Abstract
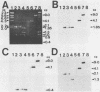
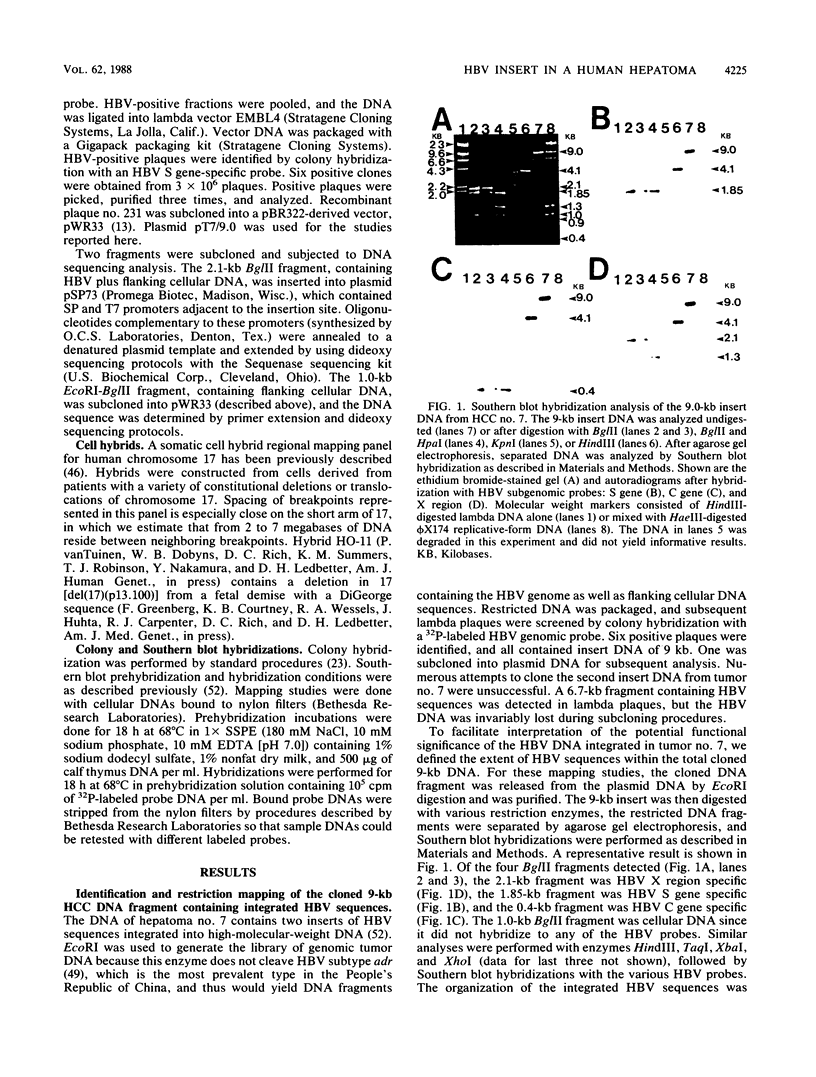
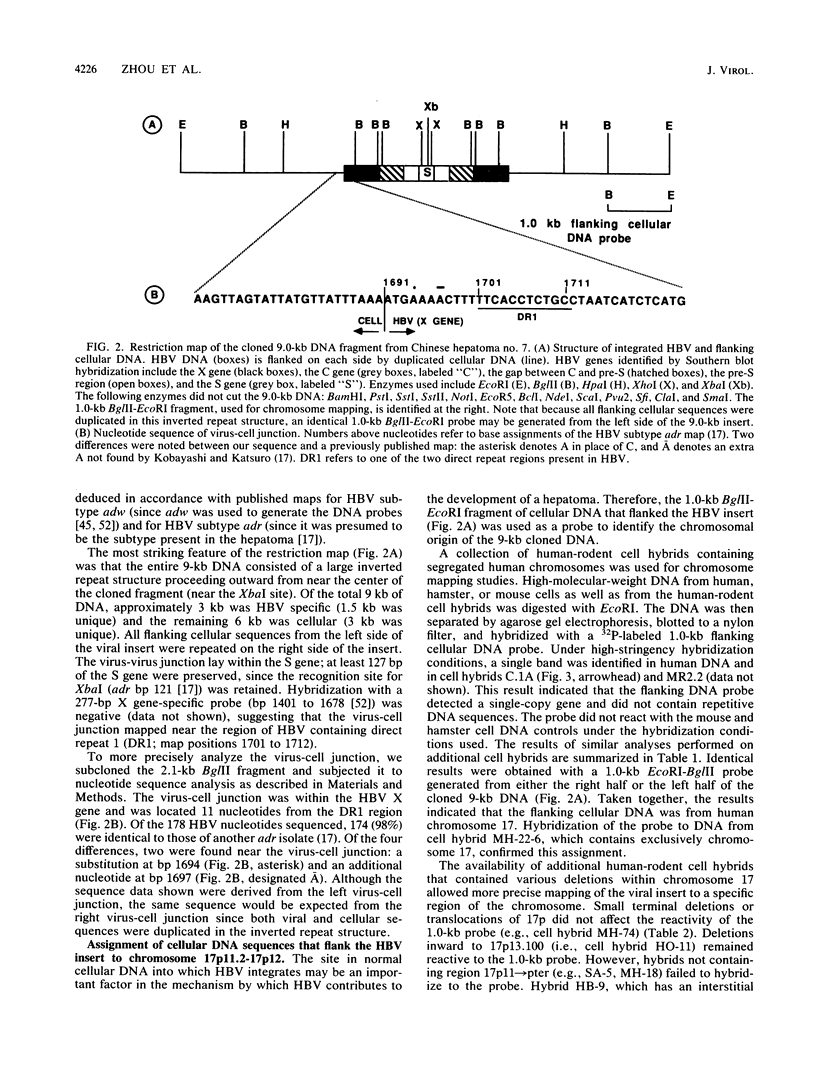
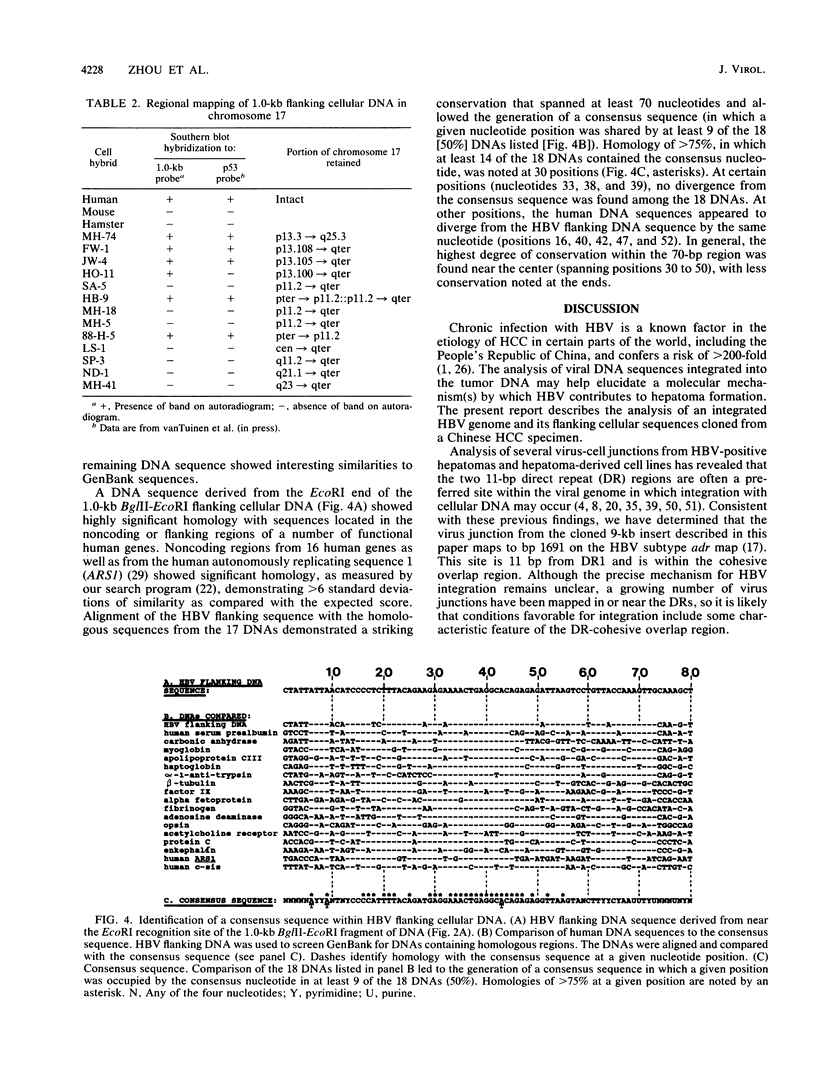
Hepatitis B virus (HBV) is clearly a factor in the development of hepatocellular carcinoma, but its mechanism of action remains obscure. One possibility is that the HBV integration event alters the expression of a nearby growth-regulatory cellular gene. A 9-kilobase (kb) DNA fragment containing an HBV insert plus flanking cellular sequences was cloned from a hepatoma specimen from Shanghai, People's Republic of China. Restriction mapping of the insert revealed a large inverted repeat structure consisting of both viral sequences (encompassing all of the core and pre-S regions and portions of the X and S genes) and at least 3 kb of unique cellular sequences. The virus-cell junction mapped 11 nucleotides from the DR1 region, in a position within the HBV X gene and included in the cohesive overlap region. A probe generated from 1.0 kb of the flanking cellular DNA mapped the viral insert to chromosome 17 in the region designated 17p11.2-17p12, which is near the human proto-oncogene p53. Sequence data from a portion of the flanking cellular DNA revealed a stretch of approximately 70 base pairs that showed highly significant homology with a conserved region of a number of functional mammalian DNAs, including the human autonomously replicating sequence 1 (ARS1).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Hammer R. E., Brinster R. L., Palmiter R. D., Townes T. M. Two 3' sequences direct adult erythroid-specific expression of human beta-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7056–7060. doi: 10.1073/pnas.84.20.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulla G. A., Siddiqui A. The hepatitis B virus enhancer modulates transcription of the hepatitis B virus surface antigen gene from an internal location. J Virol. 1988 Apr;62(4):1437–1441. doi: 10.1128/jvi.62.4.1437-1441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron de Fromentel C., May-Levin F., Mouriesse H., Lemerle J., Chandrasekaran K., May P. Presence of circulating antibodies against cellular protein p53 in a notable proportion of children with B-cell lymphoma. Int J Cancer. 1987 Feb 15;39(2):185–189. doi: 10.1002/ijc.2910390211. [DOI] [PubMed] [Google Scholar]
- Choo K. B., Liu M. S., Chang P. C., Wu S. M., Su M. W., Pan C. C., Han S. H. Analysis of six distinct integrated hepatitis B virus sequences cloned from the cellular DNA of a human hepatocellular carcinoma. Virology. 1986 Oct 30;154(2):405–408. doi: 10.1016/0042-6822(86)90467-8. [DOI] [PubMed] [Google Scholar]
- Dejean A., Bougueleret L., Grzeschik K. H., Tiollais P. Hepatitis B virus DNA integration in a sequence homologous to v-erb-A and steroid receptor genes in a hepatocellular carcinoma. Nature. 1986 Jul 3;322(6074):70–72. doi: 10.1038/322070a0. [DOI] [PubMed] [Google Scholar]
- Dejean A., Brechot C., Tiollais P., Wain-Hobson S. Characterization of integrated hepatitis B viral DNA cloned from a human hepatoma and the hepatoma-derived cell line PLC/PRF/5. Proc Natl Acad Sci U S A. 1983 May;80(9):2505–2509. doi: 10.1073/pnas.80.9.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean A., Sonigo P., Wain-Hobson S., Tiollais P. Specific hepatitis B virus integration in hepatocellular carcinoma DNA through a viral 11-base-pair direct repeat. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5350–5354. doi: 10.1073/pnas.81.17.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliyahu D., Raz A., Gruss P., Givol D., Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984 Dec 13;312(5995):646–649. doi: 10.1038/312646a0. [DOI] [PubMed] [Google Scholar]
- Fowler M. J., Thomas H. C., Monjardino J. Cloning and analysis of integrated hepatitis B virus DNA of the adr subtype derived from a human primary liver cell carcinoma. J Gen Virol. 1986 Apr;67(Pt 4):771–775. doi: 10.1099/0022-1317-67-4-771. [DOI] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Hino O., Shows T. B., Rogler C. E. Hepatitis B virus integration site in hepatocellular carcinoma at chromosome 17;18 translocation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8338–8342. doi: 10.1073/pnas.83.21.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe M., Emanuel B. S., Givol D., Oren M., Croce C. M. Localization of gene for human p53 tumour antigen to band 17p13. Nature. 1986 Mar 6;320(6057):84–85. doi: 10.1038/320084a0. [DOI] [PubMed] [Google Scholar]
- Jameel S., Siddiqui A. The human hepatitis B virus enhancer requires trans-acting cellular factor(s) for activity. Mol Cell Biol. 1986 Feb;6(2):710–715. doi: 10.1128/mcb.6.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Koike K. Complete nucleotide sequence of hepatitis B virus DNA of subtype adr and its conserved gene organization. Gene. 1984 Oct;30(1-3):227–232. doi: 10.1016/0378-1119(84)90124-0. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Miller C., Nicolson M. A., Ranyard J., Bosselman R. A. Increased expression of p53 protein in human leukemia cells. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4035–4039. doi: 10.1073/pnas.83.11.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosche K. A., Dobkin C., Bank A. DNA sequences regulating human beta globin gene expression. Nucleic Acids Res. 1985 Nov 11;13(21):7781–7793. doi: 10.1093/nar/13.21.7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy R., Koch S., von Loringhoven A. F., Kahmann R., Murray K., Hofschneider P. H. Integration of hepatitis B virus DNA: evidence for integration in the single-stranded gap. Cell. 1983 Aug;34(1):215–223. doi: 10.1016/0092-8674(83)90152-6. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lawrence C. B., Goldman D. A., Hood R. T. Optimized homology searches of the gene and protein sequence data banks. Bull Math Biol. 1986;48(5-6):569–583. doi: 10.1007/BF02462324. [DOI] [PubMed] [Google Scholar]
- Masuda H., Miller C., Koeffler H. P., Battifora H., Cline M. J. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7716–7719. doi: 10.1073/pnas.84.21.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride O. W., Merry D., Givol D. The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13). Proc Natl Acad Sci U S A. 1986 Jan;83(1):130–134. doi: 10.1073/pnas.83.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L. Approaching the control of primary liver cancer by means of a virus vaccine. Prog Med Virol. 1985;32:22–38. [PubMed] [Google Scholar]
- Miller C., Mohandas T., Wolf D., Prokocimer M., Rotter V., Koeffler H. P. Human p53 gene localized to short arm of chromosome 17. 1986 Feb 27-Mar 5Nature. 319(6056):783–784. doi: 10.1038/319783a0. [DOI] [PubMed] [Google Scholar]
- Mizusawa H., Taira M., Yaginuma K., Kobayashi M., Yoshida E., Koike K. Inversely repeating integrated hepatitis B virus DNA and cellular flanking sequences in the human hepatoma-derived cell line huSP. Proc Natl Acad Sci U S A. 1985 Jan;82(1):208–212. doi: 10.1073/pnas.82.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel J. F., Norbury C. J., Tuite M. F., Dobson M. J., Mills J. S., Kingsman A. J., Kingsman S. M. Characterization of human chromosomal DNA sequences which replicate autonomously in Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Jan 25;12(2):1049–1068. doi: 10.1093/nar/12.2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerenberg M., Hinrichs S. H., Reynolds R. K., Khoury G., Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987 Sep 11;237(4820):1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- Ou J., Rutter W. J. Hybrid hepatitis B virus-host transcripts in a human hepatoma cell. Proc Natl Acad Sci U S A. 1985 Jan;82(1):83–87. doi: 10.1073/pnas.82.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada L. F., Land H., Weinberg R. A., Wolf D., Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984 Dec 13;312(5995):649–651. doi: 10.1038/312649a0. [DOI] [PubMed] [Google Scholar]
- Pasquinelli C., Garreau F., Bougueleret L., Cariani E., Grzeschik K. H., Thiers V., Croissant O., Hadchouel M., Tiollais P., Bréchot C. Rearrangement of a common cellular DNA domain on chromosome 4 in human primary liver tumors. J Virol. 1988 Feb;62(2):629–632. doi: 10.1128/jvi.62.2.629-632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogler C. E., Hino O., Su C. Y. Molecular aspects of persistent woodchuck hepatitis virus and hepatitis B virus infection and hepatocellular carcinoma. Hepatology. 1987 Jan-Feb;7(1 Suppl):74S–78S. doi: 10.1002/hep.1840070713. [DOI] [PubMed] [Google Scholar]
- Rogler C. E., Sherman M., Su C. Y., Shafritz D. A., Summers J., Shows T. B., Henderson A., Kew M. Deletion in chromosome 11p associated with a hepatitis B integration site in hepatocellular carcinoma. Science. 1985 Oct 18;230(4723):319–322. doi: 10.1126/science.2996131. [DOI] [PubMed] [Google Scholar]
- Saffer J. D., Lerman M. I. Unusual class of Alu sequences containing a potential Z-DNA segment. Mol Cell Biol. 1983 May;3(5):960–964. doi: 10.1128/mcb.3.5.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y., Rutter W. J., Laub O. A human hepatitis B viral enhancer element. EMBO J. 1985 Feb;4(2):427–430. doi: 10.1002/j.1460-2075.1985.tb03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Burke K., Chou M. J., Zeldis J. B., Yang C. S., Lee C. S., Isselbacher K. J., Wands J. R., Goodman H. M. Tight clustering of human hepatitis B virus integration sites in hepatomas near a triple-stranded region. J Virol. 1987 Nov;61(11):3491–3498. doi: 10.1128/jvi.61.11.3491-3498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A., Marion P. L., Robinson W. S. Ground squirrel hepatitis virus DNA: molecular cloning and comparison with hepatitis B virus DNA. J Virol. 1981 Apr;38(1):393–397. doi: 10.1128/jvi.38.1.393-397.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandau D. F., Lee C. H. trans-activation of viral enhancers by the hepatitis B virus X protein. J Virol. 1988 Feb;62(2):427–434. doi: 10.1128/jvi.62.2.427-434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokino T., Fukushige S., Nakamura T., Nagaya T., Murotsu T., Shiga K., Aoki N., Matsubara K. Chromosomal translocation and inverted duplication associated with integrated hepatitis B virus in hepatocellular carcinomas. J Virol. 1987 Dec;61(12):3848–3854. doi: 10.1128/jvi.61.12.3848-3854.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tur-Kaspa R., Burk R. D., Shaul Y., Shafritz D. A. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1627–1631. doi: 10.1073/pnas.83.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Schloemer R. H. Transcriptional trans-activating function of hepatitis B virus. J Virol. 1987 Nov;61(11):3448–3453. doi: 10.1128/jvi.61.11.3448-3453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Wu X. F., Zhou Y. Z., Feng Z. M., Li Z. P., Xia S. Y. Cloning and restriction mapping of human HBV genome serotype adr. Sci Sin B. 1983 Sep;26(9):954–960. [PubMed] [Google Scholar]
- Yaginuma K., Kobayashi H., Kobayashi M., Morishima T., Matsuyama K., Koike K. Multiple integration site of hepatitis B virus DNA in hepatocellular carcinoma and chronic active hepatitis tissues from children. J Virol. 1987 Jun;61(6):1808–1813. doi: 10.1128/jvi.61.6.1808-1813.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma K., Kobayashi M., Yoshida E., Koike K. Hepatitis B virus integration in hepatocellular carcinoma DNA: duplication of cellular flanking sequences at the integration site. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4458–4462. doi: 10.1073/pnas.82.13.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. Z., Butel J. S., Li P. J., Finegold M. J., Melnick J. L. Integrated state of subgenomic fragments of hepatitis B virus DNA in hepatocellular carcinoma from mainland China. J Natl Cancer Inst. 1987 Aug;79(2):223–231. [PubMed] [Google Scholar]
- de Thé H., Marchio A., Tiollais P., Dejean A. A novel steroid thyroid hormone receptor-related gene inappropriately expressed in human hepatocellular carcinoma. Nature. 1987 Dec 17;330(6149):667–670. doi: 10.1038/330667a0. [DOI] [PubMed] [Google Scholar]
- van Tuinen P., Rich D. C., Summers K. M., Ledbetter D. H. Regional mapping panel for human chromosome 17: application to neurofibromatosis type 1. Genomics. 1987 Dec;1(4):374–381. doi: 10.1016/0888-7543(87)90042-5. [DOI] [PubMed] [Google Scholar]
- von Loringhoven A. F., Koch S., Hofschneider P. H., Koshy R. Co-transcribed 3' host sequences augment expression of integrated hepatitis B virus DNA. EMBO J. 1985 Jan;4(1):249–255. doi: 10.1002/j.1460-2075.1985.tb02343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]