Abstract
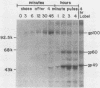
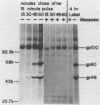
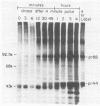
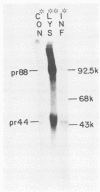
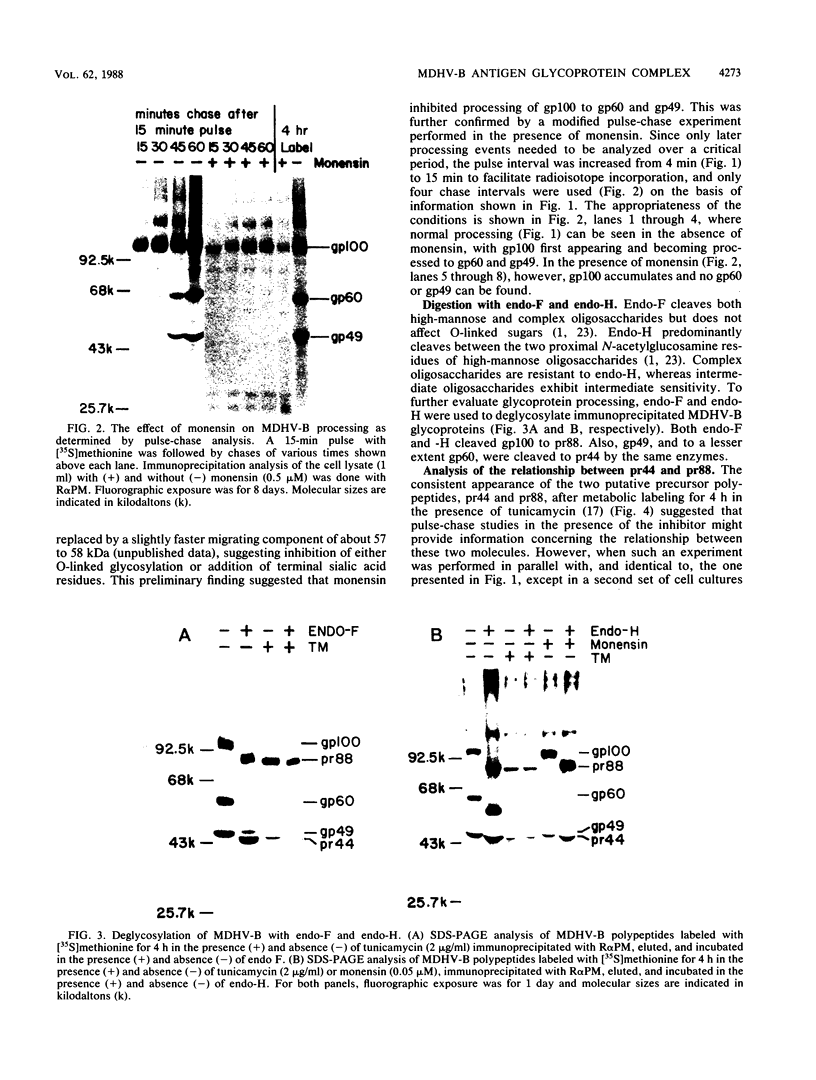
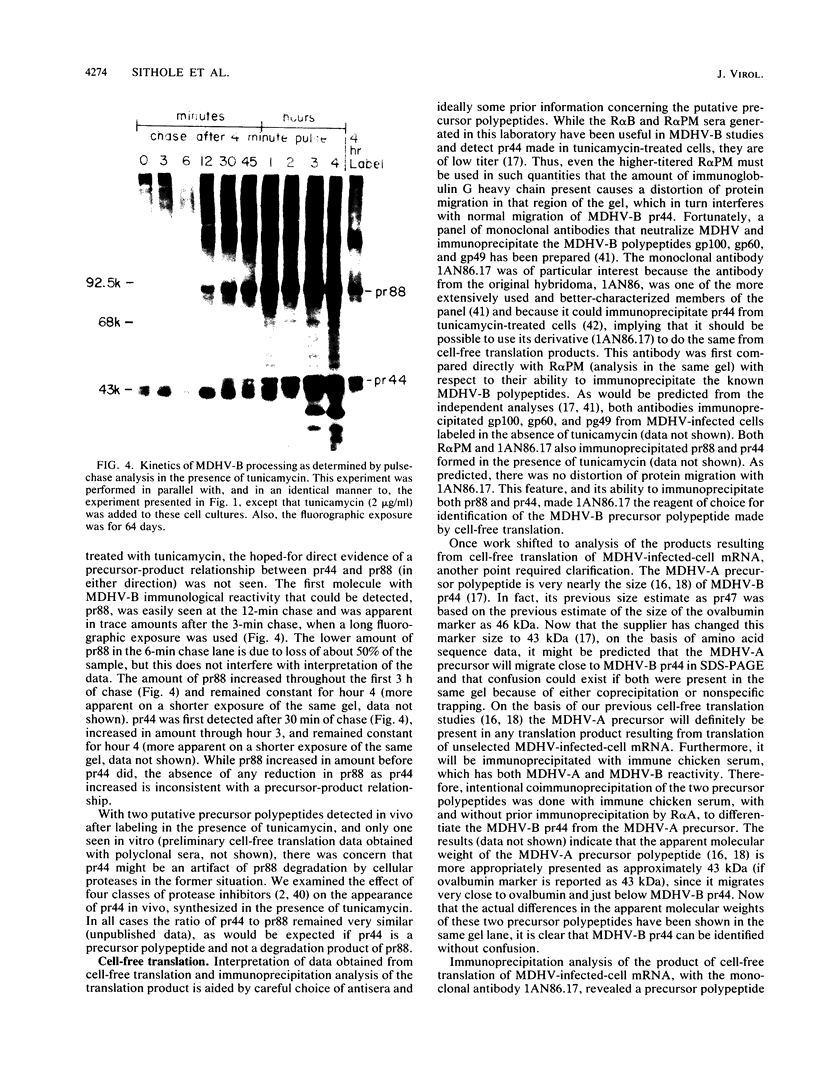
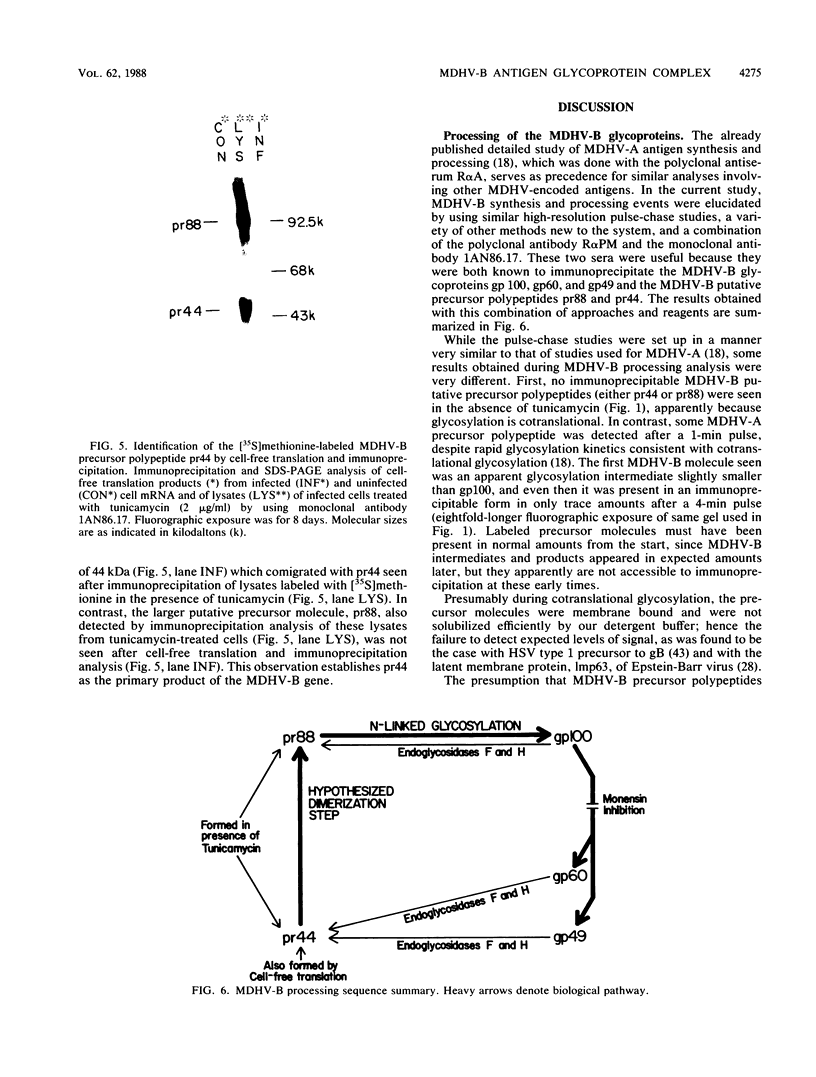
The Marek's disease herpesvirus B antigen (MDHV-B) complex was previously immunologically identified and molecularly characterized as a set of three glycoproteins designated gp100, gp60, and gp49 on the basis of apparent molecular weight and immunoprecipitation with both polyclonal and monoclonal antibodies. Immunoprecipitation analysis, previously with polyclonal and more recently with monoclonal antibodies, of infected cell lysates labeled with [35S]methionine in the presence of tunicamycin, an inhibitor of N-linked glycosylation, revealed two putative precursor molecules of 88,000 daltons (pr88) and 44,000 daltons (pr44). High-resolution pulse-chase studies revealed that gp100 was a glycosylated intermediate which was processed to yield gp60 and gp49. This cleavage was inhibited by monensin, an inhibitor of glycoprotein processing. Endo-beta-N-acetylglucosaminidases F and H (endo-F, endo-H) reduced gp100 to pr88, indicating that the latter is an intermediate in the biosynthetic pathway. These same enzymes reduced gp49, and to a lesser extent gp60, to pr44, suggesting that pr44 is their polypeptide backbone. Significant support for this concept is the fact that the same monoclonal antibody recognized all three molecules, gp60, gp49, and pr44. In the presence of monensin, terminal addition of complex sugars was also prevented, since gp60 was replaced by a slightly faster migrating component which was insensitive to both endo-F and endo-H. Cell-free translation of infected-cell mRNA, followed by immunoprecipitation analysis with either polyclonal or monoclonal antibody, resulted in detection of a putative unglycosylated precursor polypeptide of 44,000 daltons. Since pr88 was not the initial precursor polypeptide of the MDHV-B complex, its existence may have resulted from dimerization of pr44. Again, detection of both pr88 and pr44 with the same monoclonal antibody is consistent with this interpretation. These collective data obtained from the cell-free and in vivo studies with polyclonal and monoclonal antibodies reactive with MDHV-B are consistent with the concept that pr44, the initial gene product, dimerizes to form pr88 and demonstrate that pr88 is actually a processing intermediate glycosylated to gp100, another processing intermediate, which is then processed to gp60 and gp49.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balachandran N., Hutt-Fletcher L. M. Synthesis and processing of glycoprotein gG of herpes simplex virus type 2. J Virol. 1985 Jun;54(3):825–832. doi: 10.1128/jvi.54.3.825-832.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J. R., Braiterman L. T., Hubbard A. L. Biochemical characterization of domain-specific glycoproteins of the rat hepatocyte plasma membrane. J Biol Chem. 1985 Oct 15;260(23):12792–12802. [PubMed] [Google Scholar]
- Benko D. M., Gibson W. Primate cytomegalovirus glycoproteins: lectin-binding properties and sensitivities to glycosidases. J Virol. 1986 Sep;59(3):703–713. doi: 10.1128/jvi.59.3.703-713.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Britt W. J., Auger D. Synthesis and processing of the envelope gp55-116 complex of human cytomegalovirus. J Virol. 1986 Apr;58(1):185–191. doi: 10.1128/jvi.58.1.185-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L., Spear P. G. Oligomerization of herpes simplex virus glycoprotein B. J Virol. 1986 Nov;60(2):803–806. doi: 10.1128/jvi.60.2.803-806.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens P. M., Velicer L. F. Structure and complete nucleotide sequence of the Marek's disease herpesvirus gp57-65 gene. J Virol. 1988 Jul;62(7):2373–2379. doi: 10.1128/jvi.62.7.2373-2379.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Olio F., Malagolini N., Speziali V., Campadelli-Fiume G., Serafini-Cessi F. Sialylated oligosaccharides O-glycosidically linked to glycoprotein C from herpes simplex virus type 1. J Virol. 1985 Oct;56(1):127–134. doi: 10.1128/jvi.56.1.127-134.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar G. H., Greenaway P. J. Characterization of glycoprotein complexes present in human cytomegalovirus envelopes. J Gen Virol. 1986 Jul;67(Pt 7):1469–1473. doi: 10.1099/0022-1317-67-7-1469. [DOI] [PubMed] [Google Scholar]
- Glaubiger C., Nazerian K., Velicer L. F. Marek's disease herpesviruses. IV. Molecular characterization of Marek's disease herpesvirus A antigen. J Virol. 1983 Mar;45(3):1228–1234. doi: 10.1128/jvi.45.3.1228-1234.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Weigle K. A., Friedrichs W. E., McGuire W. L. Varicella-zoster virus-specific gp140: a highly immunogenic and disulfide-linked structural glycoprotein. Virology. 1984 Jan 15;132(1):138–146. doi: 10.1016/0042-6822(84)90098-9. [DOI] [PubMed] [Google Scholar]
- Hampl H., Ben-Porat T., Ehrlicher L., Habermehl K. O., Kaplan A. S. Characterization of the envelope proteins of pseudorabies virus. J Virol. 1984 Nov;52(2):583–590. doi: 10.1128/jvi.52.2.583-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Ueda S., Kato S., Hirai K. Identification with monoclonal antibodies of glycoproteins of Marek's disease virus and herpesvirus of turkeys related to virus neutralization. J Virol. 1984 Mar;49(3):1014–1017. doi: 10.1128/jvi.49.3.1014-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Ueda S., Kato S., Hirai K. Processing of glycoprotein gB related to neutralization of Marek's disease virus and herpesvirus of turkeys. Microbiol Immunol. 1984;28(8):923–933. doi: 10.1111/j.1348-0421.1984.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Isfort R. J., Kung H. J., Velicer L. F. Identification of the gene encoding Marek's disease herpesvirus A antigen. J Virol. 1987 Aug;61(8):2614–2620. doi: 10.1128/jvi.61.8.2614-2620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R. J., Sithole I., Kung H. J., Velicer L. F. Molecular characterization of Marek's disease herpesvirus B antigen. J Virol. 1986 Aug;59(2):411–419. doi: 10.1128/jvi.59.2.411-419.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R. J., Stringer R. A., Kung H. J., Velicer L. F. Synthesis, processing, and secretion of the Marek's disease herpesvirus A antigen glycoprotein. J Virol. 1986 Feb;57(2):464–474. doi: 10.1128/jvi.57.2.464-474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Diggelmann H. Extracellular cleavage of the glycoprotein precursor of Rous sarcoma virus. J Virol. 1979 Jan;29(1):285–292. doi: 10.1128/jvi.29.1.285-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukàcs N., Thiel H. J., Mettenleiter T. C., Rziha H. J. Demonstration of three major species of pseudorabies virus glycoproteins and identification of a disulfide-linked glycoprotein complex. J Virol. 1985 Jan;53(1):166–173. doi: 10.1128/jvi.53.1.166-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek-Hedayat S., Meiners S. A., Metcalf T. N., 3rd, Schindler M., Wang J. L., Ho S. C. Endogenous lectin from cultured soybean cells. Chemical characterization of the lectin of SB-1 cells. J Biol Chem. 1987 Jun 5;262(16):7825–7830. [PubMed] [Google Scholar]
- Mann K. P., Thorley-Lawson D. Posttranslational processing of the Epstein-Barr virus-encoded p63/LMP protein. J Virol. 1987 Jul;61(7):2100–2108. doi: 10.1128/jvi.61.7.2100-2108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo E. A., Grose C. Neutralization epitope of varicella zoster virus on native viral glycoprotein gp118 (VZV glycoprotein gpIII). Virology. 1986 Mar;149(2):230–241. doi: 10.1016/0042-6822(86)90124-8. [DOI] [PubMed] [Google Scholar]
- Montalvo E. A., Parmley R. T., Grose C. Structural analysis of the varicella-zoster virus gp98-gp62 complex: posttranslational addition of N-linked and O-linked oligosaccharide moieties. J Virol. 1985 Mar;53(3):761–770. doi: 10.1128/jvi.53.3.761-770.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy K. K., Calnek B. W. Pathogenesis of Marek's disease; effect of immunization with inactivated viral and tumor-associated antigens. Infect Immun. 1979 Nov;26(2):547–553. doi: 10.1128/iai.26.2.547-553.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki W., Purchase H. G., Burmester B. R. Protection against Marek's disease by vaccination with a herpesvirus of turkeys. Avian Dis. 1970 May;14(2):413–429. [PubMed] [Google Scholar]
- Ono K., Takashima M., Ishikawa T., Hayashi M., Yoshida I., Konobe T., Ikuta K., Nakajima K., Ueda S., Kato S. Partial protection against Marek's disease in chickens immunized with glycoproteins gB purified from turkey-herpesvirus-infected cells by affinity chromatography coupled with monoclonal antibodies. Avian Dis. 1985 Apr-Jun;29(2):533–539. [PubMed] [Google Scholar]
- Pereira L., Dondero D., Roizman B. Herpes simplex virus glycoprotein gA/B: evidence that the infected Vero cell products comap and arise by proteolysis. J Virol. 1982 Oct;44(1):88–97. doi: 10.1128/jvi.44.1.88-97.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J. M., Stone H. A. Genetic resistance to Marek's disease. Delineation of the response of genetically resistant chickens to Marek's disease virus infection. Avian Dis. 1972 Jul-Sep;16(4):894–906. [PubMed] [Google Scholar]
- Shin J., Ji T. H. Composition of cross-linked 125I-follitropin-receptor complexes. J Biol Chem. 1985 Oct 15;260(23):12822–12827. [PubMed] [Google Scholar]
- Silva R. F., Lee L. F. Monoclonal antibody-mediated immunoprecipitation of proteins from cells infected with Marek's disease virus or turkey herpesvirus. Virology. 1984 Jul 30;136(2):307–320. doi: 10.1016/0042-6822(84)90167-3. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zaane D., Brinkhof J. M., Gielkens A. L. Molecular-biological characterization of Marek's disease virus. II. Differentiation of various MDV and HVT strains. Virology. 1982 Aug;121(1):133–146. doi: 10.1016/0042-6822(82)90123-4. [DOI] [PubMed] [Google Scholar]
- Van Zaane D., Brinkhof J. M., Westenbrink F., Gielkens A. L. Molecular-biological characterization of Marek's disease virus. I. Identification of virus-specific polypeptides in infected cells. Virology. 1982 Aug;121(1):116–132. doi: 10.1016/0042-6822(82)90122-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Limited proteolysis of herpes simplex virus glycoproteins that occurs during their extraction from vero cells. J Virol. 1984 Apr;50(1):258–262. doi: 10.1128/jvi.50.1.258-262.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., van den Hurk J. V., Gilchrist J. E., Misra V., Babiuk L. A. Interactions of monoclonal antibodies and bovine herpesvirus type 1 (BHV-1) glycoproteins: characterization of their biochemical and immunological properties. Virology. 1984 Jun;135(2):466–479. doi: 10.1016/0042-6822(84)90201-0. [DOI] [PubMed] [Google Scholar]