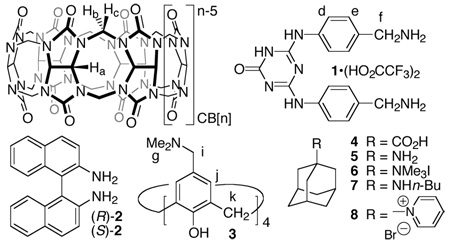
Abstract
Melamine diamine 1 is able to displace CB[5] from the CB[10]•CB[5] complex resulting in CB[10]•12 and precipitated CB[5]•1. We were able to isolate free CB[10] by treatment of CB[10]•1 with acetic anhydride followed by washing with MeOH, DMSO, and water. The spacious cavity of CB[10] is able to complex large guests including a cationic calix[4]arene derivative in its 1,3-alternate form (CB[10]•1,3-alt-3). The addition of adamantane carboxylic acid (4) to CB[10]•3 triggers a conformational change during the formation of termolecular complex CB[10]•cone-3•4.
In 1981, Mock disclosed the structure of cucurbit[6]uril (CB[6]) and subsequently delineated its outstanding binding properties toward ammonium ions in a series of elegant papers.1 Nearly 20 years later, the groups of Kim and Day reported the preparation and isolation of the CB[n] homologues CB[5], CB[7], CB[8] and CB[10] as its CB[10]•CB[5] inclusion complex.2 With their enhanced cavity size, the new members of the CB[n] family3 display a range of novel properties and applications including gas encapsulation, polarizability enhancement, and supramolecular dendrimer chemistry.4 Most notable, however, is the ability of CB[8] to simultaneously bind two aromatic guests which function as molecular machines in response to external stimuli.3b,5 In this paper we report the isolation of free CB[10] and disclose its unusual recognition properties. These results suggest that CB[10] will rival CB[8] for use as an advanced component for molecular machines and biomimetic systems.3,6
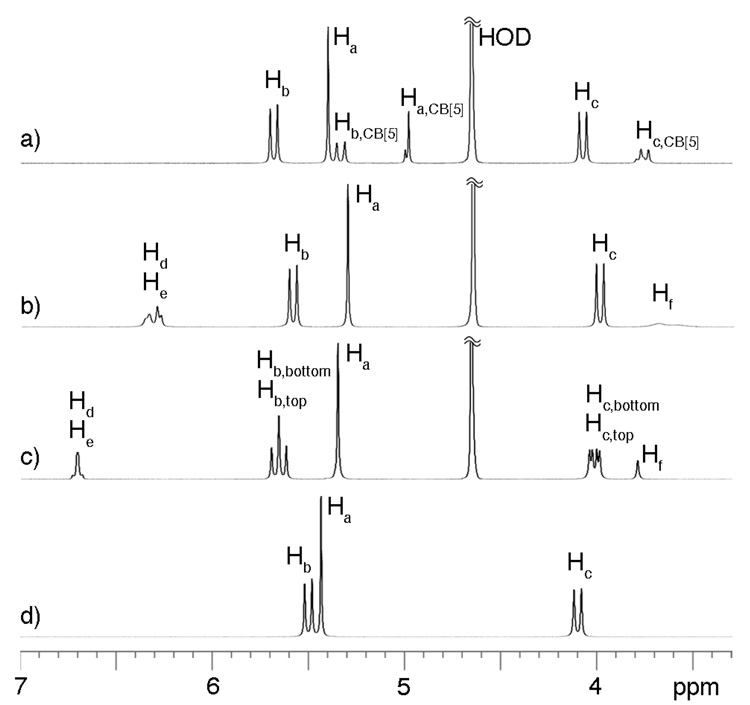
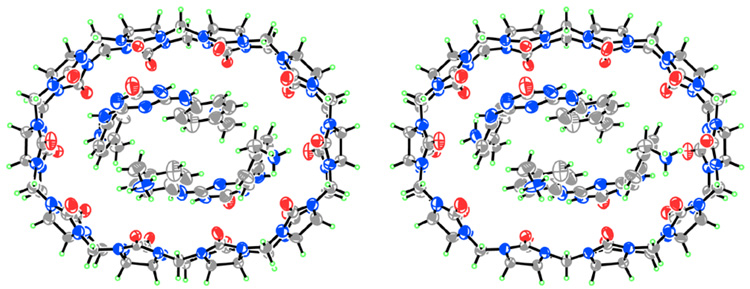
We isolated CB[10]•CB[5] in good quantities using a modification of the procedure reported by Day.2b,2c After much experimentation we discovered that treating a solution of CB[10]•CB[5] (Figure 1a) with a five equivalents of 1 results in the precipitation of the (CB[5]•1)n exclusion complex and the formation of the CB[10]•12 inclusion complex (Figure 1b). 1H NMR and x-ray crystallography indicates that 1 adopts a U-shape6 within the cavity of CB[10] (Figure 2); the two equivalents of 1 are arranged in a head-to-tail manner which results in a single set of resonances for Hb and Hc within CB[10]•12. The second equivalent of 1 is relatively weakly bound to CB[10] and can be removed by washing with MeOH to yield CB[10]•1 (Figure 1c). Once again, 1 adopts a U-shape within the CB[10]•1 complex; in this instance the top and bottom of CB[10] are differentiated and two sets of resonances are observed for Hb and Hc. Free CB[10] was obtained by heating CB[10]•1 in Ac2O followed by washing with (CH3)2SO, MeOH, and H2O (Figure 1d). CB[10] is quite stable in acidic solution (>1 month in 20% D2O/DCl at room temperature) which enabled our investigations of its molecular recognition properties.
Figure 1.
1H NMR spectra (400 MHz, D2O, 298 K) for: a) CB[10]•CB[5], b) CB[10]•12, c) CB[10]•1, d) CB[10] (20% D2O/DCl).
Figure 2.
Cross-eyed stereoview of the structure of CB[10]•12 in the crystal. Solvating water has been removed for clarity.
CB[10] is insoluble in D2O (< 50 µM) but its inclusion complexes often are nicely soluble which allows their characterization by NMR. Alternatively, CB[10] can be dissolved in 20% DCl / D2O for binding studies. An initial screen of many guests revealed that CB[10] – with its cavity volume of ≈ 870 Å3 – undergoes complexation with several chemically and biologically important substances (e.g. dyes, fluorophores, pharmaceuticals, and peptides) although some of these complexes occur as insoluble precipitates (Supporting Information). A soluble, kinetically stable complex was obtained with the more sizable and cationic guest (R)-2 which gave exclusively the termolecular complex CB[10]•(R)-22. Interestingly, when racemic (±)-2 was used, the racemic mixture of homochiral complexes (CB[10]•(R)-22 and CB[10]•(S)-22) was preferred relative to the heterochiral meso- complex (CB[10]•(R)-2•(S)-2) by a factor of three (Supporting Information). In combination, these results suggest that CB[10] may find application in drug delivery, for peptide sensing, and even to modulate the behavior of catalysts based on binaphthalene derived ligands.
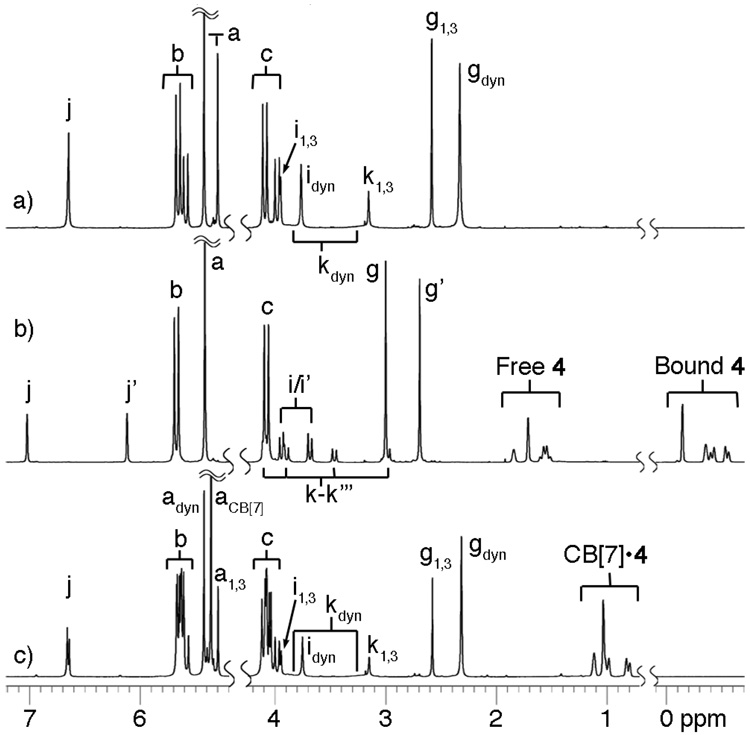
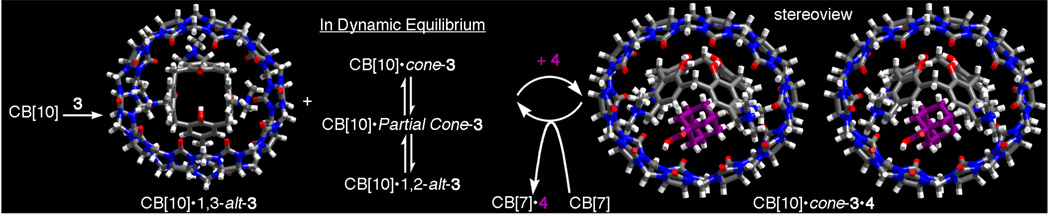
Given the vast size of the CB[10] cavity we envisioned the encapsulation of smaller host molecules like cyclodextrins, calixarenes, or even CB[6] that would merge the advantageous features of these host families. In the event, only cationic calix[4]arene derivative 3 formed a soluble stable complex (CB[10]•3 Figure 3a). Based on the number and multiplicity of resonances observed for CB[10]•3, we conclude that 3 adopts a mixture of the D2d-symmetric 1,3-alternate conformation and a rapidly equilibrating mixture of cone, 1,2-alternate and partial cone conformers within the CB[10] host. Intrigued by the possibility of using allosteric effects to control the conformation of the macromolecular complex7 we studied the binding of small molecule guests to CB[10]•3. We found that substituted adamantanes (4 – 8) – which do not bind to 3 alone – induce a dramatic change in the conformer distribution during the formation of CB[10]•cone-3•adamantane complexes (Figure 3b).8 Scheme 1 shows an MMFF minimized model of the CB[10]•cone-3•4 complex.9 One of the hallmarks of biological allostery is the reversible response of the system to activator concentration. For this purpose we added stoichiometric amounts of CB[7] which sequesters 4 as its CB[7]•4 complex3b,6d and resets the system to its original CB[10]•3 state (Figure 3c).
Figure 3.
1H NMR spectra recorded (400 MHz, D2O / DCl, RT) for: a) CB[10]•3 (1,3-alt and dynamic equilibrium beween cone, 1,2-alt and partial cone), b) CB[10]•cone-3•4 with excess 4 (0.8 equiv.), and c) CB[10]•3 and CB[7]•4. Subscripts: 1,3 = 1,3-alt-3; dyn = dynamic equilibrium of 3.
Scheme 1.
Allosteric control of the conformations of CB[10]•3 (MMFF minimized) with 4 (purple) and CB[7].
Just like the smaller CB[n] homologs, CB[10] retains the ability to bind a variety of chemically and biologically important cationic substances within its cavity. We have further demonstrated that CB[10] readily forms termolecular complexes (e.g. CB[10]•22 and CB[10]•cone-3•4); the vast cavity volume of CB[10] (≈ 870 Å3) suggests the potential formation of even higher molecularity complexes. The termolecular complexes already display a range of intriguing behavior including chiral recognition and efficient allosteric control of macromolecular geometry in response to a small molecule (e.g. 4). Overall, these results suggest that CB[10] will find broad application as an advanced component of molecular machines and biomimetic systems.
Supplementary Material
Synthetic procedures, characterization data for CB[10], and selected 1H NMR spectra for CB[10]•guest complexes (.pdf), and details of the x-ray structure of CB[10]•12 (.cif). This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgement
We thank the National Institutes of Health (GM61854) and the University of Maryland for financial support.
References
- 1.(a) Freeman WA, Mock WL, Shih N-Y. J. Am. Chem. Soc. 1981;103:7367–7368. [Google Scholar]; (b) Mock WL. Top. Curr. Chem. 1995;175:1–24. [Google Scholar]
- 2.(a) Kim J, Jung IS, Kim S-Y, Lee E, Kang J-K, Sakamoto S, Yamaguchi K, Kim K. J. Am. Chem. Soc. 2000;122:540–541. [Google Scholar]; (b) Day AI, Arnold AP, Blanch RJ, Snushall B. J. Org. Chem. 2001;66:8094–8100. doi: 10.1021/jo015897c. [DOI] [PubMed] [Google Scholar]; (c) Day AI, Blanch RJ, Arnold AP, Lorenzo S, Lewis GR, Dance I. Angew. Chem. Int. Ed. 2002;41:275–277. doi: 10.1002/1521-3773(20020118)41:2<275::aid-anie275>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.(a) Lagona J, Mukhopadhyay P, Chakrabarti S, Isaacs L. Angew. Chem. Int. Ed. 2005;44:4844–4870. doi: 10.1002/anie.200460675. [DOI] [PubMed] [Google Scholar]; (b) Lee JW, Samal S, Selvapalam N, Kim H-J, Kim K. Acc. Chem. Res. 2003;36:621–630. doi: 10.1021/ar020254k. [DOI] [PubMed] [Google Scholar]
- 4.(a) Miyahara Y, Abe K, Inazu T. Angew. Chem. Int. Ed. 2002;41:3020–3023. doi: 10.1002/1521-3773(20020816)41:16<3020::AID-ANIE3020>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (b) Kellersberger KA, Anderson JD, Ward SM, Krakowiak KE, Dearden DV. J. Am. Chem. Soc. 2001;123:11316–11317. doi: 10.1021/ja017031u. [DOI] [PubMed] [Google Scholar]; (c) Marquez C, Nau WM. Angew. Chem. Int. Ed. 2001;40:4387–4390. doi: 10.1002/1521-3773(20011203)40:23<4387::aid-anie4387>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]; (d) Moon K, Grindstaff J, Sobransingh D, Kaifer AE. Angew. Chem. Int. Ed. 2004;43:5496–5499. doi: 10.1002/anie.200460179. [DOI] [PubMed] [Google Scholar]
- 5.(a) Jeon WS, Kim E, Ko YH, Hwang I, Lee JW, Kim S-Y, Kim HJ, Kim K. Angew. Chem. Int. Ed. 2005;44:87–91. doi: 10.1002/anie.200461806. [DOI] [PubMed] [Google Scholar]; (b) Ko YH, Kim K, Kang J-K, Chun H, Lee JW, Sakamoto S, Yamaguchi K, Fettinger JC, Kim K. J. Am. Chem. Soc. 2004;126:1932–1933. doi: 10.1021/ja031567t. [DOI] [PubMed] [Google Scholar]; (c) Jeon WS, Ziganshina AY, Lee JW, Ko YH, Kang JK, Lee C, Kim K. Angew. Chem. Int. Ed. 2003;42:4097–4100. doi: 10.1002/anie.200351925. [DOI] [PubMed] [Google Scholar]; (d) Jeon YJ, Bharadwaj PK, Choi SW, Lee JW, Kim K. Angew. Chem. Int. Ed. 2002;41:4474–4476. doi: 10.1002/1521-3773(20021202)41:23<4474::AID-ANIE4474>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.(a) Lee H-K, Park KM, Jeon YJ, Kim D, Oh DH, Kim HS, Park CK, Kim K. J. Am. Chem. Soc. 2005;127:5006–5007. doi: 10.1021/ja042172s. [DOI] [PubMed] [Google Scholar]; (b) Jeon YJ, Kim H, Jon S, Selvapalam N, Oh DH, Seo I, Park C-S, Jung SR, Koh D-S, Kim K. J. Am. Chem. Soc. 2004;126:15944–15945. doi: 10.1021/ja044748j. [DOI] [PubMed] [Google Scholar]; (c) Braha O, Webb J, Gu L-Q, Kim K, Bayley H. ChemPhysChem. 2005;6:889–892. doi: 10.1002/cphc.200400595. [DOI] [PubMed] [Google Scholar]; (d) Liu S, Ruspic C, Mukhopadhyay P, Chakrabarti S, Zavalij PY, Isaacs L. J. Am. Chem. Soc. 2005;127 doi: 10.1021/ja055013x. ASAP. [DOI] [PubMed] [Google Scholar]
- 7.Castellano RK, Rudkevich DM, Rebek J., Jr J. Am. Chem. Soc. 1996;118:10002–10003. [Google Scholar]
- 8.Addition of 1 equiv. 4 to a solution of CB[10]•3 (500 µM) results in ≊ 90% formation of CB[10] • cone-3•4 reflecting the strong binding of 4 to CB[10] •3. A larger number of equivalents of 5 – 8 are required to complete the conformation change presumably because of weaker binding interactions of tetracationic CB[10] •3 with these cationic guests.
- 9.The 1H NMR spectrum of CB[10]•cone-3•4 does not show doubling of the Hg and Hj resonances as expected for the geometry shown in Scheme 1. We attribute this result to a dynamic process in which 4 reorients its CO2H group between the two portals rapidly on the chemical shift timescale. The bulkier adamantanes 7 and 8 display two sets of resonances as expected.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthetic procedures, characterization data for CB[10], and selected 1H NMR spectra for CB[10]•guest complexes (.pdf), and details of the x-ray structure of CB[10]•12 (.cif). This material is available free of charge via the internet at http://pubs.acs.org.