Abstract
Calorie restriction improves life span whereas nutrient excess leads to obesity and unfavorable metabolic consequences, supporting the role for a cellular “nutrient sensor” in aging. Hexosamine biosynthetic pathway (HBP) is a candidate nutrient-sensing pathway. We hypothesized that altered nutrient sensing (by HBP) with age may provide a link among aging, nutrient flux, and insulin resistance. Using a hyperinsulinemic clamp in young rats, we show that experimental activation of HBP, through the systemic infusion of glucosamine, induced severe insulin resistance (36% decline in peripheral insulin action; P<0.05), increased adipose tissue gene expression of fat-derived peptides (PAI-1 by 4-fold, angiotensinogen 3-fold, leptin 2-fold, resistin 4-fold, and adiponectin 4-fold; P<0.01 compared with young saline-infused), and enhanced glycosylation of transcription factors, thus mimicking a physiological and biological phenotype of aging. We further demonstrate a greater activation of nutrient-sensing HBP with age in both old ad libitum-fed and calorie-restricted rats. Interestingly, old calorie-restricted animals rapidly develop insulin resistance when exposed to glucosamine, despite their “young” phenotype. These results suggest that altered nutrient sensing by HBP with age may be the link among nutrients, insulin resistance, and age-related diabetes.—Einstein, F. H., Fishman, S., Bauman, J., Thompson, R. F., Huffman, D. M., Atzmon, G., Barzilai, N., Muzumdar, R. H. Enhanced activation of a “nutrient-sensing” pathway with age contributes to insulin resistance.
Keywords: hexosamine biosynthetic pathway, calorie restriction
Nutrient availability plays an important role in modulating the onset of various age-related diseases and, therefore, life span. An excess of nutrients leads to obesity and unfavorable metabolic consequences, whereas calorie restriction (CR) prevents age-related increases in body fat and delays the onset of age-related diseases, including diabetes (1,2,3). The beneficial effects of CR are related to the absolute number of calories consumed rather than the specific distribution of macronutrients (i.e., the percentage of fat, carbohydrate, or protein) (4). This relation suggests that at a cellular level, the availability of nutrients is sensed through a “nutrient sensor” and may explain both the beneficial effects of CR and the harmful effects of excess nutrients. In fact, biochemical pathways capable of “sensing” the availability of nutrients maintain energy homeostasis both in the organism as a whole and at a cellular level (5). In that context, it is plausible that “cellular insulin resistance” limiting the availability of glucose flux may be an adaptive response regulating nutrient storage and disposal.
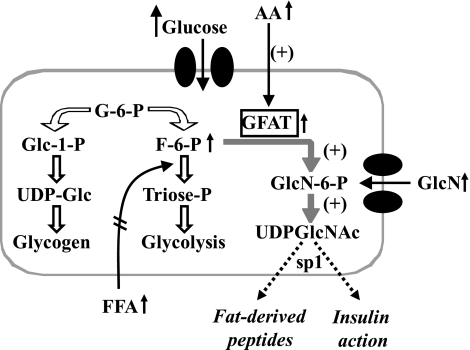
One well-established pathway for nutrient sensing is the hexosamine biosynthetic pathway (HBP), which is expressed in all cells and organs (Fig. 1) (6). Normally, only ∼1% of the intracellular glucose that is converted to fructose-6-phosphate (F-6-P) enters the HBP in most cells (excluding those in the liver) (5), and the principal end product of this pathway is uridine-diphosphoglucose-n-acetylglucosamine (UDP-Glc-NAc), a substrate for most glycosylation pathways. Changes in basic units of energy such as glucose, free fatty acids (FFAs), or amino acids, either alone or in combination, lead to an increase in flux through HBP. This may occur through the expanded pool of F-6-P, whether the result of increased phosphorylation of glucose (from hyperglycemia) or of decreased utilization of F-6-P via glycolysis (increased availability of FFAs) or increased activity of glutamine-fructose-6-phosphate-amido-transferase (GFAT; increased availability of amino acids). Increased formation of UDP-Glc-NAc results in enhanced O-linked glycosylation of several transcription factors, such as SP-1.
Figure 1.
The hexosamine biosynthetic pathway (HBP) as a nutrient sensor. Glucose is transported into the cell and phosphorylated to form glucose-6-phosphate (Glu-6-P). Glu-6-P may be converted to glycogen or further metabolized through glycolysis [Glu-6-P converted to F-6-P to triose-phosphate (Triose-P)]. A small amount (∼1%) of Glu-6-P is diverted through HBP by the enzyme GFAT to form UDP-Glc-NAc, the major substrate for O-linked or N-linked glycosylation of intracellular proteins, which induces expression of various peptides. This pathway may be activated by any nutrient: Increased glucose will increase Glc-6-P levels and flux through the HBP; increased FFAs inhibit glycolysis and increase Glc-6-P levels and flux through the HBP; and increased amino acids increase the rate-limiting enzyme GFAT. If a nutrient-dependent flux increases severalfold (equivalent to only 3–6% of intracellular glucose), it can serve as a potent switch for glycosylation of transcription factors, activating the expression of harmful peptides and the induction of insulin resistance.
An important role for nutrient sensing in aging is implicated by the extended life span offered by CR. This led us to hypothesize that mimicking excess flux through cellular nutrient sensor (activation of HBP) in young animals would result in physiological phenotype seen in aging. To experimentally induce an acute increase in flux through the HBP, we infused glucosamine (GlcN). GlcN is rapidly transported into the cell via glucose transporters, is converted to glucosamine-6-P by hexokinase, and enters the HBP, thereby increasing the cellular levels of UDP-Glc-NAc, the major end product of HBP. To identify mechanisms through which activation of the pathway exert its biological effects, we studied the result of an increase in flux through this pathway on the expression of several fat-derived peptides (FDPs) and glycosylation of transcription factors. Furthermore, we hypothesized that if this pathway is implicated in the biology of aging, it will be hyperactive in old rats. To determine the effects of HGP pathway in aging per se, we used old calorie-restricted animals, which are a lean and “metabolically young” model of aging.
MATERIALS AND METHODS
Animals:
Three groups of adult F344 × BN rats (Harlan Worldwide, Somerville, NJ, USA) were studied: 1) young ad libitum-fed (Y-AL), 3 months old, ∼280 g body weight, n = 12; 2) old ad libitum-fed (O-AL), 17–21 months old, ∼535 g body weight, n = 9; and 3) old calorie-restricted (O-CR), 22 months old, ∼288 g body weight, n = 9.
The rats were housed in individual cages and subjected to standard light (6 AM to 6 PM) and dark (6 PM to 6 AM) cycles. Y-AL and O-AL rats had free access to regular chow that consisted of 64% carbohydrate, 30% protein, and 6% fat, with a physiological fuel value of 3.3 kcal/g chow. O-CR rats were fed 60% of the standard ad libitum diet along with vitamin supplementation from 5 months of age.
One week before the in vivo study, rats were anesthetized by inhalation of methoxyflurane, and indwelling catheters were inserted in the right internal jugular vein and in the left carotid artery (7). Recovery was continued until body weight was within 3% of the preoperative weight (∼5–6 days). Chronically catheterized rats were studied while awake and unstressed ∼6–8 h after their last feeding.
In vivo hyperinsulinemic-euglycemic clamp studies
Y-AL, O-AL, and O-CR rats (n=4–6/group) were studied using the hyperinsulinemic-euglycemic clamp over 5 h (8). The protocol followed during the insulin clamp study was similar to that previously described (9,10,11). Briefly, each group received a primed continuous infusion (0.5 μCi/min bolus, 0.05 μCi/min maintenance) of high-performance, liquid chromatography-purified [3-3H] glucose throughout the study to determine glucose fluxes, as described previously (12). After a priming with 1.5 μl insulin/g body weight, continuous infusion of regular insulin (3 mU/kg/min) was administered, and an infusion of a 25% glucose solution was periodically adjusted to clamp the plasma glucose concentration at 7–8 mM. To prevent endogenous insulin secretion, somatostatin (1.5 μg/kg/min) was also infused in all the groups. Based on the study groups, the controls received saline during the period of the clamp, and the GlcN group received a continuous infusion of GlcN. Because GlcN enters the hexosamine pathway directly after being phosphorylated to GlcN-6-P without GFAT (the rate-limiting enzyme needed for conversion of F-6-P to GlcN-6-P), its intravenous infusion (17 μmol/kg/min for 5 h) (7) was used as a experimental tool to directly increase GlcN flux through the pathway without changing glucose flux.
Plasma samples for determination of 3H-glucose-specific activity were obtained at 10 min intervals throughout the insulin infusion. Plasma samples for the determination of insulin concentrations were obtained at time 0, 30, 60, 120, 240, and 300 min during the study. The total volume of blood withdrawn was ∼5.0 ml/study. To prevent volume depletion and anemia, a solution (1:1 v/v) of ∼8.0 ml of fresh blood (obtained by heart puncture from a littermate of the test animal) and heparinized saline (10 U/ml) was infused. At the conclusion of the study, rats were sacrificed using pentobarbital sodium, 60 mg/kg body weight intravenously. The abdomen was quickly opened, and adipose and muscle tissues were freeze-clamped in situ with aluminum tongs precooled in liquid nitrogen (13). The study protocol was reviewed and approved by the Animal Care and Use Committee of the Albert Einstein College of Medicine.
Assays and analytical procedures
Plasma glucose was measured by the glucose oxidase method (Glucose Analyzer II; Beckman Instruments, Inc., Palo Alto, CA, USA). Plasma insulin was measured by radioimmunoassay, using rat and porcine insulin standards. Plasma FFA concentrations were determined by an enzymatic method with an automated kit according to the manufacturer’s specification (Waco Pure Chemical, Osaka, Japan).
Calculation of whole body glucose fluxes
Plasma [3H]glucose radioactivity was measured in duplicate in the supernatants of Ba(OH) and ZnSO4 precipitates (Somogyi procedure) of plasma samples (20 μl) after they were evaporated to dryness to eliminate tritiated water. Steady-state conditions for plasma glucose-specific activity were achieved within 40–60 min after insulin in all studies. Under steady-state conditions for plasma glucose, the rate of glucose disappearance (Rd) equals the rate of glucose appearance (Ra). The latter was calculated as the ratio of the rate of infusion of [3-3H] glucose [disintegrations/min (dpm)] and the steady-state plasma [3H] glucose specific activity (dpm/mg). The rate of endogenous glucose production was calculated as the difference between Ra and the infusion rate of glucose. Because tritium on the C-3 position of glucose is lost to water during glycolysis, it can be assumed that tritium is present in either 3H2O or glucose. Plasma-tritiated water-specific activity was determined by liquid scintillation counting of the protein-free supernatant (Somogyi filtrate) before and after evaporation to dryness. The rate of glycolysis was estimated from the rate of conversion of [3H]glucose to 3H2O, as described previously (13). Glycogen synthesis was calculated as the difference between glucose uptake Rd and glycolysis (14). Data for Rd and suppression of hepatic glucose production represent the mean values during the last 90 min of the study.
RNA isolation
Total RNA was isolated from visceral fat depots obtained from individual rats by direct homogenization in 1.0 ml Trizol reagent (Gibco BRL Life Technologies, Grand Island, NY, USA). Total RNA from each depot, from each rat in the group, was obtained. The RNA was analyzed by 1% agarose gel containing 2.2 M formaldehyde before use. Further steps in the isolation process were performed according to the manufacturer’s recommended protocol. Subsequent purification and size selection of total RNA using RNeasy columns (Qiagen, Valencia, CA, USA) were also done as suggested by the manufacturer.
Quantification of expression by real-time polymerase chain reaction (PCR)
Resistin, tumor necrosis factor (TNF) -α, leptin, adiponectin, plasminogen activator inhibitor (PAI) -1, angiotensinogen (AGT), and GAPDH were assessed and quantified by quantitative real-time PCR (qRT-PCR). The qRT-PCR was performed for transcript confirmation using a hot start technique (Superscript III RT+Taq polymerase; Gibco, Gaithersburg, MD, USA), with 2 μg of total RNA, from individual rats, as starting material and an annealing temperature of 58–60°C for 40–45 cycles, depending on the individual transcript being amplified. For qRT-PCR, the first strand cDNA was synthesized from 2 μg total RNA in 20 μl final incubation volumes by using SuperScript™ Preamplification System for First Strand cDNA Synthesis (Gibco) with random primers. The qRT-PCR was carried out in a 20 μl reaction mixture containing 2 μl of the above first-strand cDNA, using a Light Cycler DNA Master SYBR green kit (Roche Applied Science, Indianapolis, IN, USA). Primer sets and PCR conditions for resistin, TNF-α, leptin, adiponectin, PAI-1, AGT, and GAPDH have been described in detail elsewhere (15). Quantification of these peptides and their signals were normalized for GAPDH to correct for loading irregularities.
Hind limb muscle UDP-Glc and UDP-Glc-NAc concentrations were obtained through 2 sequential chromatographic separations and ultraviolet detection (16,17,18). UDP-Glc-NAc colutes with UDP-Glc during solid-phase extraction. Retention time for UDP-Glc and UDP-Glc-NAc were 28.5 and 33.9 min, respectively. Plasma GlcN concentrations were determined by high-performance liquid chromatography (HPLC) after quantitative derivation with phenyl isothiocyanate as described by Anumula and Taylor (19). All HPLC analyses were performed on an HPLC system (Waters Instruments, Inc., Rochester, MN, USA) using a reverse-phase, ion-pairing isocratic method, on 2 C18T (Supelco Inc., Bellafonte, PA, USA) reverse-phase columns (0.46×25 cm) in series (20,21,22).
Sp1 glycosylation
Glycosylation of the transcription factor Sp1 in the hind limb muscle was measured as a representative marker of modifications resulting from GlcN infusion. Approximately 100 mg of skeletal muscle was mechanically homogenized in 1 ml of ice-cold buffer (1× PBS, 10 mM NaF, 1 mM Na3VO4) with 2 mM PMSF, and 50 mM O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino N-phenyl carbamate (PUGNAc) added freshly immediately before use. Samples were centrifuged for 15 min at 10,000 revolutions per minute at 4°C and assayed for protein content using the bicinchoninic acid assay. A total of 600 μg of protein was diluted in 1 ml homogenization buffer, immunoprecipitation was performed for Sp1 using 4 μg of a rabbit polyclonal anti-Sp1 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and samples were incubated with rotation overnight 4°C. A total of 20 μl of protein A–agarose beads (Roche Applied Science) was then added to the samples with rotation for 1 h. The immunoprecipitated complexes were then pelleted by centrifugation (1000 g) and washed 4–5× with wash buffer. The pellet was resuspended in 1× sample buffer, boiled, and separated on a 7.5% Tris-HCl gel. Protein was then wet-transferred onto nitrocellulose membranes, blocked for 1 h in 5% milk, and incubated overnight with a mouse monoclonal antibody to GlcNAc (1:1000; Affinity BioReagents, Neshanic Station, NJ, USA) or Sp1 (1:1000, Sigma-Aldrich Corp., St. Louis, MO, USA). After incubation with the appropriate horseradish peroxidase (HRP) -conjugated secondary antibody for 1 h, signal was detected using enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL, USA), and images were scanned into a Molecular Dynamics FluorImager (Molecular Dynamics, Sunnyvale, CA, USA) and analyzed using ImageQuant 5.5 (Amersham Pharmacia, Piscataway, NJ, USA) (15).
Statistical analysis
All values are presented as means ± se. When appropriate, data were evaluated by the 2-sample Student’s t test. A value of P ≤ 0.05 was considered significant. To take into consideration false-positive associations resulting from multiple tests of the 3 comparisons, the stringent Bonferroni correction was used; the value of P for inclusion was set to 0.05/3 = 0.016.
RESULTS
Basal metabolic characteristics of F344 × BN rats
In the individual groups (Y-AL, O-CR, and O-AL), animals that received saline or GlcN were matched in terms of body weight, amount of visceral fat, glucose,insulin, and FFA. We used a model of O-CR animals to differentiate the effects of age-associated changes in body composition from the deteriorative changes of aging per se. O-CR animals were phenotypically similar to Y-AL animals, with no significant differences in body weight, visceral fat mass, glucose levels, insulin levels, and FFA levels (Table 1). As expected, O-AL rats weighed more, had a greater amount of visceral fat, and had higher basal glucose levels than both Y-AL and O-CR rats (P<0.05). Comparisons of UDP-Glc-NAc with UDP-Glc give an indirect measurement of HBP activation compared with cellular glycogen synthesis. Basal UDP-Glc-NAc/UDP-Glc levels were 1.81 ± 0.22 mM in Y-AL rats and 2.46 ± 0.25 mM in O-CR rats (P<0.05), a 35% increase, which suggests a basal HBP activation with aging. Basal UDP-Glc-NAc compared with UDP-Glc levels were significantly higher in O-AL rats (3.15±0.21 mM) compared with the other groups (P<0.05).
TABLE 1.
Basal characteristics of F344 × BN rats
| Characteristic | Y-AL
|
O-CR
|
O-AL
|
|||
|---|---|---|---|---|---|---|
| Saline | GlcN | Saline | GlcN | Saline | GlcN | |
| n | 6 | 6 | 4 | 5 | 4 | 5 |
| Body weight (g) | 283 ± 4 | 278 ± 4 | 287 ± 4 | 289 ± 4 | 556 ± 31.9* | 516 ± 15.7* |
| Visceral fat (g) | 5.3 ± 0.6 | 5.0 ± 0.5 | 4.5 ± 0.1 | 5.0 ± 0.5 | 25.5 ± 2.3* | 24.1 ± 0.9* |
| Glucose (mmol/L) | 6.7 ± 0.1 | 6.2 ± 0.2 | 6.6 ± 0.2 | 6.1 ± 0.2 | 8.3 ± 0.16* | 9.2 ± 0.47* |
| Insulin (μU/ml) | 12.5 ± 3.8 | 12 ± 4.1 | 11 ± 2.5 | 11 ± 0.5 | 33.8 ± 3.4* | 31.9 ± 2.8* |
| FFA (mEq/l) | 0.78 ± 0.1 | 0.74 ± 0.09 | 0.71 ± 0.04 | 0.69 ± 0.04 | 1.07 ± 0.03* | 1.13 ± 0.04* |
| UDP-Glc-NAc/UDP-Glc | 1.81 ± 0.22 | 20.8 ± 1.2* | 2.46 ± 0.25 | 23.2 ± 1.5* | 3.15 ± 0.21* | 17.7 ± 2.0* |
All data expressed as means ± se.
P < 0.01 vs. Y-AL saline animals
Insulin action as assessed by hyperinsulinemic-euglycemic clamp
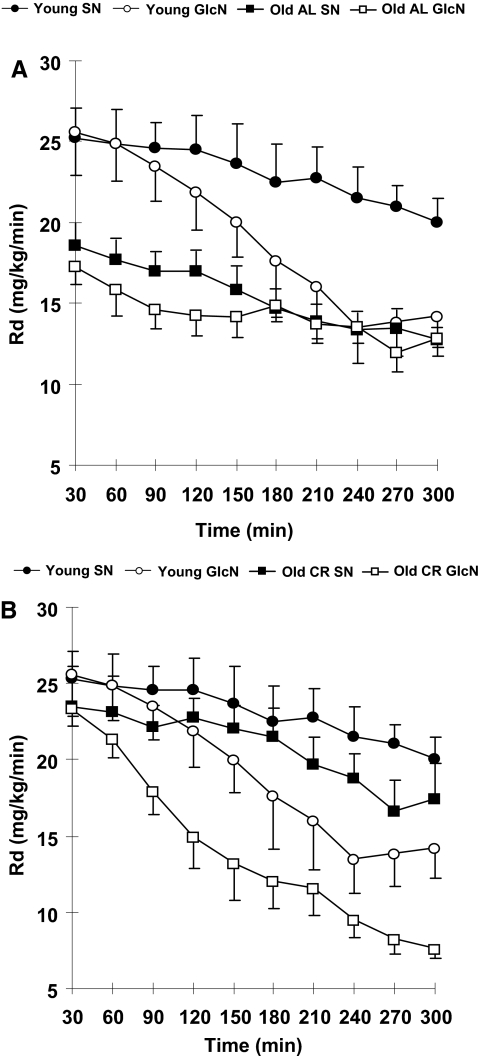
Insulin-induced peripheral Rd was greater at baseline in Y-AL (25.8±1.6 mg/kg/min) compared with O-AL (17.8±1.2 mg/kg/min; P<0.01; Fig. 2A), reflecting greater insulin sensitivity in the young rats. With infusion of GlcN, the Y-AL group developed insulin resistance, as evidenced by the significant decrease in Rd during the last 90 min of the clamp (saline 21.2±0.4 vs. GlcN 13.5±0.1 mg/kg/min, P<0.01; Table 2). Consistent with the change in Rd, glycogen synthesis was lower in the GlcN-infused group (saline 9.6±0.7 mg/kg/min vs. GlcN 5.6±1.3 mg/kg/min, P<0.01). There were no significant differences in hepatic glucose production.
Figure 2.
Rd during hyperinsulinemic clamp with and without GlcN infusion. A) Rd over 300 min in young rats (young GlcN, n=6) compared with saline control (young saline, n=6). The mean basal Rd is lower for O-AL compared with Y-AL rats (P<0.05). No further decrease in Rd was seen in the O-AL group. B) Rd over 300 min in young and O-CR rats with GlcN infusion (young GlcN and O-CR GlcN). Both developed insulin resistance, but the interval is shorter in O-CR rats (60 min vs. 210 min in young).
TABLE 2.
Clamp parameters during final 90 min of study
| Parameter | Y-AL
|
O-CR
|
O-AL
|
|||
|---|---|---|---|---|---|---|
| Saline | GlcN | Saline | GlcN | Saline | GlcN | |
| n | 6 | 6 | 4 | 5 | 4 | 5 |
| Rd | 21.2 ± 0.4 | 13.5 ± 0.1* | 17.8 ± 0.3 | 9.8 ± 0.4*,^ | 14.2 ± 0.3^ | 13.3 ± 0.1§,^ |
| GIR | 14.0 ± 1.1 | 8.5 ± 2.4 | 9.4 ± 3.8 | 3.6 ± 2.0* | 5.2 ± 1.4^ | 2.6 ± 1.2*,# |
| HGP | 5.0 ± 1.2 | 6.3 ± 1.7 | 5.2 ± 1.1 | 7.5 ± 0.4 | 8.1 ± 1.1 | 11.0 ± 0.8*,#,§ |
| Glycolysis | 10.3 ± 1.0 | 8.8 ± 1.2 | 8.8 ± 1.0 | 5.0 ± 0.6 | 4.9 ± 0.5^ | 6.0 ± 1.1 |
| Glycogen synthesis | 9.6 ± 0.7 | 5.6 ± 1.3* | 8.3 ± 2.3 | 3.8 ± 0.5* | 8.6 ± 1.9 | 6.1 ± 1.0 |
Glycolysis and glycogen synthesis values were averaged over the last 90 min of the study. All data expressed as means ± se. GIR, glucose infusion rate; HGP, hepatic glucose production.
P < 0.01 vs. saline control in same group;
P < 0.01 vs. young GlcN;
P < 0.01 vs. O-CR GlcN;
P < 0.01 vs. young saline.
The lower Rd of O-AL is consistent with insulin resistance associated with aging. There was no difference in O-AL Rd with infusion of saline (14.2±0.3 mg/kg/min) and GlcN-infused groups (13.3±0.1 mg/kg/min) at the conclusion of the clamps, suggesting that the insulin resistance of aging is the result of maximal HBP activation with ad libitum feeding. Concomitant with the lower Rd in O-AL, GIR (5.2±1.43 mg/kg/min) and glycolysis (4.9±0.53 mg/kg/min) were significantly lower compared with Y-AL (14.0±1.1 and 10.3±1.03 mg/kg/min, respectively; P<0.01).
By design, the Rd in Y-AL (25.8±1.6 mg/kg/min) and in O-CR (25.0±2.0 mg/kg/min) rats was not significantly different at baseline (Fig. 2B). Activation of the HBP (with GlcN infusion) induced insulin resistance in Y-AL and O-CR rats, but the onset of insulin resistance was significantly earlier in the O-CR compared with Y-AL rats (60 min after the beginning of clamp in O-CR compared with 210 min in Y-AL). This result, coupled with the higher baseline UDP-Glc-NAc, suggests an enhanced activation of HBP with aging.
Expression of FDPs in response to insulin with and without GlcN
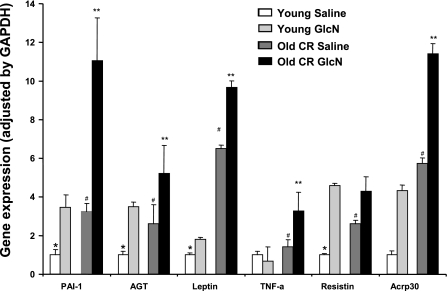
Visceral fat expression of FDPs (over GAPDH) was measured at the conclusion of the insulin clamps in Y-AL and O-CR rats with and without GlcN infusion. Several biologically active peptides have been implicated as mediators of insulin resistance (23), (TNF-α, resistin, and PAI-1), and some FDPs improve insulin sensitivity (leptin and adiponectin). PAI-1, AGT, leptin, resistin, and adiponectin genes expression increased by ∼2- to ∼4-fold with GlcN infusion in young rats, 2.5- to ∼6-fold with aging alone (O-CR with saline), and 4- to ∼12-fold with the combined effect of aging and GlcN (O-CR with GlcN; Fig. 3). TNF-α expression did not change significantly with infusion of GlcN in young rats, but there was an ∼3-fold expression increase in the O-CR GlcN-infused group. Similarities in the fold changes in gene expression in Y-AL rats infused with GlcN and O-CR rats infused with saline once again suggest the possibility of activation of HBP with aging. Both aging and HBP activation increase gene expression of FDPs compared with Y-AL rats.
Figure 3.
Expression of FDPs over GAPDH in O-CR and young rats with and without GlcN infusion. O-CR rats given GlcN demonstrate a robust increase in gene expression of several FDPs in visceral adipose tissue compared with young animals or O-CR saline controls. *P < 0.01 vs. all others; #P < 0.01 vs. age-matched GlcN; **P < 0.01 vs. young GlcN.
Glycosylation of Sp1
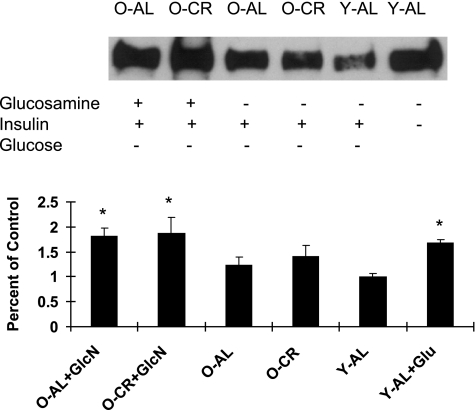
Sp1 glycosylation was quantified at the conclusion of the insulin clamp studies (saline or GlcN) in Y-AL, O-CR, and O-AL rats (Fig. 4). In addition, muscle obtained from a previous study of young rats (15) wasused as an additional control group to demonstrate the effects of hyperglycemia-induced increased flux through the HBP. Y-AL, O-CR, and O-AL rats had similar amounts of Sp1 glycosylation after 5 h of physiological hyperinsulinemia. After GlcN infusion, O-CR and O-AL rats had significantly (P<0.01) increased Sp1 glycosylation compared with Y-AL rats. Hyperglycemia induced an increase in Sp1 glycosylation in young animals compared with the Y-AL given saline alone. Experimental activation of HBP with GlcN or hyperglycemia leads to increased glycosylation of Sp1.
Figure 4.
Glycosylation of SP-1. A) Representative Western blot: the effect of GlcN, insulin, and hyperglycemia on Sp-1 O-linked GlcNAc in O-AL, O-CR, and Y-AL rats. B) Fold increase of Sp-1 O-linked GlcNAc over young saline control. *P < 0.05 vs. Y-AL.
DISCUSSION
Here, we demonstrate that an acute increase in flux through the HBP induced insulin resistance in young rodents. In addition, adipose tissue gene expression of several FDPs, such as PAI-1, AGT, leptin, TNF-α, resistin, and adiponectin were significantly increased, imitating an aging phenotype. Old rats, both ad libitum-fed and calorie-restricted, show a basal-enhanced state of activation of the HBP. O-CR rodents show heightened sensitivity to infusion of GlcN, suggesting that altered glycosylation may be a primary feature of aging per se.
Growing attention has been given to studying nutrient systems as they relate to aging in invertebrates and are modulated by CR in mammals. For example, mTOR pathway senses the amino acid component of proteins and interacts with the IGF-1/insulin-signaling pathway, playing a role in life span (24). Interaction of SIRT1 with PGC-1 is also nutritionally sensitive through glucose and insulin levels, and they are implicated in aging (25). These pathways are modulated by chronic nutrient status and are relatively specific to one nutrient source. Interestingly, when the relative contribution of nutrients was switched and rats were fed an isocaloric diet with either more amino acid, carbohydrate, or lipid, no effect was observed on longevity (26). This result suggests that the number of calories, rather than macronutrient content, is most important and led us to hypothesize that all nutrients may be sensed equally. Indeed, the sensing by HBP (Fig. 1) is linked here to a relatively acute sensing of all sources of nutrients.
Even in humans, the physiological definition of insulin resistance is obtained by provocative tests, such as glucose tolerance tests or clamp studies. We have modified such an approach to induce insulin resistance as well as to measure the degree of insulin resistance over time. Because HBP is at the crossroads between all nutrients, we use GlcN infusion as a tool to directly provoke O- or N-linked glycosylation, imitating an increase in the flux through this pathway (22). First, we demonstrate that the nutrient-sensing HBP is activated with aging by showing an increase of UDP-Glc-NAc as referenced to UDP-Glc in the basal (fasting) state. In O-AL rats, our data show that this pathway is maximally activated at baseline, resulting in insulin resistance and expression of several FDPs. However, GlcN infusion did not change insulin resistance further or alter gene expression of FDPs in O-AL animals, suggesting a maximal activity at basal state.
The age-associated changes in body composition confound the deteriorative changes of aging per se, making it impossible to discern the effects of aging from those resulting from age-associated increase in fat mass. For instance, we have previously shown that leptin resistance exhibited in old rats is independent of body fat distribution (27) and that age-related leptin resistance is likely to be centrally mediated (28). To determine if activation of HBP and heightened nutrient sensing is primary in aging and is independent of the changes in body composition, we used a model of old rats with CR (27). The animals in this group are calorie restricted from a young age and, therefore, have similar body composition, amounts of visceral fat, basal insulin and FFA levels as Y-AL rats. Moreover, the initial peripheral insulin sensitivity between these 2 groups is similar. We reasoned that if activation of HBP occurs as a feature of aging, O-CR rats would show enhanced basal activation of HBP, be more sensitive to increased flux through this pathway, and have accelerated metabolic decline compared with young weight-matched animals. Indeed, although the O-CR rats appear metabolically fit, they exhibit an increase in basal levels of products of HBP. In contrast, young calorie-restricted rats have decreased muscle hexosamine levels in association with enhanced insulin sensitivity (29), demonstrating that HBP activation is a feature of aging per se. In addition to the degree of insulin resistance, the time course associated with the induction of insulin resistance is of physiological relevance. Here, O-CR rats develop insulin resistance more rapidly with the infusion of GlcN (a direct increase in intermediates of HBP) compared with young animals. Although CR attenuates the activation of HBP in aging, it cannot completely obviate the effects of aging on the activation of this pathway.
In cultured cells, a cause–effect relationship between glycosylation and a specific action can be established using specific inhibitors of glycosylation or by modulating the rate-limiting enzyme GFAT. We have attempted in the past to increase the flux through the HBP in vivo by hyperglycemia while infusing several GFAT inhibitors, such as azaserine and 6-diazo-5-oxo-norleucin. However, meaningful data could not be collected because these agents induced rapid systemic toxic effects in rats. To demonstrate such an effect in tissue, we focused on the possibility that increased activation of HBP leads to glycosylation of transcription, factors such as Sp1, that in turn regulate the transcription of many proteins associated with metabolic syndrome (i.e., PAI-1) (30). Indeed, we demonstrate that in response to GlcN infusion, SP-1 glycosylation is increased.
Consistent with glycosylation of SP-1, O-CR rats also demonstrate increased expression of FDPs compared with weight-matched young animals. Similar to our previous studies in young rats (15, 31), this study shows that directly increasing flux through HBP in vivo increases gene expression of several FDPs involved in the metabolic syndrome. How is nutrient sensing connected to age-related diseases? PAI-1, a thrombotic factor associated with increased risk for cardiovascular events, is a clinically relevant example. We demonstrate here that PAI-1 is highly expressed with the infusion of GlcN and have previously shown that PAI-1 gene expression, plasma levels, and activity are increased by hyperglycemia (31). Post-translational reversible O-glycosylation has been shown to mediate PAI-1 gene expression and Sp1 transcriptional activity in cultured glomerular mesangial cells (32). If PAI-1 gene expression, protein level, and ultimately, activity in plasma increase in response to nutrients throughout the day and maximally at night, such increase may explain in part why most myocardial events happen around 4 AM, when PAI-1 activity is high (31). This suggests that aging per se increases sensitivity to nutrients through increased activation of HBP and may lead to the risk of age-related diseases. Similarly, other FDPs exemplify potential links to an aging phenotype (i.e., TNF-α to inflammation and insulin resistance, AGT to hypertension, and resistin to insulin resistance).
Our studies provide support for a role of a nutrient-sensing pathway in explaining major aging phenotypes. Nutrient sensing by HBP is up-regulated in aging, and an increased flux through this pathway leads to rapid onset of insulin resistance and release of potentially harmful FDPs, increasing the risk for diabetes, stroke, and cardiovascular and other age-related diseases. However, nonspecific modulation of such a pathway may present a therapeutic challenge because of its importance in building glycoproteins, proteoglycans, and most importantly, activation of transcription factors and numerous enzymes. Furthermore, although CR can slow the rate of aging, it does not completely attenuate the hyperactivity of the HBP pathway with aging.
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health to N.B. (P01-AG021654 and R01-AG18381) and to R.H.M. (K08-AG027462), from the American Association of Obstetricians and Gynecologists Foundation/Society of Maternal-Fetal Medicine to F.H.E., and by the Core Laboratories of the Albert Einstein Diabetes Research and Training Center (DK 20541).
References
- Anderson R M, Weindruch R. Calorie restriction: progress during mid-2005–mid-2006. Exp Gerontol. 2006;41:1247–1249. doi: 10.1016/j.exger.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Sohal R S. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro E J. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Masoro E J. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Rossetti L. Perspective: hexosamines and nutrient sensing. Endocrinology. 2000;141:1922–1925. doi: 10.1210/endo.141.6.7566. [DOI] [PubMed] [Google Scholar]
- Obici S, Wang J, Chowdury R, Feng Z, Siddhanta U, Morgan K, Rossetti L. Identification of a biochemical link between energy intake and energy expenditure. J Clin Invest. 2002;109:1599–1605. doi: 10.1172/JCI15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L. Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest. 1997;99:2173–2182. doi: 10.1172/JCI119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R A, Tobin J D, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Barzilai N, She L, Liu B Q, Vuguin P, Cohen P, Wang J, Rossetti L. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes. 1999;48:94–98. doi: 10.2337/diabetes.48.1.94. [DOI] [PubMed] [Google Scholar]
- Fishman S, Muzumdar R H, Atzmon G, Ma X, Yang X, Einstein F H, Barzilai N. Resistance to leptin action is the major determinant of hepatic triglyceride accumulation in vivo. FASEB J. 2007;21:53–60. doi: 10.1096/fj.06-6557com. [DOI] [PubMed] [Google Scholar]
- Muzumdar R H, Ma X, Fishman S, Yang X, Atzmon G, Vuguin P, Einstein F H, Hwang D, Cohen P, Barzilai N. Central and opposing effects of IGF-I and IGF-binding protein-3 on systemic insulin action. Diabetes. 2006;55:2788–2796. doi: 10.2337/db06-0318. [DOI] [PubMed] [Google Scholar]
- Rossetti L, Smith D, Shulman G I, Papachristou D, DeFronzo R A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L, Giaccari A. Relative contribution of glycogen synthesis and glycolysis to insulin-mediated glucose uptake. A dose-response euglycemic clamp study in normal and diabetic rats. J Clin Invest. 1990;85:1785–1792. doi: 10.1172/JCI114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G, Cases J A, She L, Ma X H, Yang X M, Hu M, Wu J, Rossetti L, Barzilai N. Ability of insulin to modulate hepatic glucose production in aging rats is impaired by fat accumulation. Am J Physiol. 2000;278:E985–E991. doi: 10.1152/ajpendo.2000.278.6.E985. [DOI] [PubMed] [Google Scholar]
- Einstein F H, Atzmon G, Yang X M, Ma X H, Rincon M, Rudin E, Muzumdar R, Barzilai N. Differential responses of visceral and subcutaneous fat depots to nutrients. Diabetes. 2005;54:672–678. doi: 10.2337/diabetes.54.3.672. [DOI] [PubMed] [Google Scholar]
- Rossetti L, Hu M. Skeletal muscle glycogenolysis is more sensitive to insulin than is glucose transport/phosphorylation. Relation to the insulin-mediated inhibition of hepatic glucose production. J Clin Invest. 1993;92:2963–2974. doi: 10.1172/JCI116919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccari A, Rossetti L. Predominant role of gluconeogenesis in the hepatic glycogen repletion of diabetic rats. J Clin Invest. 1992;89:36–45. doi: 10.1172/JCI115583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccari A, Rossetti L. Isocratic high-performance liquid chromatographic determination of the concentration and specific radioactivity of phosphoenolpyruvate and uridine diphosphate glucose in tissue extracts. J Chromatogr. 1989;497:69–78. doi: 10.1016/0378-4347(89)80006-4. [DOI] [PubMed] [Google Scholar]
- Anumula K R, Taylor P B. Quantitative determination of phenyl isothiocyanate-derivatized amino sugars and amino sugar alcohols by high-performance liquid chromatography. Anal Biochem. 1991;197:113–120. doi: 10.1016/0003-2697(91)90365-z. [DOI] [PubMed] [Google Scholar]
- Liu L, Karkanias G B, Morales J C, Hawkins M, Barzilai N, Wang J, Rossetti L. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J Biol Chem. 1998;273:31160–31167. doi: 10.1074/jbc.273.47.31160. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Wang J, Massilon D, Vuguin P, Hawkins M, Rossetti L. Leptin selectively decreases visceral adiposity and enhances insulin action. J Clin Invest. 1997;100:3105–3110. doi: 10.1172/JCI119865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L, Hawkins M, Chen W, Gindi J, Barzilai N. In vivo glucosamine infusion induces insulin resistance in normoglycemic but not in hyperglycemic conscious rats. J Clin Invest. 1995;96:132–140. doi: 10.1172/JCI118013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G S, Murray D L, Choy L N, Spiegelman B M. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid B. TOR pathway: linking nutrient sensing to life span. Sci Aging Knowl Environ. 2004;2004:PE34. doi: 10.1126/sageke.2004.36.pe34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton J C, Brown-Borg H M. Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Gerontol Biol Med Sci. 2005;60:1494–1509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Gleiser C A, Masoro E J, McMahan C A, Seo E J, Yu B P. Influence of the restriction of individual dietary components on longevity and age-related disease of Fischer rats: the fat component and the mineral component. J Gerontol. 1988;43:B13–B21. doi: 10.1093/geronj/43.1.b13. [DOI] [PubMed] [Google Scholar]
- Gabriely I, Ma X H, Yang X M, Rossetti L, Barzilai N. Leptin resistance during aging is independent of fat mass. Diabetes. 2002;51:1016–1021. doi: 10.2337/diabetes.51.4.1016. [DOI] [PubMed] [Google Scholar]
- Muzumdar R, Ma X, Yang X, Atzmon G, Bernstein J, Karkanias G, Barzilai N. Physiologic effect of leptin on insulin secretion is mediated mainly through central mechanisms. FASEB J. 2003;17:1130–1132. doi: 10.1096/fj.02-0991fje. [DOI] [PubMed] [Google Scholar]
- Gazdag A C, Wetter T J, Davidson R T, Robinson K A, Buse M G, Yee A J, Turcotte L P, Cartee G D. Lower calorie intake enhances muscle insulin action and reduces hexosamine levels. Am J Physiol. 2000;278:R504–R512. doi: 10.1152/ajpregu.2000.278.2.R504. [DOI] [PubMed] [Google Scholar]
- Goldberg H J, Whiteside C I, Fantus I G. The hexosamine pathway regulates the plasminogen activator inhibitor-1 gene promoter and Sp1 transcriptional activation through protein kinase C-beta I and -delta. J Biol Chem. 2002;277:33833–33841. doi: 10.1074/jbc.M112331200. [DOI] [PubMed] [Google Scholar]
- Gabriely I, Yang X M, Cases J A, Ma X H, Rossetti L, Barzilai N. Hyperglycemia induces PAI-1 gene expression in adipose tissue by activation of the hexosamine biosynthetic pathway. Atherosclerosis. 2002;160:115–122. doi: 10.1016/s0021-9150(01)00574-3. [DOI] [PubMed] [Google Scholar]
- Goldberg H J, Whiteside C I, Hart G W, Fantus I G. Posttranslational, reversible O-glycosylation is stimulated by high glucose and mediates plasminogen activator inhibitor-1 gene expression and Sp1 transcriptional activity in glomerular mesangial cells. Endocrinology. 2006;147:222–231. doi: 10.1210/en.2005-0523. [DOI] [PubMed] [Google Scholar]