Abstract
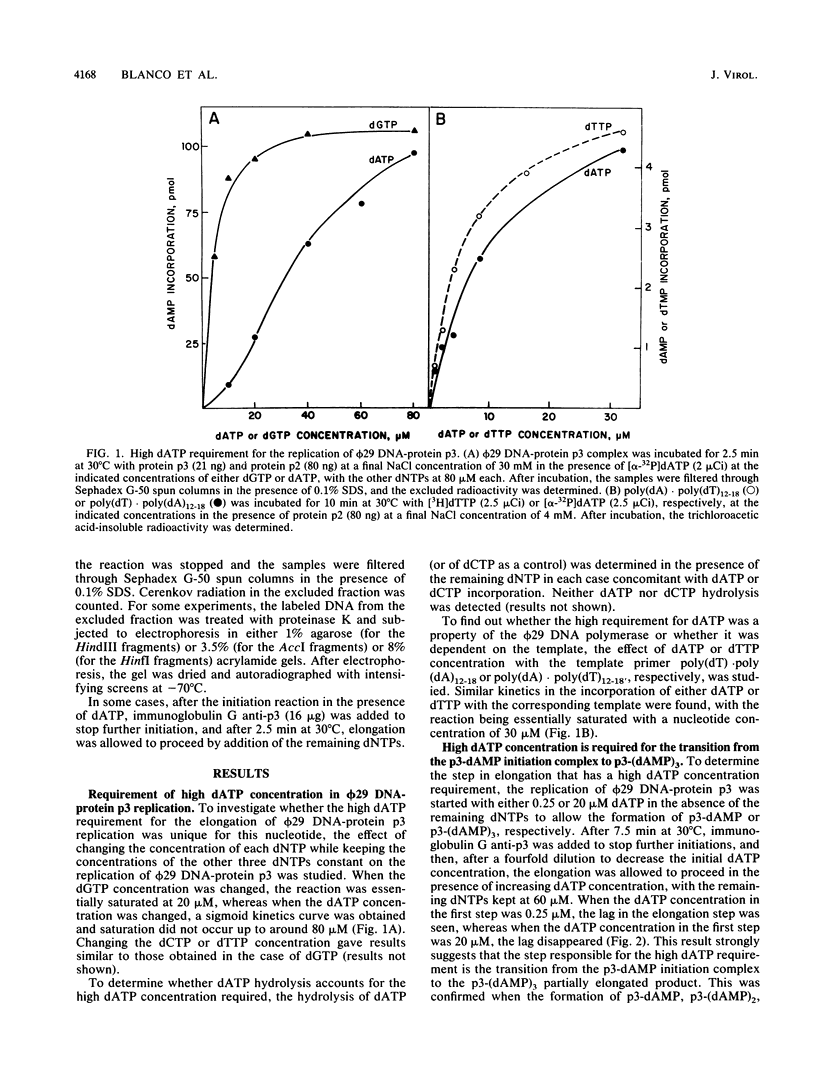
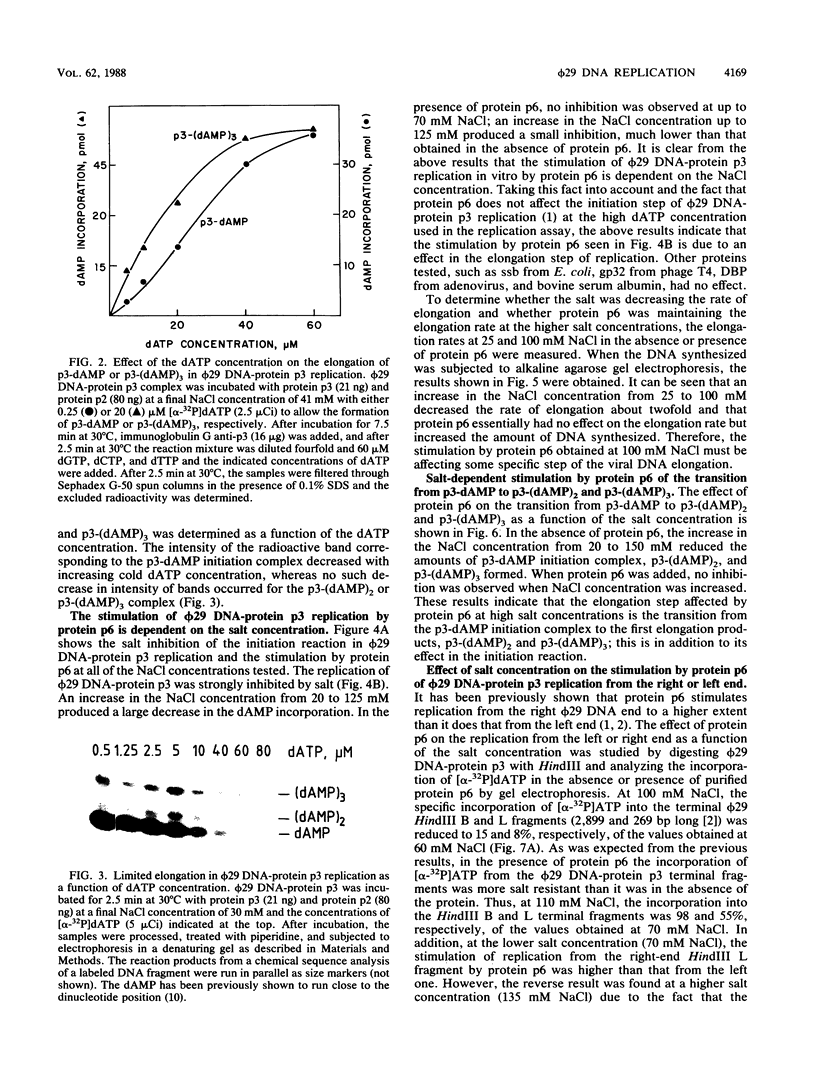
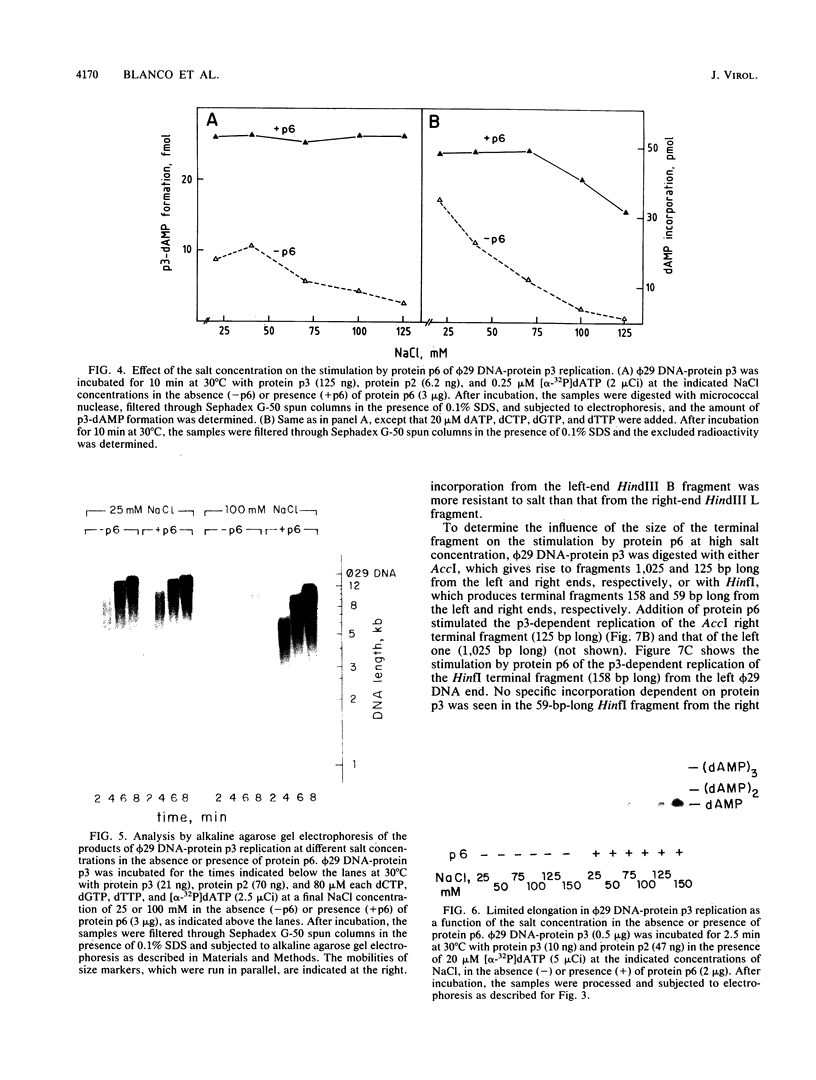
The transition step from the p3-dAMP initiation complex to the first elongated products, p3-(dAMP)2 and p3-(dAMP)3, requires a dATP concentration higher than that needed for the initiation reaction or for the further elongation of the p3-(dAMP)3 complex. The elongation in phi 29 DNA-protein p3 replication in vitro was strongly inhibited by salt. Under inhibitory salt concentration, the viral protein p6 greatly stimulated phi 29 DNA-protein p3 replication. The effect of protein p6 was not on the rate of elongation but on the amount of elongated product, stimulating the transition from initiation to formation of the first elongation products.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanco L., Gutiérrez J., Lázaro J. M., Bernad A., Salas M. Replication of phage phi 29 DNA in vitro: role of the viral protein p6 in initiation and elongation. Nucleic Acids Res. 1986 Jun 25;14(12):4923–4937. doi: 10.1093/nar/14.12.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Prieto I., Gutiérrez J., Bernad A., Lázaro J. M., Hermoso J. M., Salas M. Effect of NH4+ ions on phi 29 DNA-protein p3 replication: formation of a complex between the terminal protein and the DNA polymerase. J Virol. 1987 Dec;61(12):3983–3991. doi: 10.1128/jvi.61.12.3983-3991.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Characterization and purification of a phage phi 29-encoded DNA polymerase required for the initiation of replication. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5325–5329. doi: 10.1073/pnas.81.17.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Replication of phage phi 29 DNA with purified terminal protein and DNA polymerase: synthesis of full-length phi 29 DNA. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6404–6408. doi: 10.1073/pnas.82.19.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrascosa J. L., Camacho A., Moreno F., Jiménez F., Mellado R. P., Viñuela E., Salas M. Bacillus subtilis phage phi29. Characterization of gene products and functions. Eur J Biochem. 1976 Jul 1;66(2):229–241. doi: 10.1111/j.1432-1033.1976.tb10512.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mellado R. P., Peñalva M. A., Inciarte M. R., Salas M. The protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi 29 is involved in the initiation of DNA replication. Virology. 1980 Jul 15;104(1):84–96. doi: 10.1016/0042-6822(80)90367-0. [DOI] [PubMed] [Google Scholar]
- Pastrana R., Lázaro J. M., Blanco L., García J. A., Méndez E., Salas M. Overproduction and purification of protein P6 of Bacillus subtilis phage phi 29: role in the initiation of DNA replication. Nucleic Acids Res. 1985 May 10;13(9):3083–3100. doi: 10.1093/nar/13.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñalva M. A., Salas M. Initiation of phage phi 29 DNA replication in vitro: formation of a covalent complex between the terminal protein, p3, and 5'-dAMP. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5522–5526. doi: 10.1073/pnas.79.18.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto I., Lázaro J. M., García J. A., Hermoso J. M., Salas M. Purification in a functional form of the terminal protein of Bacillus subtilis phage phi 29. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1639–1643. doi: 10.1073/pnas.81.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto I., Serrano M., Lázaro J. M., Salas M., Hermoso J. M. Interaction of the bacteriophage phi 29 protein p6 with double-stranded DNA. Proc Natl Acad Sci U S A. 1988 Jan;85(2):314–318. doi: 10.1073/pnas.85.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas M. A new mechanism for the initiation of replication of phi 29 and adenovirus DNA: priming by the terminal protein. Curr Top Microbiol Immunol. 1984;109:89–106. doi: 10.1007/978-3-642-69460-8_4. [DOI] [PubMed] [Google Scholar]
- Salas M., Mellado R. P., Viñuela E. Characterization of a protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1978 Feb 25;119(2):269–291. doi: 10.1016/0022-2836(78)90438-2. [DOI] [PubMed] [Google Scholar]
- Talavera A., Salas M., Viñuela E. Temperature-sensitive mutants affected in DNA synthesis in phage phi29 of Bacillus subtilis. Eur J Biochem. 1972 Dec 4;31(2):367–371. doi: 10.1111/j.1432-1033.1972.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Vlcek C., Paces V. Nucleotide sequence of the late region of Bacillus phage phi 29 completes the 19,285-bp sequence of phi 29 genome. Comparison with the homologous sequence of phage PZA. Gene. 1986;46(2-3):215–225. doi: 10.1016/0378-1119(86)90406-3. [DOI] [PubMed] [Google Scholar]
- Watabe K., Leusch M., Ito J. Replication of bacteriophage phi 29 DNA in vitro: the roles of terminal protein and DNA polymerase. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5374–5378. doi: 10.1073/pnas.81.17.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley H. R., Ramey W. D., Spiegelman G. B., Holder R. D. Modulation of in vivo and in vitro transcription of bacteriophage phi 29 early genes. Virology. 1986 Dec;155(2):392–401. doi: 10.1016/0042-6822(86)90202-3. [DOI] [PubMed] [Google Scholar]






