Abstract
Groups of adult humans, previously unexposed to rabies and with no history of rabies vaccination, were inoculated with different schedules of phenolized inactivated vaccine and Flury strain chicken-embryo vaccine, with or without one inoculation of hyperimmune serum. Serum specimens of the inoculated individuals were studied for antibody up to the 28th day following the first inoculation of the vaccines and serum. The results can be summarized as follows:
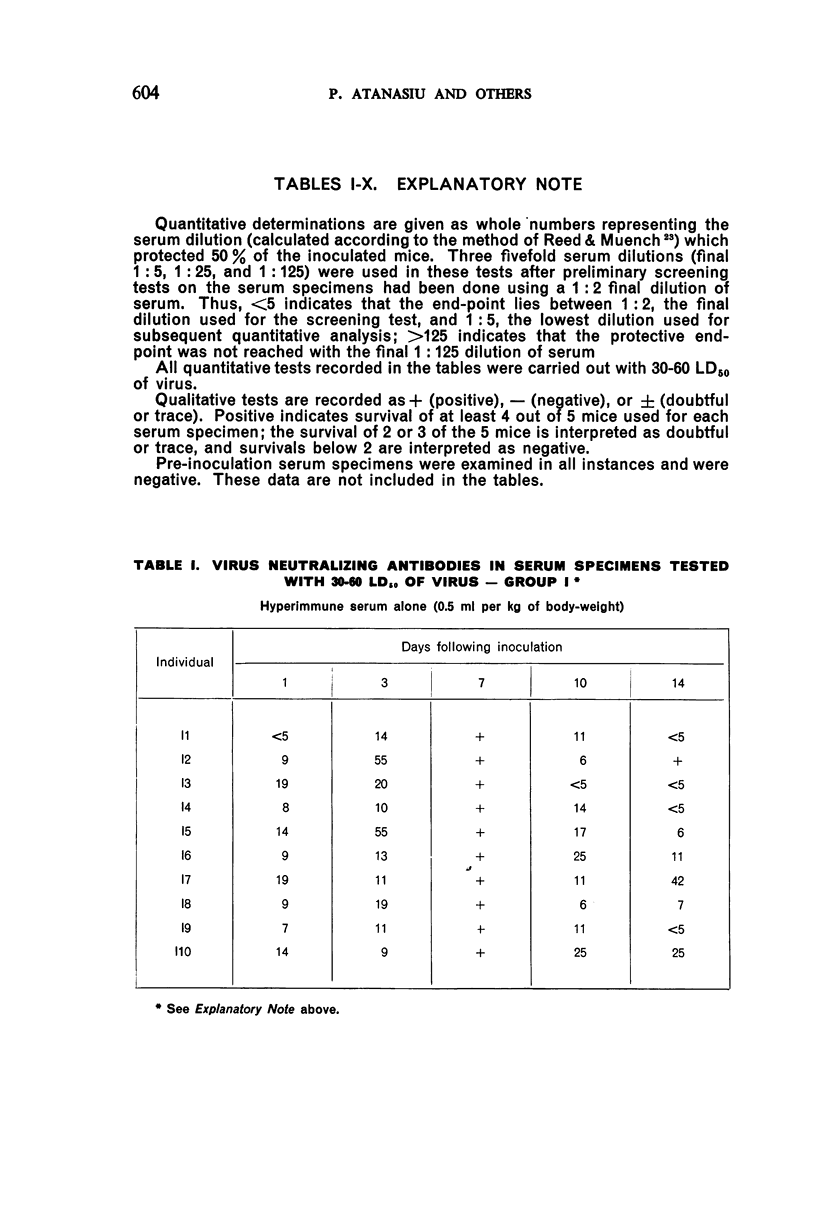
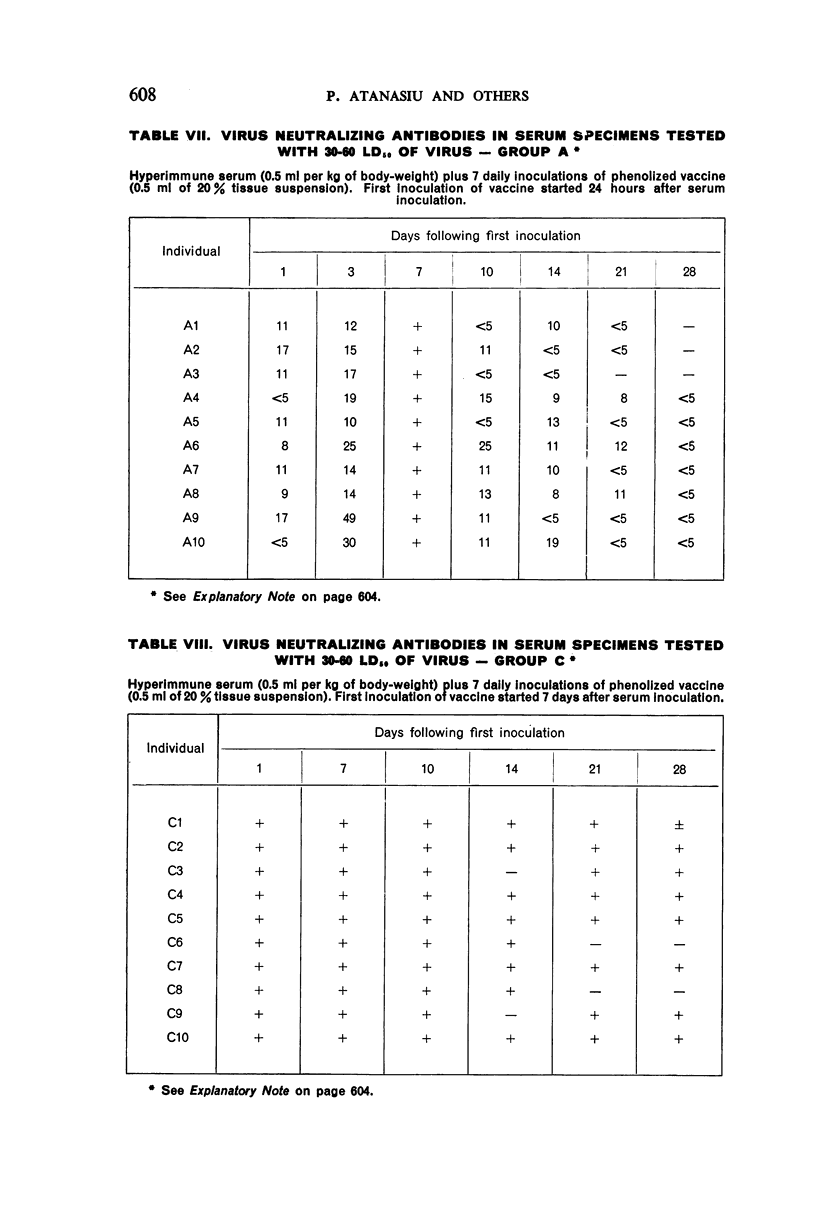
1. Passive antibody appeared in the blood-stream within one day following inoculation of hyperimmune serum. The antibody persisted at a good level for at least 10 days, but dropped slightly by the 14th day and was present in most individuals at the 21st day.
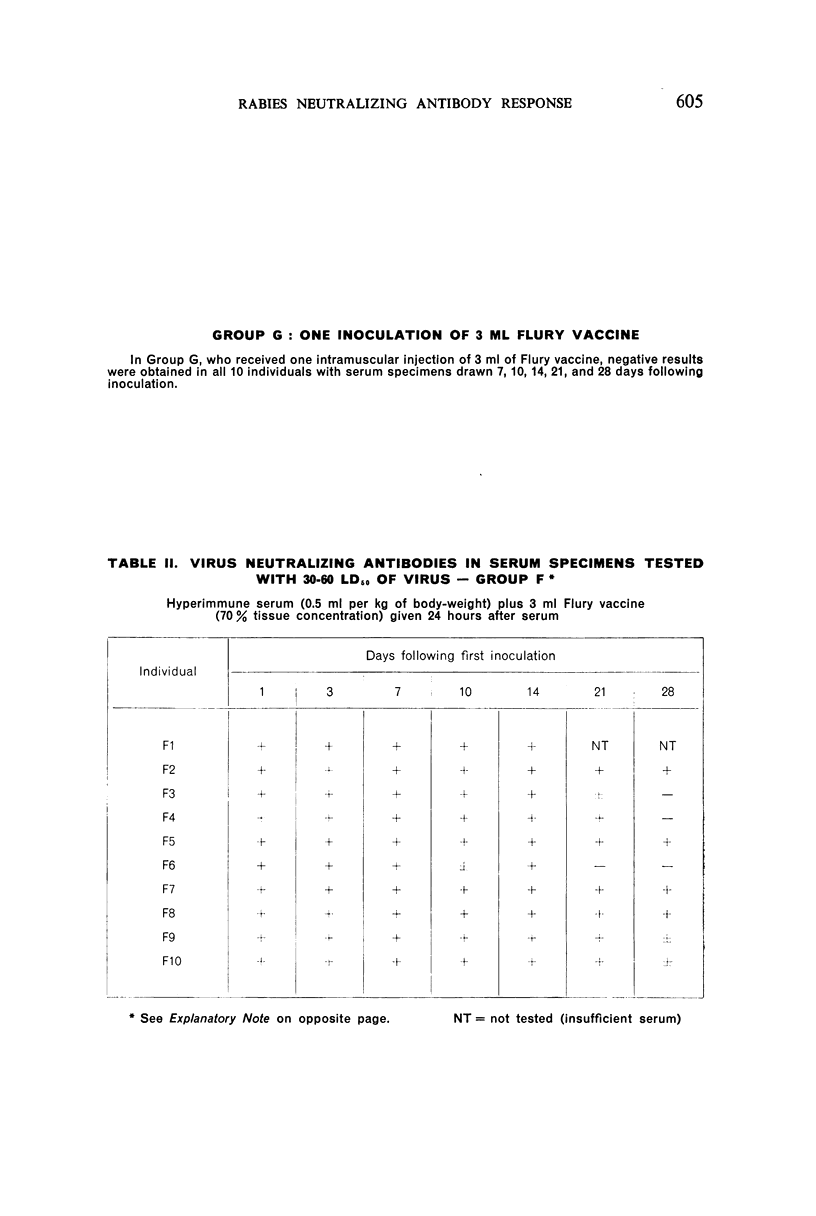
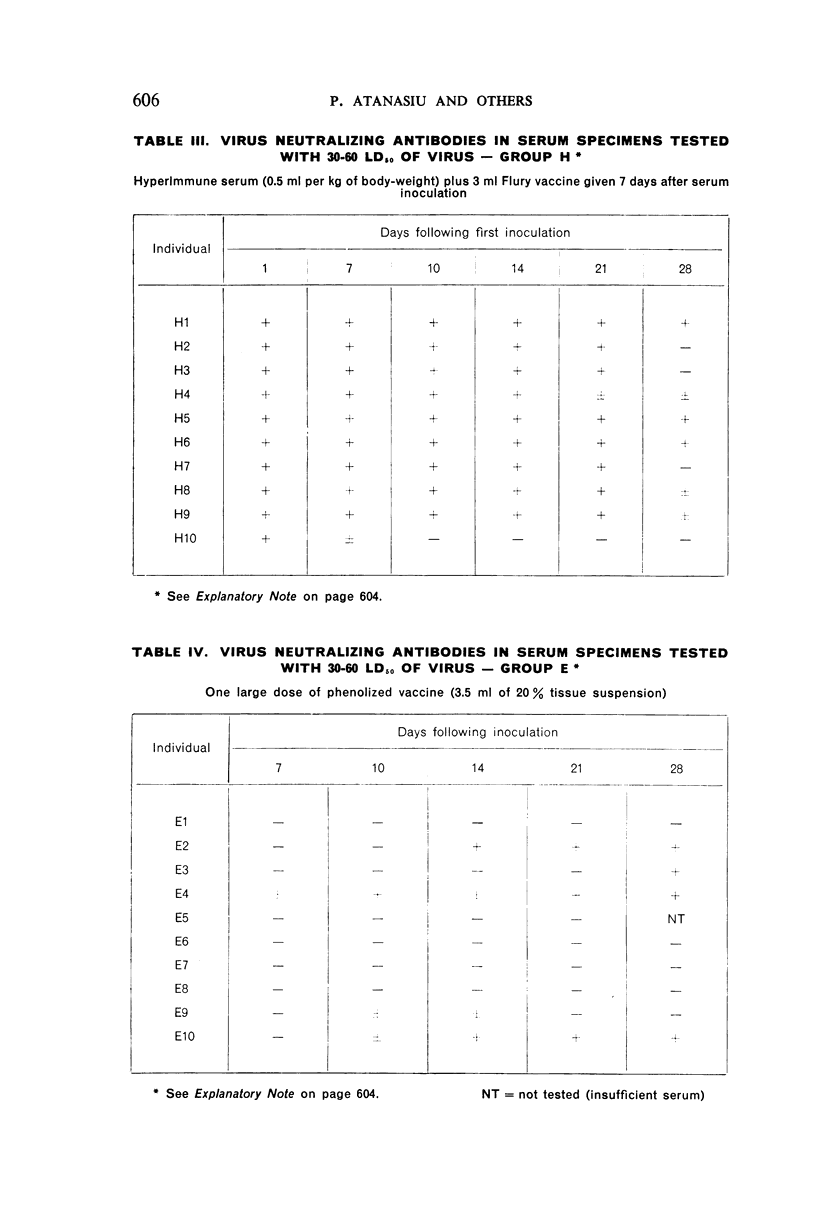
2. There was a tendency for the antibody levels at 21 and 28 days to be lower in the phenolized vaccine plus antiserum groups than in those groups which received phenolized vaccine alone.
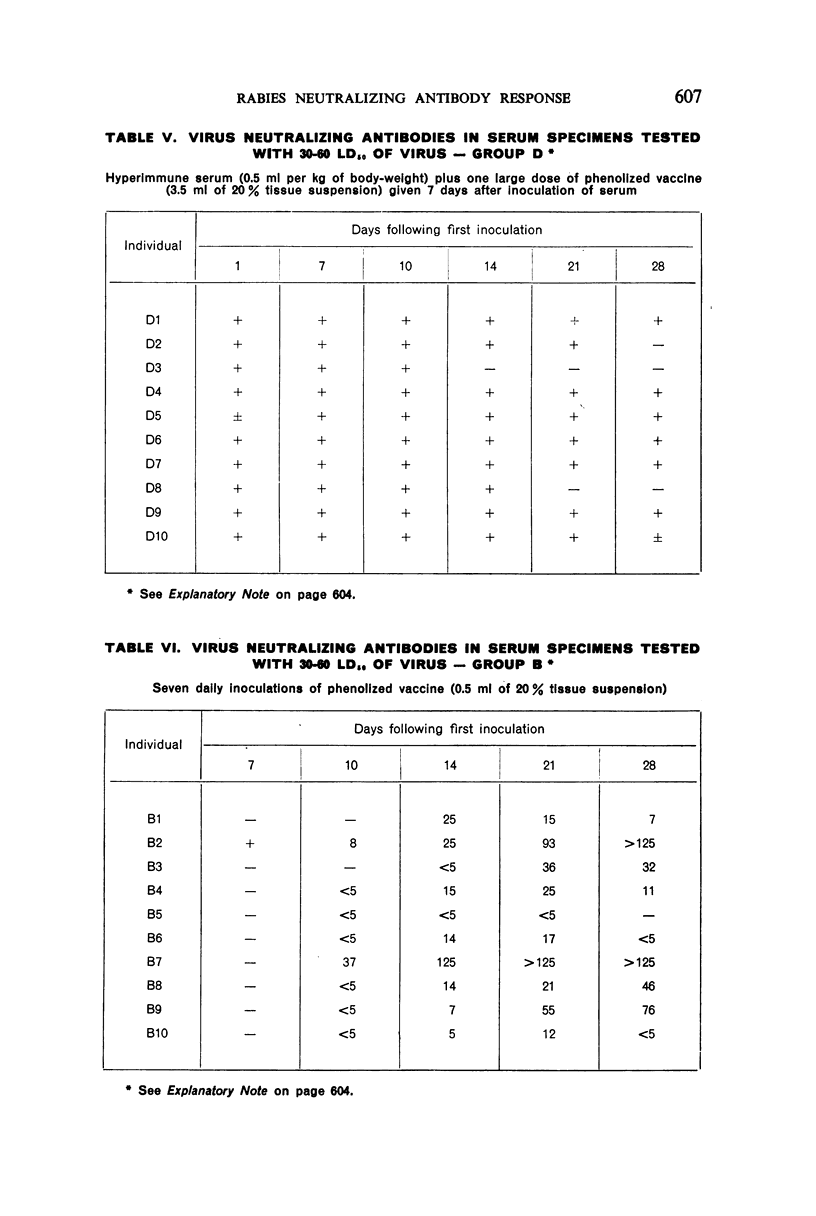
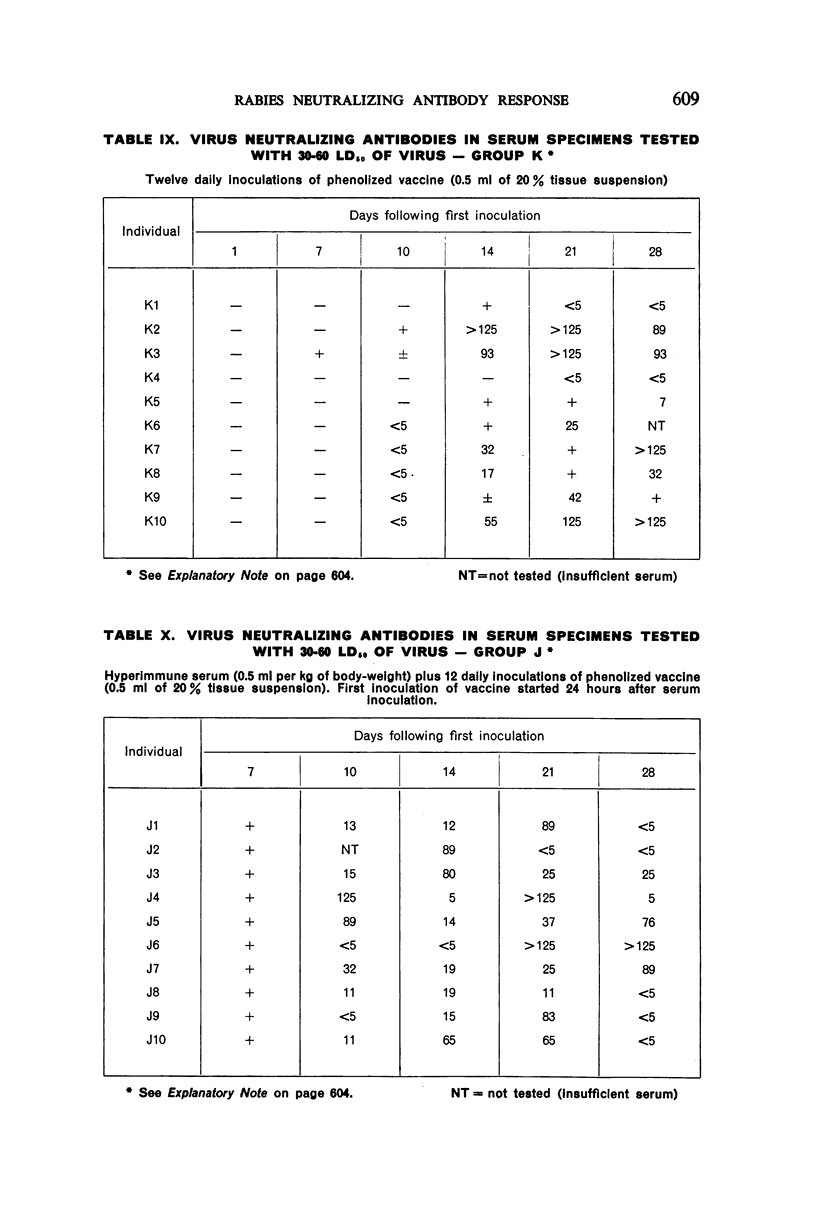
3. Seven or 12 daily inoculations of phenolized vaccine alone produced antibody in most instances by the 10th day and persisted generally through the 28th day.
4. Daily inoculations of phenolized vaccine produced a superior antibody response to that derived from the same total amount of vaccine given as a single inoculation.
5. A single inoculation intramuscularly of Flury strain chicken-embryo vaccine of high egg-passage did not produce detectable antibody.
6. The group which received hyperimmune serum followed by 12 daily inoculations of phenolized vaccine showed early and persistent antibody throughout the entire period of test. The antibody levels were comparable to those recently observed in man treated effectively with antiserum-vaccine combination after severe exposure to rabies.9
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELBAUM E., NELSON J. Neurological complications following antirabies vaccination. J Am Med Assoc. 1953 Jan 17;151(3):188–191. [PubMed] [Google Scholar]
- BALTAZARD M., BAHMANYAR M. Essai pratique du sérum antirabique chez les mordus par loups enragés. Bull World Health Organ. 1955;13(5):747–772. [PMC free article] [PubMed] [Google Scholar]
- BALTAZARD M., GHODSSI M. Prevention of human rabies; treatment of persons bitten by rabid wolves in Iran. Bull World Health Organ. 1954;10(5):797–803. [PMC free article] [PubMed] [Google Scholar]
- Casals J. INFLUENCE OF AGE FACTORS ON IMMUNIZABILITY OF MICE TO RABIES VIRUS. J Exp Med. 1940 Sep 30;72(4):453–461. doi: 10.1084/jem.72.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMEZ C., BLACK J., KOPROWSKI H. Rabies in cattle. III. Comparative studies on vaccination of cattle in Colombia with Flury virus and chloroform-inactivated vaccine. J Am Vet Med Assoc. 1955 Oct;127(943):360–363. [PubMed] [Google Scholar]
- HABEL K., KOPROWSKI H. Laboratory data supporting the clinical trial of anti-rabies serum in persons bitten by a rabid wolf. Bull World Health Organ. 1955;13(5):773–779. [PMC free article] [PubMed] [Google Scholar]
- JERVIS G. A. Experimental allergic encephalitis in animals, and its bearing upon the etiology of neuroparalytic accidents following antirabies treatment in man. Bull World Health Organ. 1954;10(5):837–844. [PMC free article] [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J. Studies on chick embryo adapted rabies virus. III. Duration of immunity in vaccinated dogs. Proc Soc Exp Biol Med. 1952 Jul;80(3):410–415. doi: 10.3181/00379727-80-19640. [DOI] [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J. Studies on chick-embryo-adapted rabies virus. IV. Immunization of guinea-pigs and description of a potency control test. J Immunol. 1954 Jan;72(1):79–84. [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J. Studies on chick-embryo-adapted rabies virus. V. Protection of animals with antiserum and living attenuated virus after exposure to street strain of rabies virus. J Immunol. 1954 Jan;72(1):85–93. [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J. Studies on chick-embryo-adapted rabies virus. VII. Immunological responses of animals to vaccination with high egg passage Flury strain. J Immunol. 1954 Jun;72(6):503–510. [PubMed] [Google Scholar]
- McFADZEAN A. J., CHOA G. H. The neuroparalytic accidents of antirabies vaccination. Trans R Soc Trop Med Hyg. 1953 Sep;47(5):372–386. doi: 10.1016/s0035-9203(53)80018-0. [DOI] [PubMed] [Google Scholar]
- Webster L. T. Diagnostic and Immunological Tests of Rabies in Mice. Am J Public Health Nations Health. 1936 Dec;26(12):1207–1210. doi: 10.2105/ajph.26.12.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]


