Kapikian A. Z., Chanock R. M. Conservation of the fourth gene among rotaviruses recovered from asymptomatic newborn infants and its possible role in attenuation. J Virol. 1986 Dec;60(3):972–979. doi: 10.1128/jvi.60.3.972-979.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Passarani N., Battaglía M., Percivalle E. Rapid serotyping of human rotavirus strains by solid-phase immune electron microscopy. J Clin Microbiol. 1984 Feb;19(2):273–278. doi: 10.1128/jcm.19.2.273-278.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Aguirre Y., Hoshino Y., Esparza J., Blumentals I., Askaa J., Thompson M., Glass R. I., Kapikian A. Z., Chanock R. M. VP7 serotype-specific glycoprotein of OSU porcine rotavirus: coding assignment and gene sequence. J Gen Virol. 1986 Nov;67(Pt 11):2445–2454. doi: 10.1099/0022-1317-67-11-2445. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Hoshino Y., Buckler-White A., Blumentals I., Glass R., Flores J., Kapikian A. Z., Chanock R. M. Conservation of amino acid sequence of VP8 and cleavage region of 84-kDa outer capsid protein among rotaviruses recovered from asymptomatic neonatal infection. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7039–7043. doi: 10.1073/pnas.83.18.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. Y., Midthun K., Gorziglia M., Hoshino Y., Kapikian A. Z., Chanock R. M., Flores J. Comparison of the amino acid sequences of the major neutralization protein of four human rotavirus serotypes. Virology. 1987 Nov;161(1):153–159. doi: 10.1016/0042-6822(87)90181-4. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Flores J., Kalica A. R., Wyatt R. G., Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983 Feb;64(Pt 2):313–320. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Flores J., Midthun K., Kapikian A. Z. Serotypic characterization of rotaviruses derived from asymptomatic human neonatal infections. J Clin Microbiol. 1985 Mar;21(3):425–430. doi: 10.1128/jcm.21.3.425-430.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Kantharidis P., Dyall-Smith M. L., Holmes I. H. Marked sequence variation between segment 4 genes of human RV-5 and simian SA 11 rotaviruses. Arch Virol. 1987;93(1-2):111–121. doi: 10.1007/BF01313897. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Cline W. L., Arrobio J. O., Brandt C. D., Rodriguez W. J., Sack D. A., Chanock R. M., Parrott R. H. Human reovirus-like agent as the major pathogen associated with "winter" gastroenteritis in hospitalized infants and young children. N Engl J Med. 1976 Apr 29;294(18):965–972. doi: 10.1056/NEJM197604292941801. [DOI] [PubMed] [Google Scholar]
- López S., Arias C. F., Bell J. R., Strauss J. H., Espejo R. T. Primary structure of the cleavage site associated with trypsin enhancement of rotavirus SA11 infectivity. Virology. 1985 Jul 15;144(1):11–19. doi: 10.1016/0042-6822(85)90300-9. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. Biochemical mapping of the simian rotavirus SA11 genome. J Virol. 1983 May;46(2):413–423. doi: 10.1128/jvi.46.2.413-423.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Stroop W. G., Twist E. M., Plotkin S. A. The cultivation of human rotavirus, strain 'Wa', to high titer in cell culture and characterization of the viral structural polypeptides. J Virol Methods. 1983 Jul;7(1):29–40. doi: 10.1016/0166-0934(83)90020-4. [DOI] [PubMed] [Google Scholar]
- Perez-Schael I., Daoud G., White L., Urbina G., Daoud N., Perez M., Flores J. Rotavirus shedding by newborn children. J Med Virol. 1984;14(2):127–136. doi: 10.1002/jmv.1890140206. [DOI] [PubMed] [Google Scholar]
- Pocock D. H. Characterisation of rotavirus isolates from sub-clinically infected calves by genome profile analysis. Vet Microbiol. 1987 Jan;13(1):27–34. doi: 10.1016/0378-1135(87)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J Gen Virol. 1985 May;66(Pt 5):1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K. Genetic reassortment between two human rotaviruses having different serotype and subgroup specificities. J Gen Virol. 1986 Aug;67(Pt 8):1551–1559. doi: 10.1099/0022-1317-67-8-1551. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]


