Abstract
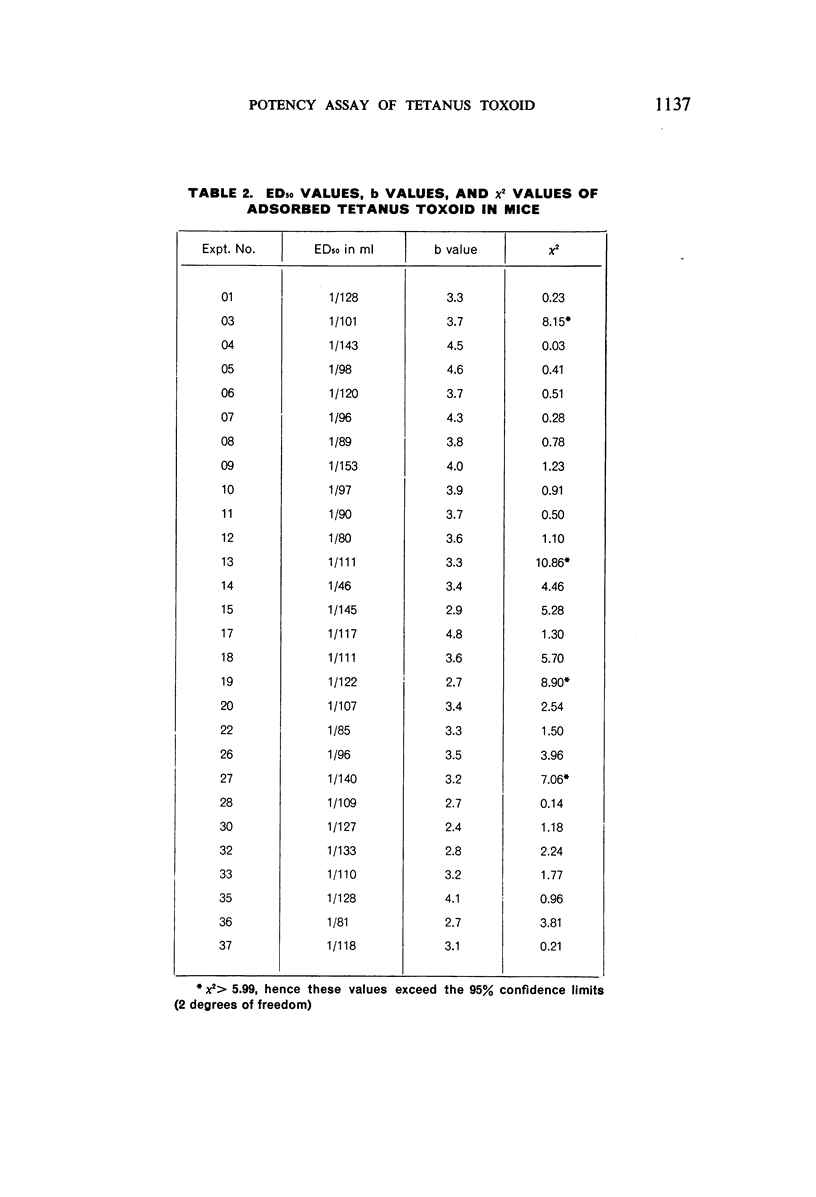
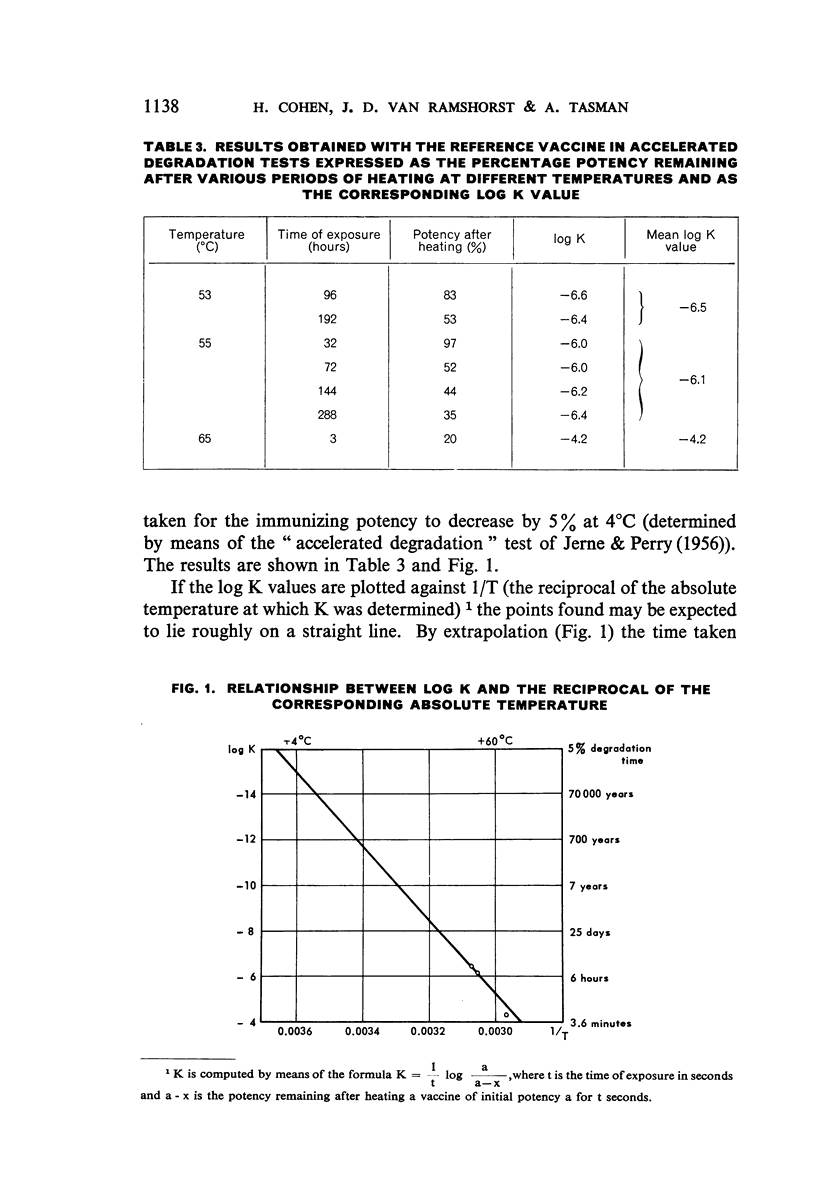
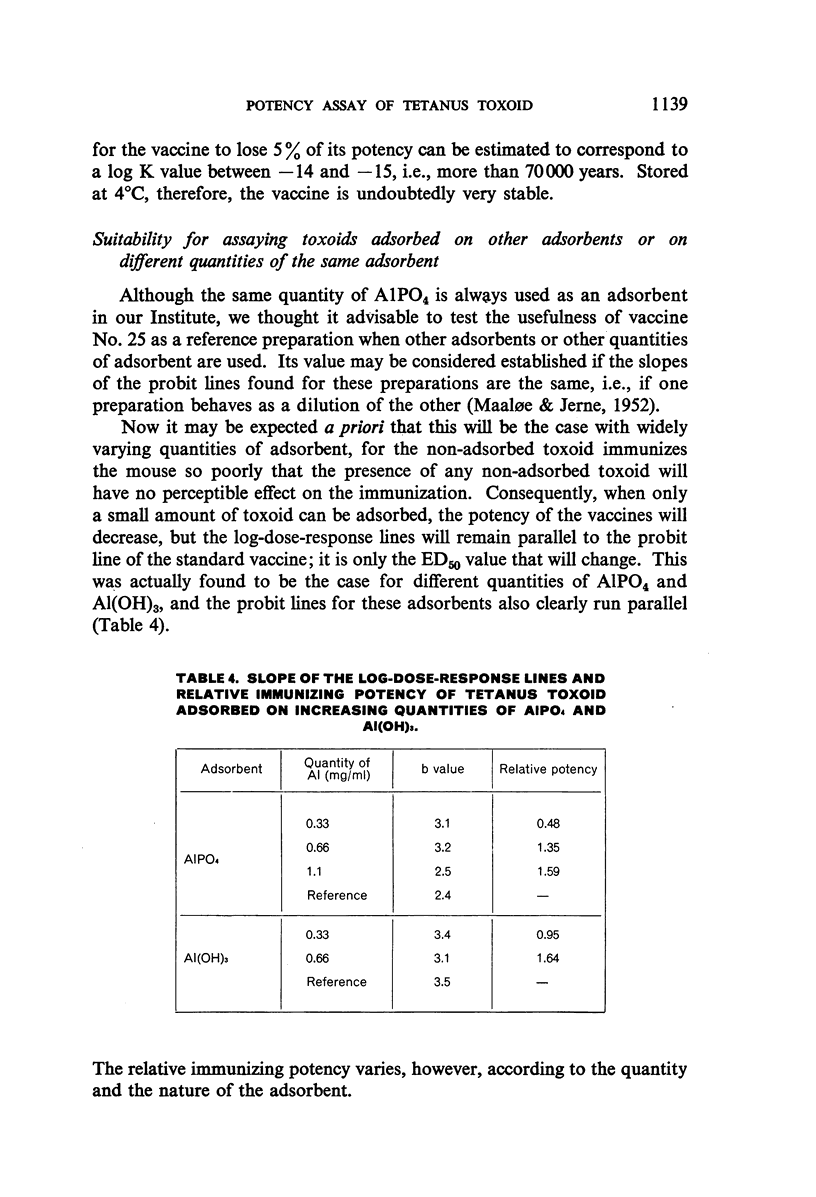
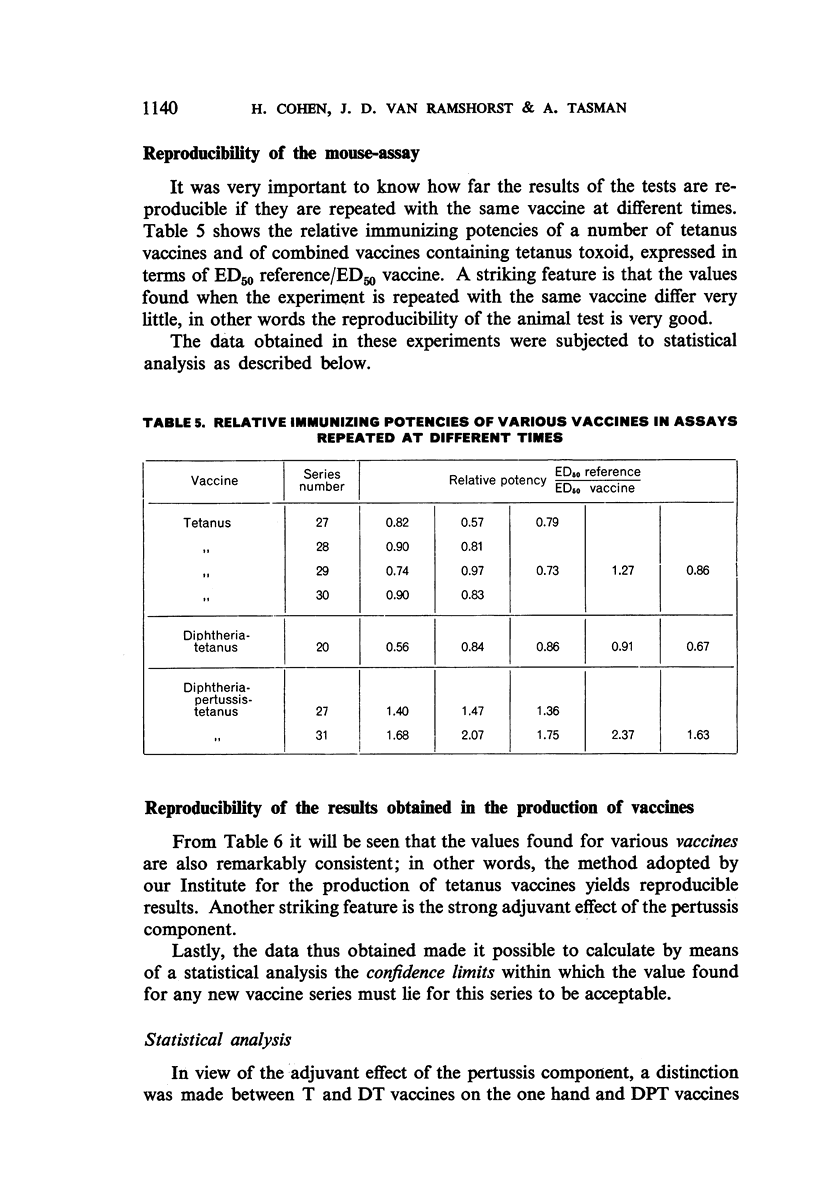
The use of mice for the assay of tetanus toxoids would offer considerable advantages over the use of guinea-pigs, but mice cannot readily be immunized with the fluid tetanus toxoid at present designated as the International Standard. This study shows, however, that the mouse is a very suitable laboratory animal for the comparison of adsorbed tetanus toxoids, and that an AlPO4-adsorbed vaccine, which is stable at 4°C, is a satisfactory reference preparation. The log-dose-response lines of toxoids adsorbed on different quantities of AlPO4 and on various quantities of another adsorbent ran parallel to those of the reference vaccine. The 95% confidence limits for the potencies of tetanus vaccines, diphtheria-tetanus vaccines, and diphtheria-pertussis-tetanus vaccines, determined by assay against the reference vaccine in mice, showed a high degree of reproducibility of the results.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMITAGE P., PERRY W. L. British standard for pertussis vaccine: its use in routine control of commercial vaccines. Br Med J. 1957 Aug 31;2(5043):501–505. doi: 10.1136/bmj.2.5043.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARR M., FULTHORPE A. J., LLEWELLYN-JONES M. The immunity responses of mice to injections of diphtheria and tetanus prophylactics. Br J Exp Pathol. 1957 Jun;38(3):312–318. [PMC free article] [PubMed] [Google Scholar]
- BROWN J. H., GERWE E. G., GREENBERG L., HENDRY J. L., HOHENADEL J., MASUCCI P., NEWMAN C., TAYLOR E. M. Precision of potency assay of alum-precipitated tetanus toxoid in mice; an inter-institutional study. J Immunol. 1953 Feb;70(2):171–180. [PubMed] [Google Scholar]
- COHEN H. H., LEPPINK G. J. Selection of Hemophilus pertussis strains for vaccine production in the mouse protection test in a balanced design; critical appreciation of the method. J Immunol. 1956 Nov;77(5):299–304. [PubMed] [Google Scholar]
- GREENBERG L., BENOIT R. Control of potency and the dosage of diphtheria and tetanus toxoids. J Am Med Assoc. 1956 Jan 14;160(2):108–113. doi: 10.1001/jama.1956.02960370018005. [DOI] [PubMed] [Google Scholar]
- IPSEN J., Jr Bio-assay of four tetanus toxoids (aluminum precipitated) in mice, guinea pigs and humans. J Immunol. 1953 Apr;70(4):426–434. [PubMed] [Google Scholar]
- JERNE N. K., PERRY W. L. The stability of biological standards. Bull World Health Organ. 1956;14(1):167–182. [PMC free article] [PubMed] [Google Scholar]
- LEVINE L., STONE J. L. Factors affecting the efficiency of combined prophylactics. I. Effect of diphtheria toxoid and pertussis vaccine on tetanus toxoid. J Immunol. 1954 Apr;72(4):258–262. [PubMed] [Google Scholar]
- LEVINE L., STONE J. L., WYMAN L. Factors affecting the efficiency of the aluminum adjuvant in diphtheria and tetanus toxoids. J Immunol. 1955 Oct;75(4):301–307. [PubMed] [Google Scholar]
- MAAL|OE O., JERNE N. K. The standardization of immunological substances. Annu Rev Microbiol. 1952;6:349–366. doi: 10.1146/annurev.mi.06.100152.002025. [DOI] [PubMed] [Google Scholar]
- MUELLER J. H., MILLER P. A. Variable factors influencing the production of tetanus toxin. J Bacteriol. 1954 Mar;67(3):271–277. doi: 10.1128/jb.67.3.271-277.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIGGE R. Die Beziehung zwischen dem Antigengehalt und der Wirksamkeit von Diphtherie- und Tetanus-Impfstoffen; Untersuchungen über eine biologische Konstante. Arb Paul Ehrlich Inst Georg Speyer Haus Ferdinand Blum Inst Frankf A M. 1954;51:108–123. [PubMed] [Google Scholar]
- PRIGGE R. Die Beziehung zwischen dem Antigengehalt und der Wirksamkeit von Diphtherie- und Tetanus-Impfstoffen; Untersuchungen über eine biologische Konstante. Arb Paul Ehrlich Inst Georg Speyer Haus Ferdinand Blum Inst Frankf A M. 1954;51:108–123. [PubMed] [Google Scholar]
- TASMAN A., VAN RAMSHORST J. D. Bereiding en eigenschappen van een nieuwe tetanusentstof, het tetanus-P.T. Geneeskd Gids. 1952 Oct 30;30(22):469–474. [PubMed] [Google Scholar]


