Abstract
The α-subunits of the trimeric Go class of GTPases, comprising the splice variants Go1α and Go2α, are abundantly expressed in brain and reside on both plasma membrane and synaptic vesicles. Go2α is involved in the vesicular storage of monoamines but its physiological relevance is still obscure. We now show that genetic depletion of Go2α reduces motor activity induced by dopamine-enhancing drugs like cocaine, as repeated injections of cocaine fail to provoke behavioral sensitization in Go2α−/− mice. In Go2α−/− mice, D1 receptor signaling in the striatum is attenuated due to a reduced expression of Golfα and Gsα. Following cocaine treatment, Go2α−/− mice have lower D1 and higher D2 receptor amounts compared to wild-type mice. The lack of behavioral sensitization correlates with reduced dopamine levels in the striatum and decreased expression of tyrosine hydroxylase. One reason for the neurochemical changes may be a reduced uptake of monoamines by synaptic vesicles from Go2α−/− mice as a consequence of a lowered set point for filling. We conclude that Go2α optimizes vesicular filling which is instrumental for normal dopamine functioning and for the development of drug-induced behavioral sensitization.—Brunk, I., Blex, C., Sanchis-Segura, C., Sternberg, J., Perreau-Lenz, S., Bilbao, A., Hörtnagl, H., Baron, J., Juranek, J., Laube, G., Birnbaumer, L., Spanagel, R., Ahnert-Hilger, G. Deletion of Go2α abolishes cocaine-induced behavioral sensitization by disturbing the striatal dopamine system.
Keywords: vesicular regulation, VMAT2, locomotor activity, psychostimulants
A great variety of brain functions, including motor control, cognition, and emotions, as well as drug-induced reinforcement and sensitization, is linked to dopaminergic neurotransmission. Dopamine is synthesized by tyrosine hydroxylase (TH) and DOPA decarboxylase, and cytosolic dopamine is concentrated into synaptic vesicles (SVs) of dopaminergic neurons by the action of the vesicular monoamine transporter 2 (VMAT2). Cytosolic dopamine can be degraded by monoaminooxidases (MAOs). Released dopamine exerts its physiological response on dopaminergic receptors of the dopamine 1 (D1) -like or D2-like subfamilies. Dopamine is regained by the plasma membrane dopamine transporter (DAT). In contrast to DAT, VMAT2 is not selective for dopamine but also transports serotonin, noradrenaline, and adrenaline with comparable efficiency (1, 2). A perfect balance between synthesis, degradation, and reuse guarantees adequate amounts of monoamines for their diverse physiological functions. An alteration in any of the components involved in dopamine synthesis, transport, and degradation usually results in a disturbance at the systemic level, as observed by pharmacological interventions or knockout models. The DAT knockout, for example, most prominently shows a hyperactive phenotype (3). Deletion of VMAT2 is fatal, and even mice heterozygous for VMAT2 display altered drug-induced locomotor activity and MPTP toxicity (4). Indeed, compensatory mechanisms exist that counteract imbalances of dopamine homeostasis, particularly with respect to vesicular storage. Thus, TH expression is increased in VMAT2 knockout animals (5), and enhanced monoamine synthesis is observed when VMAT2 activity is blocked by reserpine (6).
We have recently shown that vesicular storage of dopamine, as well as of other monoamines, is controlled by Go2α (7). Go1α and Go2α are splice variants of Goα, which when associated with Gβγ dimers constitute 1 to 2% of total brain membrane protein (8). Besides their occurrence at the plasma membrane, Go proteins have been found on endomembranes, including SVs (9, 10) suggesting an effect on vesicular function. Indeed monoamine storage mediated by VMAT2 is regulated by SV-associated Go2α but not by Go1α, thus confirming that the two splice variants exert different functions. In this signaling pathway, VMAT2 assumes the role of a G-protein-coupled receptor that senses intravesicular transmitter concentrations and provides a direct feedback loop between the degree of vesicle filling and uptake activity (7, 11).
Recently, mouse strains have been characterized that are deficient in either Go1α or Go2α. Although Go1α knockouts are severely impaired, Go2α−/− mice do not present obvious phenotypical changes (12,13,14). Considering the role of Go2α in regulating vesicular monoamine storage discussed above, there are two possible explanations for the lack of gross phenotypical and behavioral changes in these mice. First, compensatory mechanisms may exist that substitute for Go2α, thus ensuring that vesicular monoamine storage is maintained within a normal range. Alternatively, storage is altered, but the resulting effects are too subtle to be obvious without closer analyses.
In the present study, we examined whether in Go2α-deficient mice dopaminergic dysfunctions become apparent when dopamine homeostasis is pharmacologically challenged. We found that Go2α−/− mutants lack behavioral sensitization following repeated injections of cocaine, probably caused by an imbalance in D1 receptor signaling within the striatum and impaired dopamine storage dynamics. Our results provide evidence that fine-tuning of vesicular dopamine content is important in modulating the effects of psychostimulants in the striatal dopaminergic system.
MATERIALS AND METHODS
Antibodies
Mouse monoclonal antibodies against Goα proteins were previously described (15). A monoclonal antibody against synaptophysin (16) and polyclonal antibodies against VMAT2 (17) and against the 116-kDa subunit of the vacuolar proton pump were obtained from Synaptic Systems (Göttingen, Germany). Antibodies against TH (rabbit), DAT (rabbit), dopamine D1 (mouse) and D2 (rabbit) receptors were from Chemicon International (Temecula CA, USA). Antibodies against Gqα (rabbit) were purchased from Calbiochem (La Jolla, CA, USA), against Golfα and Gsα (both rabbit) and Goα (sc13532) from Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively. A polyclonal antiserum against G-protein β-subunits 1–4 (18) was a generous gift from B. Nürnberg (Heinrich-Heine-Universität, Düsseldorf, Germany).
Secondary antibodies, horse anti-mouse and goat anti-rabbit conjugated with horseradish peroxidase, were purchased from Vector Laboratories (Burlingame, CA, USA). Fluorescent dye-labeled secondary antibodies (goat anti-mouse IgG Cy2 or Cy3 and goat anti-rabbit IgG Cy2 or Cy3) were obtained from Dianova (Hamburg, Germany).
Chemicals
Guanolyl 5′-imidodiphosphate [GMP-P(NH)P], dopamine, serotonin, reserpine, nigericine, and bafilomycine were purchased from Sigma-Aldrich (St. Louis, MO, USA). 5-Hydroxy-[3H]tryptamine trifluoroacetate (serotonin; specific activity 4.33 TBq/mmol) and 7,8-[3H]dopamine (specific activity 1.78 TBq/mmol) were obtained from GE Healthcare (New York, NY, USA). GBR12909 was purchased from Tocris Bioscience (Bristol, UK). Cocaine was purchased from Sigma-Aldrich. Streptolysin O (SLO) from β-hemolytic streptococci was purified as described previously (19).
G-protein deletion mutants
Go1α and Go2α splice variant-specific deletion mutants as well as Goα deletion mutants were generated and bred as described previously (12, 14). The respective wild-type littermates (129/SvxC57/BL6) were derived from heterozygous breeding. All experimental procedures were approved by the Committee on Animal Care and Use and were carried out in accordance with the local Animal Welfare Act and the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Synaptosomes and SVs
Synaptosomes were either prepared from whole mouse brain or from the striatal area, as described previously (20). SVs were prepared from whole mouse brain, as described previously (15).
Monoamine uptake
Uptake was performed into either SVs or permeabilized synaptosomes. SVs were resuspended in potassium glutamate (KG) buffer (150 mM KG; 20 mM 1,4-piperazinediethanesulfonic acid; 4 mM EGTA; 2.9 mM MgCl2, equivalent to 1 mM free Mg2+; and 2 mM ATP, adjusted to pH 7.0 with KOH) by passing 10 times through a 27-gauge needle, before they were divided into individual reaction cups. Uptake was started by adding KG-ATP buffer, supplemented with 1 mM ascorbic acid and 40 nM [3H]serotonin or [3H]dopamine. Additives such as GMP-P(NH)P (100 μM), cocaine (5 μM), or reserpine (6 μM, to determine nonspecific uptake), were applied during this step. Incubation was performed for 10 min at 25°C and stopped by addition of 500 μl of ice-cold KG buffer followed by rapid centrifugation at 440,000 g for 10 min at 4°C. The pellets were lysed in 0.4% Triton X-100 to determine radioactivity by liquid scintillation counting and protein content using the bicinchoninic acid method.
For permeabilization, synaptosomes were resuspended in KG buffer supplemented with 5000 hemolytic units (HE)/ml SLO and incubated for 10 min at 4°C. Under these conditions, SLO monomers bind to the cholesterol of the plasma membrane. After removal of unbound SLO by centrifugation (16,000 g, 2 min, 4°C), oligomerization and pore formation can be initiated by elevating the temperature, thereby restricting permeability to the plasma membrane. The pellet containing permeabilized synaptosomes was resuspended in KG buffer, and monoamine uptake was performed for 10 min at 37°C as described for SVs.
Dopamine uptake by intact synaptosomes was performed in sodium buffer (10 mM glucose; 5 mM KCl; 140 mM NaCl; 5 mM NaHCO3;1 mM MgCl2; 1.2 mM Na2HPO4; 20 mM HEPES, adjusted to pH 7.4 with NaOH), and nonspecific uptake was determined in the presence of 20 μM GBR 12909.
HPLC analysis
Wild-type and Go2α−/− mice of different ages [adult, postnatal day (P) 2, 4, 8, or 12] were decapitated; after removal, brains were immediately frozen on dry ice and kept at −80°C until use. From frozen P12 and adult brains, different areas (bulbus olfactorius, frontal cortex, striatum, hypothalamus, hippocampus, and cerebellum) were dissected on a cold plate (–15°C). Samples were weighed and stored at −80°C until homogenization. After adding of 10–20 vol of deionized water, frozen samples were homogenized by ultrasonication at 4°C. Homogenates were added to an equal volume of 0.2 N perchloric acid and centrifuged for 10 min at 25,000 g and 4°C. The supernatant was used for determination of monoamines by HPLC with electrochemical detection, as described previously (21).
Immunoblot analysis
Subcellular fractions from brain homogenates were resuspended in sucrose buffer, analyzed for protein content, and subjected to SDS-PAGE. After transfer to nitrocellulose membranes, proteins were analyzed using the indicated antibodies and the enhanced chemiluminescence (ECL) detection system (GE Healthcare). ECL-processed films were scanned, and protein bands were densitometrically quantified using the Labimage 1D 2006 program (Kapelan GmbH, Halle, Germany). It was ensured that signals were in the linear range of the ECL detection system. Quantification of proteins between wild-type and knockout samples was performed from one gel using either synaptophysin or synaptobrevin as internal standards.
Measurement of MAO activities
Activities of MAO A and B were determined with the Amplex Red MAO assay kit from Molecular Probes, Invitrogen (Tübingen, Germany).
Immunofluorescence microscopy
Anesthesized mice were transcardially perfused by a fixative containing 4% formaldehyde, 0.05% glutaraldehyde, and 0.2% picric acid and dissolved in 0.1 M phosphate buffer (pH 7.4) for 30 min. The fixative was removed by a subsequent perfusion with 0.15 M sucrose in 0.1 M phosphate buffer (pH 7.4). Immunocytochemistry was performed on cryostat sections (20 or 30 μm), as described previously (17).
Cocaine-induced conditioned place preference and sensitization
The procedure of conditioning and testing of cocaine-induced conditioned place preference (CPP) was conducted as described previously (22). During the course of this experiment, the effects of cocaine on acute locomotion and on locomotor sensitization were also measured. CPP was conducted in six white acrylic chambers (32 cm long, 16 cm wide, 22 cm high) using two different types of floors, spaced metal bars (rod) or holed metal plate (hole), as conditioning stimulus (CS). The whole procedure consisted of 6 phases: 1 preconditioning session and 8 conditioning sessions of 30 min, and 1 session of 15 min for the preference test. All sessions were monitored by a video-tracking system (Ethovision 2.0; Noldus, Wageningen, The Netherlands) to determine locomotion and spatial placement of each mouse each 0.2 s across the whole session. On the first day (preconditioning session), all subjects were placed inside the conditioning chamber with a distinctive floor. The conditioning phase started the next day and followed an unbiased pavlovian conditioning procedure in which mice belonging to the different genotype-based groups were randomly assigned to one of two experimental conditions (CS+: rod+ or hole+; or CS−: rod− or hole−). During the conditioning phase, mice had access to the entire apparatus, but only one type of floor was presented. On CS+ trials, each mouse received an injection of cocaine (10 mg/kg i.p.), whereas in the CS− trials, animals were injected with equivalent volumes of saline. Immediately after injection, mice were placed in the conditioning apparatus containing the corresponding floor. The effects of an acute drug challenge on mice locomotion were assessed by comparing the distance traveled (cm/30 min) during the first CS+ and the first CS− trial. Furthermore, drug-induced behavioral sensitization was assessed by analyzing the changes in locomotion across the four CS+ trials. The preference test took place 24 h after the last conditioning trial. To conduct this test, both types of floor were presented in each chamber, and mice were placed in the center of the chamber without any previous injection. The relative position (left vs. right) of each floor was counterbalanced. During the 15 min of the preference test session, the time spent on CS+ and CS− floor was assessed. The mice were killed immediately after the end of the CPP test. Their brains were collected, frozen in an isopentane solution for 2 min, and stored at −80°C for further analyses.
Data analysis
All biochemical and in vivo experiments were repeated at least 3 times, and samples were run in triplicate. Data are presented as means ± sd (biochemical experiments) or means ± se (HPLC and behavioral experiments), and a significance level of P ≤ 0.05 was applied throughout this study. The behavioral data were analyzed by 1- or 2-way ANOVA, with repeated measures when necessary, followed by Duncan’s post hoc tests, when appropriate. CPP was analyzed by means of a paired Student’s t test for independent samples; for biochemical data, a Student’s t test for independent samples was applied.
RESULTS
Lack of cocaine-induced behavioral sensitization and reduced dopamine D1 receptor signaling in Go2α−/− mice
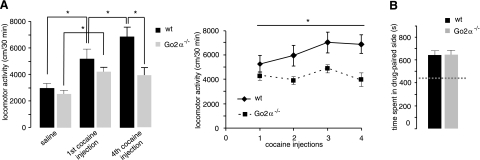
Go2α−/− mice show normal basal motor activity. Alterations in motor activity might become apparent following a pharmacological challenge of the dopaminergic system. An acute injection of cocaine increased activity, but no significant changes could be observed between the genotypes (Fig. 1A). Repeated injections of cocaine led to the development of behavioral sensitization in wild-type mice (Fig. 1A). In contrast, Go2α−/− mice did not develop cocaine-induced behavioral sensitization (Fig. 1A). The observed phenotypical changes were specific for locomotor-stimulating effects because reinforcing effects of cocaine measured by a conditioned place preference test (23) revealed no differences between the genotypes (Fig. 1B).
Figure 1.
Cocaine-induced behavior in wild-type (wt) and Go2α−/− mice. A) Effects of acute and repeated intermittent injections of cocaine on locomotor activity of wt (n=16) and Go2α−/− (n=15) littermates. Single administration of cocaine increased locomotor activity in both genotypes (cocaine F1, 27=44.77, P<0.0001); however, there were only slight, but not significant, differences between the genotypes (genotype F1, 27=1.43, P=0.24). Subsequently, daily injections of cocaine led to an increase in locomotor activity (behavioral sensitization) in wild-type mice (Trial F3, 81=0.04, P<0.0001). In contrast, Go2α−/− mice showed no development of sensitization (genotype F1, 27=7.79, P<0.01). *P < 0.05. B) Both genotypes did not differ in the conditioned place preference induced by cocaine (T26=0.059, P=0.95). The dashed line indicates the time spent in the drug-paired side before drug administration.
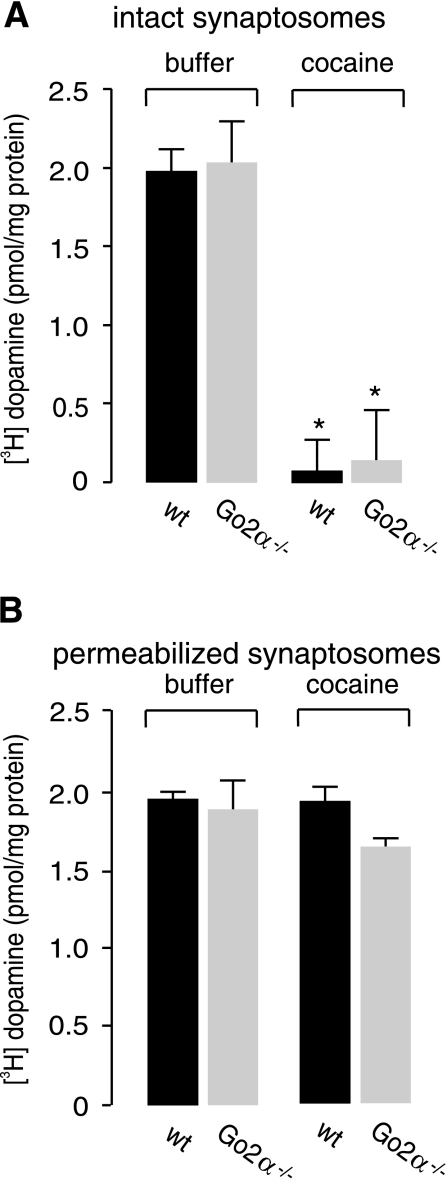
Cocaine mainly exerts its effect by inhibition of the plasma membrane transporter DAT, thereby increasing the extracellular amount of dopamine. To exclude an altered effect of cocaine on plasma membrane and vesicular transporters in Go2α−/− compared to wild-type mice, we examined the effects of cocaine on dopamine uptake, mediated either by DAT or VMAT2, into striatal synaptosomes. As expected, cocaine completely blocked DAT-mediated dopamine uptake into intact synaptosomes but had no effect on VMAT2-mediated uptake following SLO permeabilization, with no difference between synaptosomes derived from the two genotypes (Fig. 2).
Figure 2.
In vitro effects of cocaine on synaptosomes from wild-type (wt) and Go2α−/− mice. A) Synaptosomes from wt or Go2α−/− mouse brains were loaded with [3H]dopamine for 10 min at 37°C, either in the absence or presence of 5 μM cocaine. Nonspecific uptake using 20 μM GBR 12909, a specific inhibitor of DAT, was subtracted. As expected, cocaine completely blocked uptake into synaptosomes with no difference between the genotypes. *P < 0.05. B) Synaptosomes from both wild-type and Go2α−/− mice were permeabilized by SLO, and vesicular uptake was determined in the absence or presence of 5 μM cocaine. Nonspecific uptake in the presence of reserpine was subtracted. Vesicular uptake was not affected by cocaine, and there was no difference between the genotypes. Data represent means ± sd of 3 determinations.
Reduced motor activity in Go2α−/− mice suggests changes at the level of G proteins or their upstream dopamine receptors. Goα has been linked to dopamine receptors; however, the effect of the splice variants remained unclear (24). Motor activity mainly depends on functional D1 receptors. In the striatum, coupling of dopamine D1 receptors to adenylyl cyclase is mediated by Golfα and Gsα, and alterations in these G proteins can affect locomotor responses of psychostimulants (25, 26). Therefore, we asked whether the levels of Go1α, Golfα, and Gsα were altered in Go2α−/− mice compared to wild-type animals.
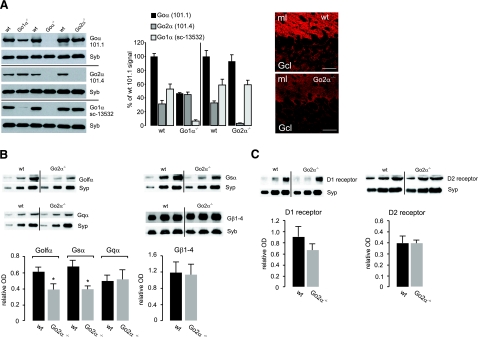
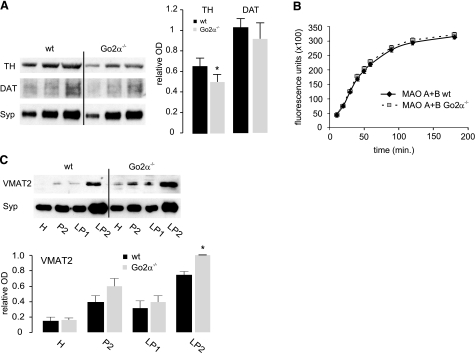
First, the amounts of Goα subunits were analyzed by Western blot analysis from synaptosomal preparations of wild-type, Goα −/−, Go1α−/−, and Go2α−/− mice using a monoclonal antibody that recognizes both splice variants (101.1), a Go2α-specific antibody (101.4) (15), and a commercially available Goα antibody (sc13532) that preferentially recognizes Go1α. Go2α represents on average one-third of total Goα in mouse brain (Fig. 3A). No significant up-regulation of Go1α was observed in Go2α−/− mice (according to the Go1α antibody). Parallel quantification of actin, synaptophysin, and synaptobrevin showed no changes and served as internal controls. In cerebellar slices, the immunosignal for Goα (using the 101.1 antibody) was reduced by 30 to 40% in Go2α−/− mice compared to their wild-type littermates (Fig. 3A and data not shown). Next, we compared the amounts of Golfα and Gsα in striatal membrane preparations from wild-type and Go2α−/− mice. A significant decrease in both G-protein subunits was observed in the knockout animals, whereas Gqα or Gβ subunits were not affected (Fig. 3B).
Figure 3.
Expression of trimeric G-protein subunits and D1- and D2-receptor proteins in wild-type (wt) and Go2α−/− mice. A) Ratio of Go1α and Go2α expression. Blots: synaptosomal fractions (P2) from whole brains of Go1α−/− (P20), Goα−/− (P30), or Go2α−/− (adult) and of the corresponding wt littermates were analyzed using the monoclonal antibody clone 101.1 (recognizing both splice variants), the Go2α-specific clone 101.4 (only applicable for Western blot analysis), and the sc13532 Goα antibody preferentially recognizing Go1α. Note that in Goα−/− preparations, none of the antibodies gives a signal, whereas signals for clone 101.4 or sc13532 antibodies are almost or completely absent in Go2α−/− or Go1α−/− mice, respectively. Go2α represents one-third of Goα in P20 (left bars) or adult (right bars) brains, as revealed by quantification using synaptobrevin (Syb) as an internal standard. Go1α is not up-regulated in Go2α−/− mice compared to wt littermates. Immunofluorescence: cerebellar sections were labeled by an antibody (clone 101.1), recognizing both Goα subunits (15). The mean immunofluorescence/100 μm2 of Goα subunits checked was significantly reduced in the molecular layer from 105.2 ± 6.8 in wt to 72.8 ± 9.4 in Go2α−/− mice and in the granular cell layer from 41.7 ± 9.0 (wt) to 21.9 ± 1.8 (knockout) (means ± sd). B) Quantification was performed with synaptosomal preparations from striata of four pairs of wt and Go2α−/− mice. The amounts of Golfα and Gsα, but not of Gqα or Gβ subunits, in general (Gβ1–4), were reduced in Go2α−/− mice. C) There was no significant difference in the amounts of D1- and D2-receptor protein between wt and Go2α−/− mice. For each quantification in B and C, 5, 10, and 15 μg of protein of the respective sample was loaded. Quantification is given as relative optical density (OD) and was performed using synaptophysin (Syp) or synaptobrevin (Syb) as an internal control. Graphs show the quantification from the 10 μg protein load. Values are expressed as means ± sd. *P < 0.05.
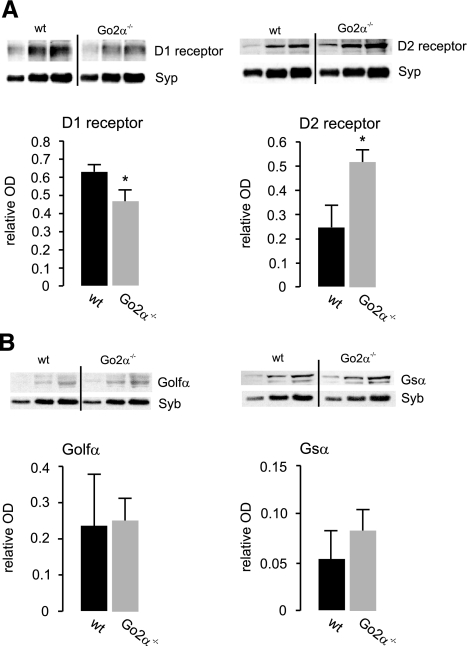
These data indicate a possible reduction in D1-receptor signaling in the knockout animals as a result of lowered levels of Golf and Gs. The amounts of D1 receptors showed a tendency toward being reduced that was not significant, whereas D2 receptors did not differ between genotypes (Fig. 3C). These initial findings were changed when analyzing striatal membrane preparations of the two genotypes following cocaine treatment. Now the amount of D1 receptor was significantly reduced, and the amount of D2 receptor was increased in Go2α−/− mice (Fig. 4A), whereas the differences in the protein levels of Golfα and Gsα were leveled out between the genotypes (Fig. 4B).
Figure 4.
Expression of dopamine (D1, D2) receptor proteins and G-protein α-subunits in wild-type (wt) and Go2α−/− mice following cocaine treatment. Western blot analyses were performed from postnuclear membrane preparation from frozen striata of both genotypes using cocaine-treated animals presented in Fig. 1. A) After treatment with cocaine, amounts of D1 receptor were lower and of D2 receptor were higher in striata of Go2α−/− mice compared to wt littermates. B) The expression of Golfα and Gsα leveled out between the genotypes following cocaine treatment. For each quantification, 5, 10, and 15 μg of protein of the respective sample was loaded; the graphs show the quantification from the 10-μg protein load. Quantification is given as relative OD and was performed using either Syp or Syb as an internal control. Values are expressed as means ± sd. *P < 0.05.
Reduced striatal dopamine, decreased amounts of TH, and increased amounts of VMAT2 in Go2α−/− mice
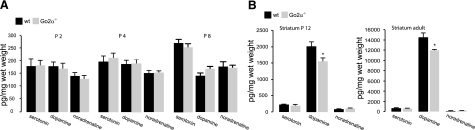
The data described so far suggest that lack of cocaine-induced sensitization in Go2α−/− mice is caused by an impairment of D1-receptor-mediated signaling and a shift toward D2-receptor-mediated effects. In addition to dopamine receptor and G-protein expression, we also analyzed the overall amounts of dopamine, noradrenaline, and serotonin, including their metabolites during postnatal development in Go2α−/− mice and their wild-type littermates. Analysis of whole brains from days P2, 4, and 8 revealed no differences between wild-type and knockout littermates (Fig. 5A). However, on P12 and in adult Go2α−/− mice, striatal dopamine levels were significantly reduced, while levels of serotonin and noradrenaline remained unchanged (Fig. 5B). Analysis of dopamine, noradrenaline, and serotonin in all other regions, including forebrain, hippocampus, bulbus olfactorius, hypothalamus, and cerebellum, revealed no differences between the genotypes (Supplemental Table 1).
Figure 5.
Monoamine levels in brain areas of wild-type (wt) and Go2α−/− mice. Serotonin, noradrenaline, and dopamine were analyzed in extracts from frozen brains or brain areas using HPLC. A) Until day P8, there are no significant differences in whole brain monoamine concentrations between wt and Go2α−/− mice. B) In the striatum of P12 and adult Go2α−/− animals, dopamine amounts are decreased compared to wt littermates. Values represent means ± se; n = 6. *P < 0.05.
To explore reasons for the reduced dopamine levels in the striatum, we examined whether synthesizing or degrading enzymes or an alteration of the respective transporters for dopamine are changed in Go2α−/− mice. We found reduced amounts of TH in Go2α−/− mice (Fig. 6A), but no changes in MOA A or B activities (Fig. 6B). The reduction in TH expression was not affected by cocaine (data not shown). As discussed in the introduction, TH is up-regulated in VMAT-deficient mice, suggesting that compensatory regulatory mechanisms exist to rescue an impairment of vesicular storage. The fact that TH is reduced in Go2α-deficient mice raised the question whether the reverse is also true, i.e., whether VMAT2 is up-regulated when TH levels are low. Indeed, VMAT2 amounts were increased in SV preparations from Go2α−/− mice (Fig. 6C), which may compensate for the reduced dopamine synthesis and allow for sufficient vesicular loading (see above). Comparable to the reduction of TH, up-regulation of VMAT2 was not affected by cocaine treatment (data not shown).
Figure 6.
Dopamine synthesizing and metabolizing enzymes, as well as transporters in wild-type (wt) and Go2α−/− mice. A) Amounts of TH and DAT were analyzed in synaptosomal preparations (whole brain) from wt and Go2α−/− mice. Amount of TH is reduced in Go2α−/− mice. Values represent means ± se; n = 4. B) Determination of MAO A + B activity in synaptosomes from wt and Go2α−/− mice revealed no difference between the genotypes. Values represent the means ± se; n = 3. C) VMAT2 expression in wt and Go2α−/− mice. Subcellular fractionation [H, homogenate; P2, synaptosomes; LP1, lysed synaptosomal pellet 1 at 29,000 g; LP2, lysed synaptosomal pellet 2 (SVs) at 360,000 g] of wt and Go2α−/− mice revealed that VMAT2 is enriched in the SV fraction (LP2) in both genotypes. Surprisingly, more VMAT2 is expressed in the SV fraction of Go2α−/− mice compared to wt littermates. For each quantification, 5, 10, and 15 μg of protein of the respective sample was loaded. Quantification is given as relative optical density (OD) and was performed using Syp as a reference. Graphs show the quantification from 10-μg protein load. Values represent means ± se; n = 4 animals/genotype. *P < 0.05.
The data so far show that Go2α−/− mice have diminished dopamine levels in the striatum. This reduction disturbs dopaminergic signaling and leads to alterations in the development of cocaine-induced sensitization. We next asked whether the down-regulation of TH and the resulting reduction of striatal dopamine levels are primarily caused by a dysregulation of vesicular dopamine storage.
Reduced vesicular monoamine uptake in Go2α−/− mice
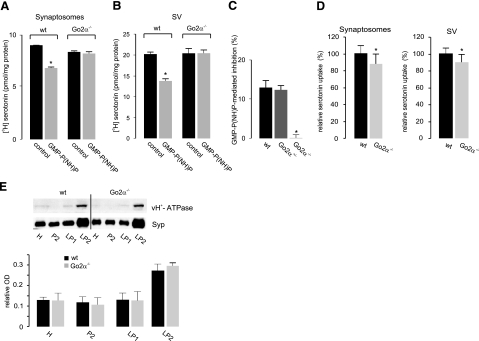
We have previously shown that persistent G-protein activation by the GTP-analog GMP-P(NH)P diminishes vesicular uptake of monoamines, as seen both in permeabilized synaptosomes and in isolated SVs. This effect of GMP-P(NH)P was absent in preparations from knockout animals and unchanged in preparations derived from heterozygous mice (Fig. 7A–C). These data show that Go2α is the neuronal regulator for vesicular monoamine storage, thereby confirming previous findings (17). However, the overall amount of monoamine uptake was slightly (∼10%) but significantly reduced in Go2α−/− mice, regardless of whether SLO-permeabilized synaptosomes or isolated SVs were analyzed (Fig. 7D).
Figure 7.
Go2α modulates [3H] monoamine uptake into VMAT2-containing SVs. A, B) SLO-permeabilized synaptosomes (A) or isolated SVs (B) of either wt or Go2α−/− mice were subjected to [3H] serotonin uptake in the absence or presence of 5 or 100 μM of GMP-P(NH)P, respectively. GMP-P(NH)P failed to modulate vesicular uptake in knockout animals. C) SVs from either wt, heterozygous, or knockout animals were subjected to serotonin uptake in the absence or presence of 100 μM GMP-P(NH)P. Values are expressed as the GMP-P(NH)P-mediated inhibition (%). Note that there is no inhibition in the knockout-derived SVs. D) [3H]serotonin uptake into permeabilized synaptosomes or isolated SVs is moderately decreased in Go2α−/− animals compared to wt littermates. Values were taken from 10 different experiments, each run in triplicate. The reserpine-sensitive uptake of wt animals was set at 100%. Bars indicate means ± sd. E) The amount of the vacuolar ATPase in various subcellular fractions analyzed using an antibody against the 116-kDa subunit revealed an enrichment in the SV fraction (LP2), with no difference between wt- and Go2α−/−-derived membranes. Graphs show the quantification from 10-μg protein load. Data were normalized to Syp; means ± sd; n = 4 animals/genotype. *P < 0.05.
Next, we tested whether uptake was reduced because of a perturbation of the electrochemical proton gradient ΔμH+ that drives monoamine uptake by VMAT2. In addition to providing the driving force for uptake, the low intravesicular pH also traps monoamines in the vesicles and thus may contribute to dopamine storage by an independent mechanism (27). No differences between wild-type and knockout animals were detected when the pH dependence of monoamine uptake was measured. Furthermore, the level of the vacuolar proton ATPase was unchanged (Fig. 7E and data not shown).
Taken together, loss of Go2α diminishes dopamine uptake by SVs by an as yet unknown mechanism.
DISCUSSION
The α-subunit of Go2 is an important regulator of dopamine homeostasis. Deletion of the protein results in an imbalance of dopamine pools due to altered levels of TH and VMAT2 and reduced uptake by SVs. As a consequence, cocaine-induced behavioral sensitization is abolished. These behavioral changes can be attributed to a diminished dopamine D1-receptor-mediated signal transduction via Golfα and Gsα.
Mechanisms underlying behavioral consequences
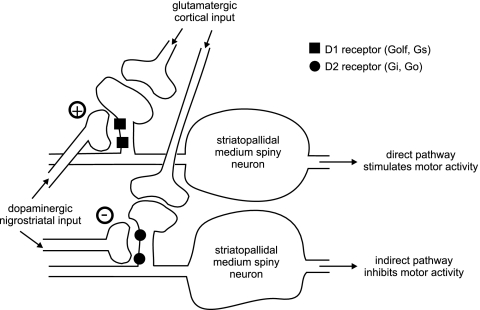
Motor activity is regulated by neuronal circuits involving the basal ganglia. Dopamine promotes motor activity by activating the D1-receptor-mediated direct pathway and by blocking the D2-receptor-mediated indirect pathway (Fig. 8). Actually, the D1 receptor has a low affinity, thus requiring high levels of dopamine to get activated. Because of the high affinity of D2 receptors, the indirect pathway is blocked, even under resting conditions when striatal dopamine concentration is low (Fig. 8).
Figure 8.
Effects of D1- and D2-receptor activation on motor activity. Dopamine enhances motor activity both by promoting the direct pathway via the stimulatory D1 receptor signaling and by slowing down the indirect pathway via the inhibitory D2-receptor signaling.
Expression of D1 receptor is decreased in Go2α−/− mice compared to wild-type animals, especially after cocaine treatment, indicating a reduction of activity within the D1-receptor pathway. As a consequence, the development of cocaine-induced behavioral sensitization is absent. This is the first phenotypical change that can be directly linked to Go2 at the systemic level. Drug-induced behavioral sensitization is a dopamine-dependent, very robust phenomenon that has been observed across several species and that is frequently used as a test for the initiation of a compulsive, drug-seeking behavior (28). The observed phenotypical changes in Go2α−/− mice were specific for locomotor-stimulating effects, because reinforcing effects of cocaine measured by a conditioned place preference test (23) revealed no differences in genotypes. In this context, it is important to note that cocaine reinforcement is not strictly dependent on dopamine. For example, DAT knockout animals develop normal conditioned place preference following cocaine application. Cocaine-induced place preference is only abolished in dopamine/serotonin transporter double knockouts (29). Furthermore, two recent meta-analyses showed that cocaine-induced dopamine overflow is linearly increased over a dose range of 5–30 mg/kg (30). However, cocaine-induced place preference does not parallel this dose-response relationship (31), demonstrating that dopamine levels do not correlate well with drug reinforcement measures.
Coupling of dopamine D1 receptors to adenylyl cyclase is mediated by Golfα and Gsα, and reduced striatal levels of these stimulatory G proteins have pronounced effects on locomotor response to psychostimulants (25, 26, 32). Psychostimulant-mediated effects appear to rely more on the presence of Golfα, because genetic down-regulation of Golfα, but not of D1 receptor, severely affects behavioral responses to cocaine (26). In line with this observation, Go2α −/− mice exhibit reduced Golfα levels before cocaine treatment and show a lack in the development of behavioral sensitization following repeated intermittent injections of cocaine. However, cocaine treatment offsets the reduced Golfα and Gsα levels in Go2α−/− mice, while D1 receptors are decreased, suggesting that these G proteins may also couple to other receptors. Indeed, following cocaine treatment, D2 receptors are higher in Go2α−/− mice compared to wild type. The differences in the amount of key molecules of dopamine signaling between wild-type and knockout animals under resting condition and following cocaine treatment are summarized in Table 1.
TABLE 1.
Differences in the amount of key molecules of the dopaminergic system between wild-type and Go2α−/− (knockout) mice
| Molecule | Untreated | Following cocaine treatment |
|---|---|---|
| D1 receptor | wt = ko | wt > ko |
| D2 receptor | wt = ko | wt < ko |
| Gsα | wt > ko | wt = ko |
| Golfα | wt > ko | wt = ko |
| TH | wt > ko | wt > ko |
| VMAT2 | wt < ko | wt < ko |
wt, wild type; ko, knockout.
Following cocaine treatment, the ratio between D1 and D2 receptors is lower in Go2α−/− compared to wild-type mice. This finding may be explained in two ways. An increase in functional D2 receptors could enhance the inhibition of the indirect pathway. This may provide a compensatory mechanism for a reduced stimulation of the direct pathway due to a lack of D1 receptor signaling. An increase of the interaction of D2 receptor with the NMDA receptor subunit NR2B after acute cocaine administration has been described recently (33). By complexing NR2B, the D2 receptor prevents the interaction of NR2B with the Ca2+/calmodulin-dependent protein kinase II and suppresses the inhibitory indirect pathway (33). This mechanism may amplify D2-receptor-mediated effects in Go2α−/− mice.
Alternatively, increased amounts of D2 receptor in Go2α−/− mice may reflect accumulated nonfunctional receptors, which lack G-protein coupling. In this scenario, an increase in D2-receptor expression represents an unsuccessful compensating mechanism in Go2α−/− mice. In addition, D2 receptors may not be able to interact with the NR2B receptor in the absence of Go2, thereby potentiating negative effects on motor activity following cocaine stimulation.
In summary, the absence of cocaine-induced sensitization is due to an imbalance in the dopamine system, caused by changes in both D1- and D2-receptor signaling.
Mechanisms involved in Go2α-mediated regulation of monoamine storage
Go2 heterotrimers are localized to SVs (10, 34), including monoaminergic vesicles (17). We could show in the past that Go2α or Gqα specifically modulate vesicular monoamine uptake mediated by VMAT2 in neurons or platelets, respectively (9, 11). In these in vitro approaches, both Gα subunits proved to be negative regulators of monoamine uptake. The loss of a GMP-P(NH)P-mediated effect in Go2α−/− mice clearly proves that Go2α regulates vesicular monoamine uptake. The absence of Go2 regulation decreases the overall monoamine uptake and probably increases the amount of cytosolic dopamine. The permanently increased concentration of dopamine in the cytosol may lead to a reduction of the expression of TH and increase the expression of VMAT2 in order to keep cytosolic dopamine concentrations tolerable, while sufficient vesicular loading is ensured. A correlation between VMAT2 and TH expression is also seen in VMAT2 deletion mutants that show an increase in TH expression (5). Thus, the ratio between cytosolic and vesicular dopamine appears to be a sensitive regulator for VMAT2 and TH expression and is altered in Go2α−/− mice.
Surprisingly, enhanced VMAT2 expression in Go2α deletion mutants is accompanied by reduced monoamine uptake, as well as striatal dopamine content in vivo (35). This discrepancy may be explained by two, so far, hypothetical and controversial models.
As one possibility, VMAT2 in the presence of nonactivated Go2 may be constitutively more active, whereas the activation of Go2α (mediated by the vesicular filling) reduces the activity of the transporter. In this scenario, the reduced uptake in Go2α−/− mice would reflect the dissociation of an activated Go2α from VMAT2 or from other vesicular compounds in wild-type mice. So far, we have not found an indication for a direct interaction between VMAT2 and Go2α, when performing immunoprecipitation using the respective antibodies. Besides VMAT2, other vesicular or vesicle-associated proteins may be bound by Go2 and released by an activation of the G protein subsequently reducing vesicular storage.
As a second possibility, Go2α regulates the set point of vesicular filling. Monoaminergic vesicles exhibit nonspecific leakage of transmitters not mediated by VMAT2. A high ΔpH provided by the proton pump and chloride transporters counteracts this leakage by trapping monoamines in their protonated form in the vesicular lumen. Increased amounts of VMAT2 increase quantal size, provided the driving force for uptake, that is, the H+ electrochemical gradient, is comparable. A slight decrease in the H+ electrochemical gradient caused by loss of Go2α could increase the efflux of monoamines, which may be counteracted by an increased expression of VMAT2 (27). So the decreased monoamine uptake observed in SVs of Go2α−/− mice may indicate a disturbance of the H+ electrochemical gradient. At the moment, we can only speculate on how and by which steps Go2α interferes with vesicular storage, but its main target appears to reside on the vesicle.
The present findings show the effects of subtle changes at the level of SVs for systemic functions. In conclusion, Go2α is responsible for the fine-tuning of vesicular dopamine content and is important for modulating the effects of psychostimulants like cocaine and probably also of other pharmacological compounds that do affect the striatal dopamine system.
Supplementary Material
Acknowledgments
This work was supported by the Deutsche Forshungsgemeinschaft (AH 67/3–3), the Forschungsförderung der Charité to G.A.H., by the German Federal Ministry of Education and Research (BMBF; Nationales Genomforschungsnetz) to R.S., and by the Intramural Research Program of the National Institutes of Health to L.B. (Z01ES01643). Technical assistance of Marion Möbes and Birgit Metze (both Charité) is gratefully acknowledged.
References
- Erickson J D, Schäfer M K, Bonner T I, Eiden L E, Weihe E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93:6166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl G R, Li S, Takahashi N, Itokawa K, Lin Z, Hazama M, Sora I. The VMAT2 gene in mice and humans: amphetamine responses, locomotion, cardiac arrhythmias, aging, and vulnerability to dopaminergic toxins. FASEB J. 2000;14:2459–2465. doi: 10.1096/fj.00-0205rev. [DOI] [PubMed] [Google Scholar]
- Gainetdinov R R, Sotnikova T D, Caron M G. Monoamine transporter pharmacology and mutant mice. Trends Pharmacol Sci. 2002;23:367–373. doi: 10.1016/s0165-6147(02)02044-8. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Miner L L, Sora I, Ujike H, Revay R S, Kostic V, Jackson-Lewis V, Przedborski S, Uhl G R. VMAT2 knockout mice: Heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci U S A. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y M, Gainetdinov P R, Fumagalli F, Xu F, Jones S R, Bock C B, Miller G W, Wightman R M, Caron M G. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine. Neuron. 1997;19:1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- German D C, McMillen B A, Sanghera M K, Saffer S I, Shore P A. Effects of severe dopamine depletion on dopamine neuronal impulse flow and on tyrosine hydroxylase regulation. Brain Res Bull. 1981;6:131–134. doi: 10.1016/s0361-9230(81)80037-8. [DOI] [PubMed] [Google Scholar]
- Brunk I, Blex C, Rachakonda S, Höltje M, Winter S, Pahner I, Walther D, Ahnert-Hilger G. The first lumenal domain of the vesicular monoamine transporter 2 mediates G-protein-dependent regulation of transmitter uptake. J Biol Chem. 2006;281:33373–33385. doi: 10.1074/jbc.M603204200. [DOI] [PubMed] [Google Scholar]
- Sternweis P C, Robishaw J D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- Ahnert-Hilger G, Höltje M, Pahner I, Winter S, Brunk I. Regulation of vesicular transmitter transporter. Rev Physiol Biochem Pharmacol. 2003;150:140–160. doi: 10.1007/s10254-003-0020-2. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke E A, Gronborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller S A, Rammner B, Gräter F, Hub J S, De Groot B L, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Brunk I, Höltje M, von Jagow B, Winter S, Sternberg J, Blex C, Pahner I, Ahnert-Hilger G. Regulation of vesicular monoamine and glutamate transporters by vesicle associated trimeric G proteins—new jobs for long known signal transduction molecules. Handbk Exp Pharmacol. 2006;175:305–325. doi: 10.1007/3-540-29784-7_15. [DOI] [PubMed] [Google Scholar]
- Jiang M, Gold M S, Boulay G, Spicher K, Pexton M, Brabet P, Srinivasan Y, Rudolph U, Ellison G, Birnbaumer L. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc Natl Acad Sci U S A. 1998;95:3269–3274. doi: 10.1073/pnas.95.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Lyubarsky A, Jiang M, Pugh E N, Birnbaumer L, Sterling P, Vardi N. The light responses of ON bipolar neurons require Gαo. J Neurosci. 2001;20:9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Jiang M S, Wang T L, Lyubarsky A, Savchenko A, Bar-Yehudopamine T, Sterling P, Birnbaumer L, Vardi N. Light response of retinal ON bipolar cells requires a specific splice variant of G alpha(o) J Neurosci. 2002;22:4878–4884. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter S, Brunk I, Walther D J, Höltje M, Jiang M, Peter J U, Takamori S, Jahn R, Birnbaumer L, Ahnert-Hilger G. G alpha(o2) regulates vesicular glutamate transporter activity by changing its chloride dependence. J Neurosci. 2005;25:4672–4680. doi: 10.1523/JNEUROSCI.0549-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Schiebler W, Oimet C, Greengard P. A 38,000 Dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985;82:4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje M, von Jagow B, Pahner I, Lautenschlager M, Hörtnagl H, Nürnberg B, Jahn R, Ahnert-Hilger G. The neuronal monoamine transporter VMAT2 is regulated by the trimeric GTPase Go(2) J Neurosci. 2000;20:2131–2141. doi: 10.1523/JNEUROSCI.20-06-02131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopoldt D, Harteneck C, Nürnberg B. G proteins endogenously expressed in Sf9 cells: interactions with mammalian histamine receptor. Naunyn-Schmiedebergs Arch Pharmacol. 1997;356:116–120. doi: 10.1007/pl00005044. [DOI] [PubMed] [Google Scholar]
- Weller U, Müller L, Messner M, Palmer M, Valeya A, Tranum-Jensen J, Agrawal P, Biermann C, Dobereiner A, Kehoe M A, Bhakdi S. Expression of active streptolysin O in Escherichia coli as a maltose-binding-protein-streptolysin-O-fusion protein. The N-terminal 70 amino acids are not required for hemolytic activity. Eur J Biochem. 1996;236:34–39. doi: 10.1111/j.1432-1033.1996.00034.x. [DOI] [PubMed] [Google Scholar]
- Becher A, Drenckhahn A, Pahner I, Margittai M, Jahn R, Ahnert-Hilger G. The synaptophysin-synaptobrevin complex: a hallmark of synaptic vesicle maturation. J Neurosci. 1999;19:1922–1931. doi: 10.1523/JNEUROSCI.19-06-01922.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörtnagl H, Berger M L, Havelec L, Hornykiewicz O. Role of glucocorticoids in the cholinergic degeneration in rat hippocampus induced by ethylcholine aziridinium (AF64A) J Neurosci. 1993;13:2939–2945. doi: 10.1523/JNEUROSCI.13-07-02939.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke T. Measuring reward with the conditioned place preference paradigm: update of the last decade. Addict Biol. 2007;12:227–463. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. Most central nervous system D2 dopamine recpetors are coupled to their effectors by Go. Proc Natl Acad Sci U S A. 2001;98:3577–3582. doi: 10.1073/pnas.051632598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Belluscio L, Hen R. G(olf)alpha mediates dopamine D1 receptor signaling. J Neurosci. 2000;20:91–95. doi: 10.1523/JNEUROSCI.20-16-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol J C, Valjent E, Pascoli V, Robin A, Stipanovich A, Luedtke R R, Belluscio L, Girault J A, Herve D. Quantitative changes in Gαolf protein levels, but not D1 receptor, alter specifically acute responses to psychostimulants. Neuropsychopharmacology. 2007;32:1109–1121. doi: 10.1038/sj.npp.1301230. [DOI] [PubMed] [Google Scholar]
- Edwards R H. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioral assessment of drug-reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Sora I, Hall F S, Andrews A M, Itokawa M, Li X F, Wei H B, Wichems C, Lesch K P, Murphy D L, Uhl G R. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci U S A. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S T, Krumm B, Spanagel R. Cocaine-induced dopamine overflow within the nucleus accumbens measured by in vivo microdialysis: A meta-analysis. Synapse. 2008;62:243–252. doi: 10.1002/syn.20489. [DOI] [PubMed] [Google Scholar]
- Bardo M T, Rowlett J K, Harris M J. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Hummel M, Unterwald E M. D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action. J Cell Physiol. 2002;191:17–27. doi: 10.1002/jcp.10078. [DOI] [PubMed] [Google Scholar]
- Liu X Y, Chu X P, Mao L M, Wang M, Lan H X, Li M H, Zhang G C, Parelkar N K, Fibuch E E, Haines M, Neve K A, Liu F, Xiong Z G, Wang J Q. Modulation of D2R-N2RB interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Pahner I, Höltje M, Winter S, Takamori S, Bellochio E, Spicher K, Laake P, Nürnberg B, Ottersen O P, Ahnert-Hilger G. Functional G-protein heterotrimers are associated with vesicles of putative glutamatergic terminals: implications for regulation of transmitter uptake. Mol Cell Neurosci. 2003;23:398–413. doi: 10.1016/s1044-7431(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Pothos E N, Larsen K E, Krantz D E, Liu Y-j, Haycock J W, Setlik W, Gershon M D, Edwards R H, Sulzer D. Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J Neurosci. 2000;20:7297–7306. doi: 10.1523/JNEUROSCI.20-19-07297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.