Abstract
2-Nitrosoamino-3,8-dimethylimidazo[4,5-f]quinoxaline (N-NO-MeIQx) is a nitrosation product of the food carcinogen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) and proposed to form in vivo under inflammatory conditions. This study evaluated the stability and reactivity of N-NO-MeIQx to assess its possible role in initiation of colon cancer by MeIQx. 14C-N-NO-MeIQx (4 μM) was incubated for 4 hours over a range of pH values and its stability monitored by HPLC. At pH values from pH 7.4 to 9.0, N-NO-MeIQx was very stable with no detectable change observed. Glutathione (1 mM) did not alter stability at pH 7.4. As pH decreased, this nitrosamine was less stable with only 48 ± 1 % remaining at pH 5.5 and none remaining at pH 3.5 or 2.0. Major products identified by electrospray ionization mass spectrometry were 3,8-dimethylimidazo[4,5-f]quinoxaline and 2-hydroxy-3,8-dimethylimidazo[4,5-f]quinoxaline. MeIQx was a minor product. At pH 2.0, the t1/2 for N-NO-MeIQx was reduced from 2.1 ± 0.2 to 1.2 ± 0.1 min with 10 mM NaN3. This effect of azide was due to formation of 2-azido-MeIQx. The binding of 14C-N-NO-MeIQx to DNA increased with decreasing pH. The 10-fold increase in binding observed at pH 2.0 compared to pH 5.5 was completely inhibited by 10 mM NaN3 due to 2-azido-MeIQx formation. The reactivity of N-NO-MeIQx was compared to N-OH-MeIQx by evaluating adduct formation with 2′-deoxyguanosine 3′-monophosphate (dGp) by 32P-postlabeling. N-OH-MeIQx formed a single major adduct, N-(deoxyguanosin-8-yl)-MeIQx (dG-C8-MeIQx). Incubation of N-NO-MeIQx under inflammatory conditions (pH 5.5 ± HOCl) produced dG-C8-MeIQx along with 4 to 6 other adducts. dG-C8-MeIQx formation increased in the presence of HOCl. Liver from a MeIQx-treated mouse contained dG-C8-MeIQx and two other adducts detected with N-NO-MeIQx, but not N-OH-MeIQx. These results suggest that N-NO-MeIQx could be genotoxic, is activated by conditions that mediate inflammatory responses, and is a possible cancer risk factor for individuals with inflammation of the colon.
Introduction
Reactive nitrogen oxygen species (RNOS)1 generated by inflammatory cells cause DNA and tissue damage, which contribute to multistage carcinogenesis (1). Damage can be caused by nitration, oxidation, and nitrosation. To understand the pathobiochemistry resulting from RNOS, it is necessary to identify targets of their reaction which can be measured and assess their effects on the carcinogenic process. This study assesses a RNOS-derived nitrosation product of the food carcinogen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx).
Heterocyclic amines (HCAs) are formed during the cooking of proteinaceous food at high temperatures (2). The formation of HCAs is the result of a Maillard reaction which occurs when amino acids (proteins) and reducing sugars (carbohydrates) are heated together. More than 20 HCAs have been identified. MeIQx is one of the most prevalent HCAs found in cooked meat and is one of seventeen new entries in the 2005 Eleventh Report on Carcinogens (3). In healthy volunteers eating a normal diet, the daily exposure to MeIQx is estimated to be 0.2 to 2.6 μg/person and was not detected in parenterally fed individuals (4). MeIQx is a strong mutagen in the Ames assay (5) and at high doses can induce tumors at multiple sites in rodents (6). In vivo mutagenicity of low dose MeIQx in transgenic rats has been demonstrated in the liver, Zymbal gland, and colon, but not in eight other tissues examined (7). This is consistent with aberrant crypt foci in the colon of MeIQx-treated rats (8). There is a strong association between well-done red meat intake and colorectal cancer risk (9, 10). The presence of HCAs in well-done red meat (2, 11) and their initiation of colon cancer (8) suggest that these amines may be responsible for some of the increased risk of colon cancer associated with this dietary component. When the intake of three major HCAs (MeIQx, 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline, and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) commonly found in well-done meat was examined, only MeIQx was shown to be a risk factor for colorectal adenomas (12).
HCAs, like aromatic amines, require metabolic activation to be mutagenic or carcinogenic. A major activation pathway is N-oxidation by cytochrome P450 enzymes, i.e. P450 1A2 (13, 14). The resulting N-hydroxylamine product can react directly with DNA by formation of nitrenium ions. In vitro and in vivo studies have demonstrated that N-(deoxyguanosin-8-yl)-MeIQx (dG-C8-MeIQx) is the major DNA adduct formed (15, 16). A linear relationship between the dose of MeIQx and the MeIQx-DNA adduct level has been reported in studies using either rats or mice and with different methods of adduct analysis (17, 18). Most mutations reported for HCAs, including MeIQx, involve guanine residues (19). Using a plasmid specifically modified to contain dG-C8-MeIQx adducts, > 90% of the mutations in E. coli occurred at G-C base pairs (20). In livers from Big Blue transgenic rats, MeIQx most frequently induced G frameshifts, followed by G to T transversions (7). Among six squamous cell carcinomas of rat zymbal gland induced by MeIQx, two possessed point mutations of Ha-ras, one had a G to T transversion in codon 13 and the other had an A to T transversion in codon 61 (21). These observations suggest that modification of guanine bases are involved in the gene alterations induced by MeIQx and that MeIQx primarily binds to guanine residues of DNA in vivo.
Chronic inflammation/infection and injury play an important role in colon cancer (22). The incidence of colorectal carcinoma in patients with inflammatory bowel disease, a chronic inflammatory disease, is 20-fold higher and has an average age of onset 20 years younger than the general population (23). High levels of nitric oxide (NO) are observed during inflammation due to upregulation of inducible nitric oxide synthase (iNOS) (24). By reacting with superoxide to form peroxynitrite anions or with oxygen (autoxidation), NO produces RNOS such as nitrogen dioxide radicals (NO2•) and dinitrogen trioxide (N2O3). Inflammatory bowel disease is associated with marked mucosal infiltration of macrophages, lymphocytes, and neutrophils in which high levels of iNOS and 3-nitrotyrosine, a marker of RNOS, are detected (25–27). Genotoxic effects of autoxidizing NO in both human colorectal tumors and colons of individuals with ulcerative colitis correlate with increased iNOS detected in these tissues (25, 28). NO also inhibits DNA repair (29, 30). Increased formation of N-nitrosamines and nitrate have been reported in vivo in human subjects with infection and inflammation (31–33). This elevation in endogenous nitrosation potential might be responsible for reduced levels of cytochrome P450 enzyme observed during infection/inflammation (for review see (34)). N-Hydroxylamine products of cytochrome P450 and accompanying adduct formation would also be reduced. Thus, despite considerable research, the biotransformation pathways during infection/inflammation need further study.
We have recently demonstrated NO-dependent nitrosation of MeIQx and its potentiation by hemin and myeloperoxidase (35). We propose the nitrosation product 2-nitrosoamino-3,8-dimethylimidazo[4,5-f]quinoxaline (N-NO-MeIQx) to be an alternative to N-hydroxy-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (N-OH-MeIQx) in initiating colon cancer in individuals with colitis. During an inflammatory response, stimulated neutrophils infiltrate the site of inflammation and undergo a respiratory burst to produce oxidants, including hypochlorous acid (HOCl), a cytotoxic agent (36). In addition, the acidic environment (pH 5.3 to 5.5) created by inflammatory conditions may influence adduct formation (37, 38). To provide insight into its carcinogenicity, the stability and reactivity of N-NO-MeIQx were evaluated as a function of pH with and without nucleophiles. Different components of the inflammatory response were used to compare adduct formation by N-NO-MeIQx to that by N-OH-MeIQx. Results demonstrate that N-NO-MeIQx is genotoxic and a potential colon cancer risk factor in individuals with colitis.
Experimental Procedures
Caution
MeIQx is carcinogenic to rodents and should be handled in accordance with NIH Guidelines for the Laboratory Use of Chemical Carcinogens (39).
Materials
[2-14C]-MeIQx (50 mCi/mmol, >98% radiochemical purity) and N-OH-MeIQx were purchased from Toronto Research Chemicals (Toronto, ON). NaOCl, NaN3, ascorbic acid, H2O2, horseradish peroxidase, calf thymus DNA, dGp, glutathione, and diethylenetriaminepentaacetic acid (DETAPAC) were purchased from Sigma-Aldrich Corporation (St. Louis, MO). Nuclease P1 (EC 3.1.30.1) and spleen exonuclease (EC 3.1.16.1) were from Boehringer Mannheim, Indianapolis, IN and T4 polynucleotide kinase (EC 2.7.1.78) from U.S. Biochemical Corp. (Cleveland, OH). Diethylamine NONOate, a NO donor, was purchased from Calbiochem (San Diego, CA). The concentration of this NO donor was determined at 250 nm with ε = 8,000 M−1cm−1 (40). N-NOMeIQx (50 mCi/mmol, >98% radiochemical purity) was prepared by incubating 0.24 mM 14CmeIQx with 0.4 mM diethylamine NONOate, 0.4 mM H2O2, and 0.4 mg/ml horseradish peroxidase at pH 7.4 and purified by a combination of Sep Pak and HPLC methods. 2-Azido-3,8-dimethylimidazo[4,5-f]quinoxaline (2-Azido-MeIQx) was synthesized, like 2-azido-3-methylimidazo[4,5-f]quinoline (35), by incubating 0.1 mM N-NO-MeIQx with 10 mM NaN3 in citrate phosphate buffer pH 2.0 for 60 min at 37°C. Ultima-Flo AP was purchased from PerkinElmer LAS, Inc., Shelton, CT.
Animals and dosing
Male, 21 day old, C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, MA). Food and deionized water were provided ad libitum. Animals were administered 20 mg/kg MeIQx in corn oil by gavage once a day for 10 successive days. The animals were euthanized by cervical dislocation 24 hours after the last dose and DNA was isolated from the liver as previously described (41).
Determination of N-NO-MeIQx Reaction Products
14C-N-NO-MeIQx (4 μM, 0.05 μCi) with 0.1 mM DETAPAC and 100 mM phosphate buffer was incubated at 37°C for various times at different pH values in the presence and absence of nucleophiles. Experiments containing 10 mM NaN3 were like to those assessing the reactivity of N-NO-IQ (35). Reactions (0.05 mL) were stopped by neutralizing with concentrated NH4OH and dimethylformamide (0.05 mL). Reaction products were analyzed by HPLC as described below.
HPLC Analysis of Products
Products were assessed using a Beckman HPLC with System Gold software and a 5 μm, 4.6 × 150 mm C-18 ultrasphere column attached to a guard column. The mobile phase contained 20 mM ammonium formate pH 4.0 in 10% acetonitrile, 0–3 min; 10–13% acetonitrile, 3–9 min; 13–35% acetonitrile, 16–21 min; 35–10% acetonitrile, 25–30 min; flow rate 1 mL/min. Radioactivity in HPLC eluents was measured using a FLO-ONE (PerkinElmer LAS, Inc., Shelton, CT) radioactive flow detector. Data are expressed as a percent of total radioactivity or pmol recovered by HPLC.
DNA Binding
Binding of 14C-N-NO-MeIQx (0.03 mM, 2 μCi) to DNA was assessed as a function of pH. Reaction mixtures (0.25 mL) were incubated for 60 min as described above, except 1 mg/mL DNA was present (42). Calf thymus DNA was purified before use by extracting with phenol and a 24 : 1 chloroform/isoamyl alcohol mixture. The reaction was stopped by precipitation of DNA due to adjusting the concentration of NaCl to 0.25 M, addition of 2 volumes of ethanol, and being left at −20°C for 4 hours (43). The DNA pellet was washed twice with 70% ethanol, dissolved in water, and the process of precipitation and washing repeated. The radioactivity of the water-dissolved DNA was determined. The purity and quantity of DNA were assessed by the absorbance at 260 and 280 nm with a A260/A280 ratio of approximately 1.7 demonstrating high purity of DNA in each sample. Blank values were obtained by stopping the pH 7.4 incubations at zero time. Binding is expressed as nmol/mg DNA/h.
Reaction of N-OH-MeIQx with dGp
N-OH-MeIQx (0.06 mM) was incubated in 100 mM phosphate buffer pH 7.4 with 0.1 mM DETAPAC and 1 mg/ml dGp in a total volume of 0.5 mL for 30 min at 37°C. Previous experiments have shown that no adducts formed in the absence of N-OH-MeIQx. After 30 min at 37°C, samples were placed on ice and immediately applied to Sep-Pak columns (Presep C18 from Fisher Scientific) pre-washed with methanol followed by 1 mM phosphate buffer pH 3.0. Samples were diluted with 1 ml of 1 mM Na2HPO4 pH 3.0, applied, and columns washed with 6 ml phosphate buffer pH 3.0 followed by two 6 ml water washes. Adducts were then eluted with 5 ml of methanol : ammonium hydroxide, 9:1. This column procedure separates most of the unreacted dGp from adducts. Solvent was evaporated under N2 to dryness and the residue was used for 32P-postlabeling analysis.
Reaction of N-NO-MeIQx with dGp
14C-N-NO-MeIQx (0.06 mM) was incubated in 100 mM phosphate buffer pH 5.5 with 0.1 mM DETAPAC and 1 mg/ml dGp in a total volume of 0.5 mL for 30 min at 37°C. Hypochlorous acid (HOCl, 0.1 mM) oxidation was initiated by its addition. Incubations were stopped by placing on ice and applied to Sep-Pak columns as described above. Previous experiments have shown that no adducts formed when N-NO-MeIQx was not included in the incubations.
Adduct Analysis by 32P-postlabeling
The dGp adducts were analyzed by 32P-postlabeling, using standard (ATP-saturating) conditions (41, 44), as previously described (45). dGp-adducts were labeled with [γ-32P]ATP to form 3′,5′-bisphosphate nucleotides (d32pGp-adduct), using T4 polynucleotide kinase. 32P-Postlabeled nucleotide adducts were separated from normal nucleotides by chromatography on polyethyleneimine cellulose thin-layer sheets (prepared in the laboratory) using the following solvents: D1, 2.3 M sodium phosphate (pH 5.8); D3, 3.5 M lithium formate and 7.0 M urea (pH 3.5); D4, 0.7 M sodium phosphate and 7.0 M urea, (pH 8.0); and D5, 1.0 M magnesium chloride. The labeled adducts were detected by autoradiography and quantitated using Cerenkov counting. Adduct levels are expressed as Relative Adduct Labeling (RAL) values.
Mass Spectral Analysis
Electrospray ionization (ESI) mass spectrometry (MS) analyses were performed on a Finnigan TSQ-7000 triple stage quadrupole mass spectrometer (San Jose, CA) equipped with a Finnigan ESI source and controlled by Finnigan ICIS software operated on a DEC alpha workstation. Samples were loop injected onto the ESI source with a Harvard syringe pump, which is continuously infused with methanol at a flow rate of 5uL/min. The skimmer was at ground potential, and the electrospray needle was at 4.5 kV. The heated capillary temperature was 250°C. To obtain collisionally activated dissociation (CAD) tandem mass spectra, the collision energy was set at 22 eV, and argon (2.3 mTorr) was used as target gas. The product ion spectra were acquired in the profile mode at the scan rate of one scan per 3 sec.
Statistical Analysis
Data are expressed as a mean ± S.E. For Table 2, the method of analysis was one-way analysis of variance. Homogeneity of variance was tested using the Levene statistic and was not rejected. There was a significant condition effect (p < 0.001). That is, the DNA Binding results varied by condition. Initial Bonferroni post hoc analyses revealed a significant difference in DNA binding between the condition pH 2.0 and the other conditions (p < 0.001).
Table 2.
Binding of N-NO-MeIQx to DNA
| Condition | DNA Binding |
|---|---|
| nmol bound/mg DNA/h | |
| pH 2.0 | 0.68 ± 0.04 |
| pH 2.0 ± 3 mM NaN3 | NDa |
| pH 3.5 | 0.14 ± 0.02b |
| pH 5.5 | 0.07 ± 0.01b |
| pH 7.4 | ND |
C-N-NO-MeIQx (0.03 mM, 50 mCi/mmol) was incubated at various pH values for 1 hour with 1 mg/ml DNA. Values represent mean ± S.E. (n = 3 to 6). The limit of detection is 0.03 nmol/mg DNA/h.
ND, not detected.
p < 0.001 vs pH 2.0
Results
Reaction Product Separation by HPLC and Identification by Tandem Mass Spectrometry
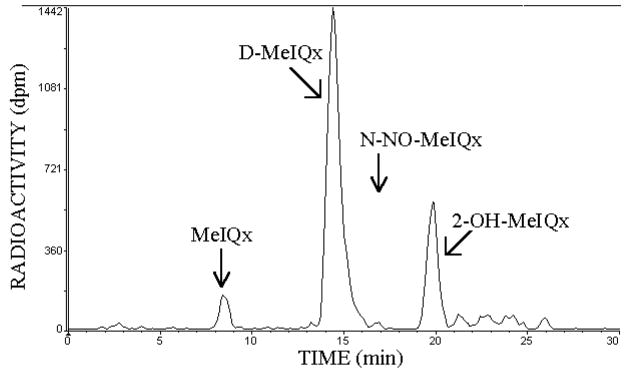
The stability of 14C-N-NO-MeIQx (4 μM) was evaluated in phosphate buffer from pH 2.0 to 9.0 for 4 hours at 37°C. Illustrated in Figure 1 is an HPLC of the pH 3.5 reaction mixture. Less than 1% of N-NO-MeIQx (16.7 min) remained with major peaks observed at 8.4 min (MeIQx), 14.4 min (D-MeIQx), and 19.8 min (2-OH-MeIQx). These major peaks accounted for 89% of the radioactivity recovered by HPLC. The structures of the reaction products were assessed by ESI CAD tandem mass spectrometry.
Figure 1.
Reaction products of 14C-N-NO-MeIQx at pH 3.5. Illustrated is the stability of N-NO-MeIQx (2 nmoles in 0.05 ml) at 37°C after a 4 h-incubation.
The D-MeIQx peak eluting at 14.4 min (Figure 1) was purified by HPLC and analyzed by ESI/MS. Mass spectrum acquired in the positive ion mode revealed the product exhibited [M + H]+, [M + Na]+, and [M + K]+ ions at m/z 199, 221, and 237, respectively (Figure 2, upper panel). For the product-ion spectrum of m/z 199 (Figure 2, low panel), fragmentation of the quinoxaline ring yielded m/z 158 (loss of CH3CN), which produced ions at m/z 131 and 104 due to consecutive losses of HCN. Ions at m/z 184, 157, and 116 represent loss of CH3 and consecutive losses of HCN. This is consistent with the product being 3,8-dimethylimidazo[4,5-f]quinoxaline, D-MeIQx. The early peak eluting at 8.4 min was identified as MeIQx by its co-elution on several different solvent systems with commercially available radiolabeled and unlabeled MeIQx (not shown).
Figure 2.
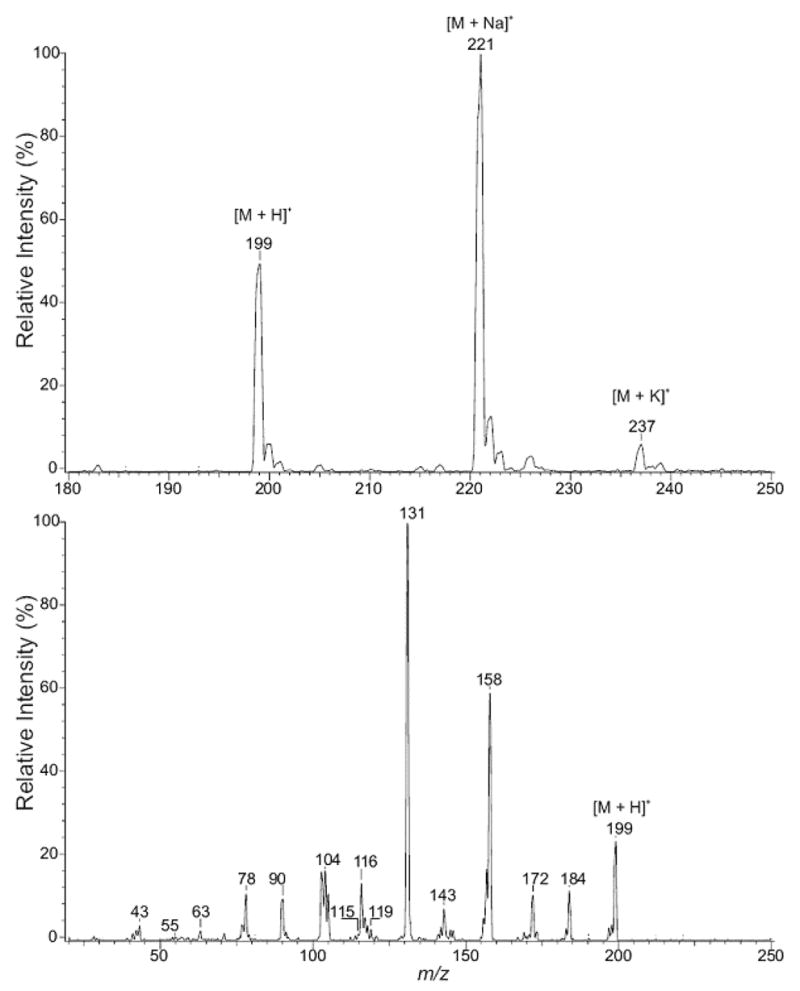
Positive-ion ESI mass spectrometric analysis of D-MeIQx. The upper panel illustrates a mass spectral scan from m/z 180 to 250. The lower panel illustrates the CAD product-ion spectra of m/z 199.
The late eluting peak, 2-OH-MeIQx, in Figure 1 was identified. ESI/MS in the positive ion mode yielded [M + H]+, [M + Na]+, and [M + K]+ ions at m/z 215, 237, and 253, respectively (Figure 3, upper panel). The product-ion spectrum of m/z 215 contains prominent ions at m/z 187, 160, and 133, representing loss of CO and consecutive losses of HCN (Figure 3, lower panel). Ions at m/z 200 and 172 correspond to loss of CH3 and CO, respectively. These results are consistent with the product being 2-hydroxy-3,8-dimethylimidazo[4,5-f]quinoxaline, 2-OH-MeIQx.
Figure 3.
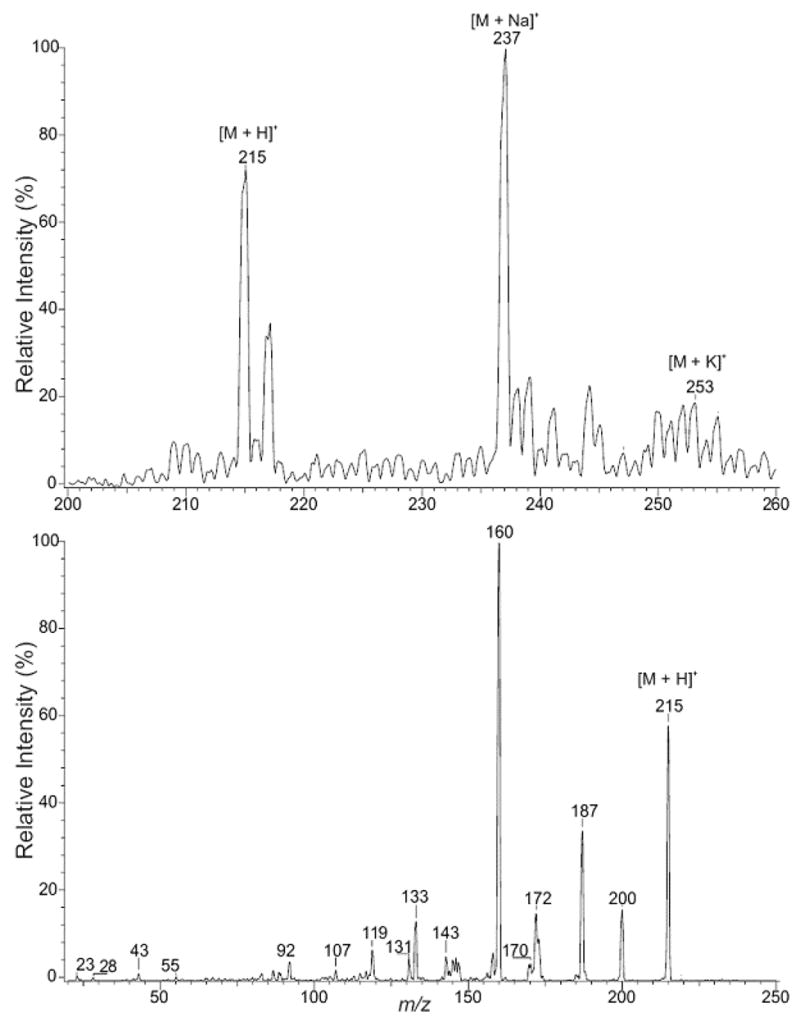
Positive-ion ESI mass spectrometric analysis of 2-OH-MeIQx. The upper panel illustrates a mass spectral scan from m/z 200 to 260. The lower panel illustrates the CAD production spectra of m/z 215.
The product formed during the incubation of N-NO-MeIQx at pH 2.0 with NaN3 (see below) was identified. With ESI/MS in the positive-ion mode, the product exhibited [M + H]+, [M + Na]+, and [M + K]+ ions at m/z 240, 262, and 278, respectively (Figure 4, upper panel). The product-ion spectrum of m/z 240 contains a prominent ion at m/z 212, arising from loss of N2, a characteristic loss unique to an azido compound (Figure 4, lower panel). The spectrum is also dominated by ions at m/z 185 and 158, arising from consecutive losses of HCN from m/z 212. Thus, this product is 2-azido-MeIQx. The different products formed by N-NO-MeIQx provide additional structural support for it being the N-nitroso product of MeIQx.
Figure 4.
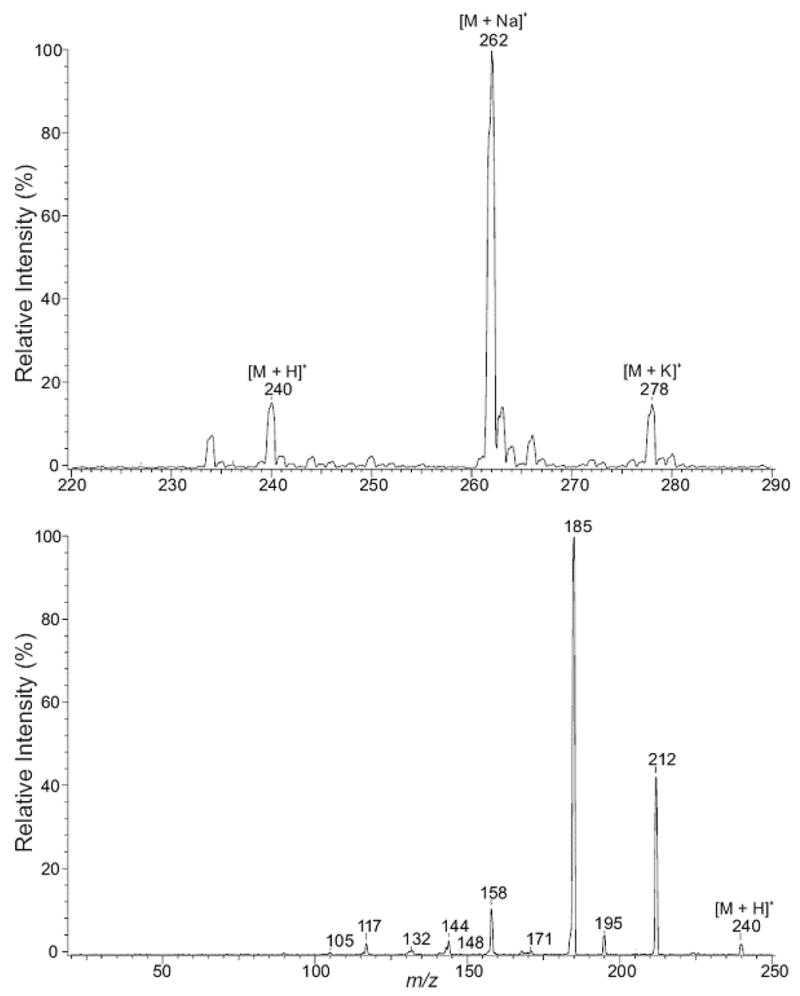
Positive-ion ESI mass spectrometric analysis of product derived from incubation of N-NO-MeIQx at pH 2.0 with NaN3. The upper panel illustrates a mass spectral scan from m/z 220 to 290. The lower panel illustrates the CAD product-ion spectra of m/z 240.
Stability of N-NO-MeIQx from pH 2.0 to 9.0
Products of N-NO-MeIQx hydrolysis in aqueous solution at pH 2.0 to 9.0 are expressed in Table 1. N-NO-MeIQx (4 μM) is quite stable from pH 7.4 to 9.0 with greater than 95% of this compound recovered after a 4-hour incubation at 37°C. Inclusion of 1mM glutathione in the incubation mixture at pH 7.4 did not alter its stability. At acidic pH values, the stability of N-NO-MeIQx decreased. At pH 5.5, only 48% of this nitrosamine remained after the 4 hour incubation. Below pH 5.5, less than 1% of the nitrosamine was detected. The products of hydrolysis depended upon the pH. At pH 5.5 and 3.5, the major product was D-MeIQx, while at pH 2.0 the major product was 2-OH-MeIQx. MeIQx represented only a small amount of product at acidic pH values.
Table 1.
Reaction Products of N-NO-MeIQx at Different pH values
| Conditions | MeIQx | D-MeIQx | N-NO-MeIQx | 2-OH-MeIQx | |
|---|---|---|---|---|---|
| % of total | |||||
| pH 9.0 | NDa | ND | 95 ± 3 | ND | |
| pH 7.4 | ND | ND | 95 ± 2 | ND | |
| pH 7.4 + 1 mM GSH | ND | ND | 91 ± 2 | ND | |
| pH 5.5 | 1.8 ± 0.6 | 35 ± 1 | 48 ± 1 | 1.8 ± 0.2 | |
| pH 3.5 | 1.5 ± 1.5 | 65 ± 1 | ND | 19 ± 0.7 | |
| pH 2.0 | 4 ± 0.1 | 21 ± 1 | ND | 59 ± 1 | |
Incubations contained 14C-N-NO-MeIQx (4 μM, 50 mCi/mmol) at 37°C for 4 hours with the indicated pH in 100 mM phosphate buffer. Samples were analyzed by HPLC and values represent mean ± SE (n = 3). The limit of detection is 0.5% of total radioactivity recovered by HPLC.
ND, not detected
Reactivity of N-NO-MeIQx with Nucleophiles
The time course of stability and reactivity of 14C-N-NO-MeIQx (4 μM) were further evaluated at pH 2.0 in the presence or absence of 10 mM NaN3 (Figure 5). Breakdown of N-NO-MeIQx followed first order kinetics with half lives of 2.1 ± 0.2 and 1.2 ± 0.1 min in the absence and presence of 10 mM NaN3, respectively. Azide significantly (p < 0.05) decreased the half live for N-NO-MeIQx by 43%. After a 10 min-incubation with 10 mM NaN3, greater than 90% of the recovered radioactivity elutes with the 2-azido-MeIQx standard. The results are consistent with nucleophilic N3− reacting with a rate determining intermediate of N-NO-MeIQx hydrolysis.
Figure 5.
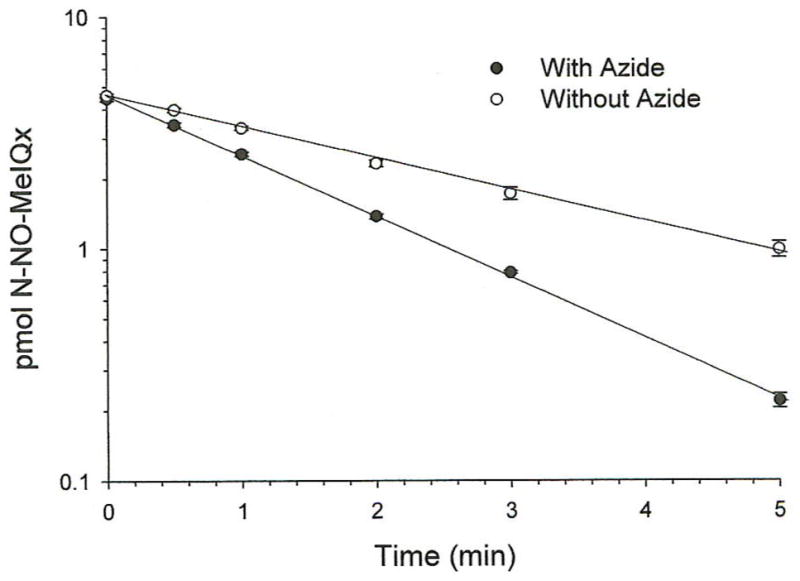
Time course of 14C-N-NO-MeIQx stability at pH 2.0. Incubations were conducted with (closed circles) or without 10 mM NaN3 (open circles) at pH 2.0 with citrate/phosphate buffer. Samples were analyzed by HPLC. Illustrated is an analysis of values representing means ± SEM of duplicate determinations from one of three independent experiments analyzed.
To determine if electrophile(s) produced by N-NO-MeIQx at acidic pH could bind DNA, 14C-N-NO-MeIQx (0.03 mM) was incubated at various pH values for 1 hour with 1 mg/ml DNA (Table 2). While binding was not detected at pH 7.4, it increased with decreasing pH. Binding at pH 2.0 is about 10-fold more than that at pH 5.5. Binding at pH 2.0 was completely inhibited with 10 mM NaN3. HPLC analysis of the reaction mixture supernatant indicated that 2-azido-IQ accounted for 88 % of the total recovered radioactivity. Thus, N-NO-MeIQx forms an electrophile(s) at acidic pH, which can bind DNA.
To evaluate the ability to N-NO-MeIQx to form DNA adducts, this nitrosamine (0.06 mM) was incubated with 3 mM dGp for 1 hour at pH 5.5 and results compared to that observed with N-OH-MeIQx (0.06 mM) at pH 7.4. Adduct formation was measured by 32P-postlabeling (Figure 6). Incubation of N-OH-MeIQx with dGp resulted in the formation of one major adduct, adduct 1 (402 × 105 RAL). This adduct has been previously identified as dG-C8-MeIQx (15). With N-NO-MeIQx, at least 7 adducts were observed. One of these adducts corresponded to adduct 1 (0.5 × 105 RAL), dG-C8-MeIQx. Incubation of N-NO-MeIQx with 0.1 mM HOCl, a mediator of inflammation, resulted in increased amounts of adducts. However, only 5 adducts were detected with this condition. Formation of adduct 1 (11 × 105 RAL) increased with HOCl treatment. Adducts formed by N-NO-MeIQx and N-OH-MeIQx were compared to that formed in liver from a male mouse treated with MeIQx. In addition to adduct 1 (0.2 × 105 RAL), two other adducts were detected in the liver sample. The latter two adducts appear to be identical to adduct a and 2 detected in N-NO-MeIQx incubations. Increased amounts of these adducts were also detected following HOCl treatment. Thus, N-NO-MeIQx, like N-OH-MeIQx, forms dG-C8-MeIQx, and N-NO-MeIQx, but not N-OH-MeIQx, may contribute to the formation of additional adducts detected in liver.
Figure 6.
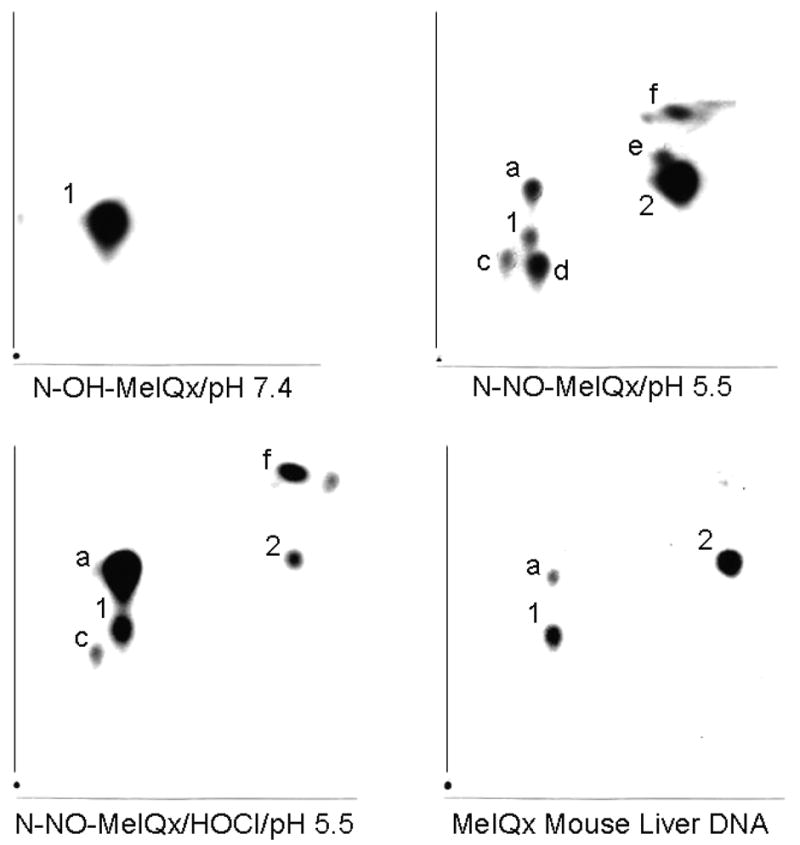
32P-Postlabeled Adducts. Incubations contained 0.06 mM N-OH-MeIQx or N-NO-MeIQx, 3 mM 2′-deoxyguanosine 3′-monophosphate, 0.1 mM DETAPAC, 100 mM potassium phosphate buffer. N-OH-MeIQx was incubated at pH 7.4. N-NO-MeIQx was incubated in the absence and presence of 0.1 mM HOCl (pH 5.5). Liver DNA was from a mouse administered 20 mg/kg MeIQx by gavage for 10 successive days. Exposure times were 15 min for N-OH-MeIQx, 6 hrs for N-NO-MeIQx, 1 hour for N-NO-MeIQx/HOCl, and 16 hrs for liver DNA.
Discussion
This study examines the stability and reactivity of N-NO-MeIQx in an effort to determine its possible role in carcinogenesis. This nitrosamine was completely stable for 4 hours from pH 7.4 to 9. While biologically relevant nucleophiles, such as glutathione and ascorbic acid, can increase the hydrolysis of N-nitroso and S-nitroso compounds (46), glutathione did not influence N-NO-MeIQx stability at pH 7.4. A decrease in pH resulted in decreased stability of N-NO-MeIQx with new products identified as 3,8-dimethylimidazo[4,5-f]quinoxaline and 2-hydroxy-3,8-dimethylimidazo[4,5-f]quinoxaline. MeIQx was only a minor product. The presence of the nucleophilic azide anion under acidic conditions resulted in the formation of 2-azido-MeIQx. Azide anion shortened the t1/2 of N-NO-MeIQx at pH 2.0 by forming the 2-azido derivative. Binding to DNA increased with decreasing pH. The decreased stability and increased reactivity of N-NO-MeIQx at acidic pH values is consistent with formation of a reactive electrophile(s) at acidic pH, which binds DNA forming adducts. This hypothesis was tested directly by comparing adduct formation of N-OH-MeIQx to N-NO-MeIQx in the presence of dGp. Using conditions that mimic an inflammatory response, acid pH ± HOCl (36–38), 5 to 7 adducts were produced by the nitrosamine. One of these adducts corresponded to dG-C8-MeIQx, which is the major adduct produced by N-OH-MeIQx. Liver from a mouse treated with MeIQx contained dG-C8-MeIQx and two other adducts detected with N-NO-MeIQx, but not N-OH-MeIQx These results suggest N-NO-MeIQx can be activated under inflammatory conditions to form dG-C8-MeIQx. Thus, N-NO-MeIQx could be genotoxic.
Azide has been used to evaluate the reactivity of esters of N-acetylhydroxamic acids or their corresponding hydroxylamines for aromatic and heterocyclic amine carcinogens (47, 48). Azide trapping did not influence the hydrolysis of these esters, since trapping occurs after the rate-limiting formation of nitrenium ions. For aromatic amine nitrenium ions, azide anions are primarily directed at the ortho carbon of the aromatic ring rather than on nitrogen. For the nitrenium ions of 2-amino-3-methylimidazo[4,5-f]quinoxaline and MeIQx, azide substitution is at position 5. The reactivity of nucleophilic azide anion with N-NO-MeIQx is different from that of carcinogenic N-OH aromatic and heterocyclic amines.
The stability and reactivity of N-NO-MeIQx is similar to that reported for some diazotates. They can be formed by the reaction of the heterocyclic amine, such as 2-deoxycytidine, with nitrous acid or nitric oxide (49). This product, 1-(beta-D-2′-deoxyribofuranosyl)-2-oxopyrimidine-4-diazotate, is stable at neutral pH. At pH 3.7 and 37°C, the diazotate is hydrolyzed exclusively to 2′-deoxyuridine by a first order reaction with a t1/2 of 24 min. With pyridine-4-diazotate, the hydrolysis product observed with 1.15 M perchloric acid is 4-pyridone and with 5.5 M perchloric acid, the product is 4-aminopyridine (50). With chloride ion, 4-chloropyridine is the major product. The hydrolysis of N-NO-MeIQx may occur by a mechanism similar to diazotates, such as the following: diazohydroxide (R-N=N-OH), diazotate (R-N=N-O−), diazonium (R-N=N+), and alkyl cation (R+) (50, 51). The alkyl cation or its precursor (diazo species) reacts with nucleophiles to form the observed products. Formation of the primary amine at lower pH is attributed to a reversible nitrosation reaction, denitrosation (51).
To assist in the quantitation and identification of adducts, dGp was used instead of DNA for adduct formation. The use of this mononucleotide simplifies the determination of adduct recovery compared to that which occurs when DNA or polynucleotides are used, since the latter require micrococcal nuclease/spleen phosphodiesterase hydrolysis. In addition with dGp, multiple radiolabeled products represent unique guanine adducts and not micrococcal nuclease/spleen phosphodiesterase-resistant oligomers (19). These adducts may represent oxidation products of NNO-MeIQx formed before and/or after its binding to guanine.
These results are consistent with previous studies evaluating the stability and reactivity of 2-nitrosoamino-3-methylimidazo[4,5-f]quinoline (N-NO-IQ) (52). N-NO-IQ also reacts with nucleophiles under acidic conditions to form 2-substituted derivatives and exhibits increased binding to DNA and adduct formation. N-(Deoxyguanosin-8-yl)-2-amino-3-methylimidazo[4,5-f]quinoline, which is thought to be responsible for the mutagenicity and carcinogenicity of 2-amino-3-methylimidazo[4,5-f]quinoline (19), is a prominent adduct. The major differences in the reactivity of these nitrosamines are that N-NO-IQ has a much larger t1/2 (10 ± 2 min) at pH 2.0 compared to N-NO-MeIQx (2.1 ± 0.2 min) and that N-NO-IQ does not form a 2-OH derivative under acidic conditions. Preliminary studies have demonstrated that both nitrosamines are mutagenic (53, 54).
This study demonstrates that dG-C8-MeIQx is formed by N-NO-MeIQx under conditions that mediate inflammatory responses, such as acidic pH (pH 5.3 to 5.7) and HOCl (36–38). This adduct is thought to be responsible for the mutagenicity and carcinogenicity of MeIQx, and is the major adduct present in human colon (55, 56). Inflammatory diseases, such as colitis, have a complex matrix of cells (macrophages, lymphocytes, and neutrophils) and inflammatory mediators (NO, H2O2, HOCl, myeloperoxidase, NO2−, NO2•) (25–27). The temporal and spatial associations necessary for the various conditions to both produce and then activate N-NO-MeIQx may exist in individuals with colitis. In addition, inflammation can further aid in the initiation of N-NO-MeIQx colon carcinogenesis by inhibiting DNA repair mechanisms (29, 30). These results are consistent with our hypothesis that N-NO-MeIQx is an alternative to N-OH-MeIQx in the initiation of colon cancer in individuals with colitis. Thus, inflammation provides a versatile milieu for facilitating the incorporation of MeIQx mutations into the genome.
Acknowledgments
We thank Priscilla Jones for excellent technical assistance. This work was supported by the Department of Veterans Affairs (T.V.Z.) and National Cancer Institute Grant CA72613 (T.V.Z.). Mass spectrometry was performed at Washington University School of Medicine, through National Institutes of Health Grants P41-RR00954, P30 DK56341, and P60-DK20579. We appreciate Dr. Katherine S. Virgo’s assistance with statistical analysis of our data.
Footnotes
Abbreviations: MeIQx, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline; 2-azido-MeIQx, 2-azido-3,8-dimethylimidazo[4,5-f]quinoxaline; CAD, collisionally activated dissociation; dGp, 2′-deoxyguanosine 3′-monophosphate; DETAPAC, diethylenetriaminepentaacetic acid; D-MeIQx, 3,8-dimethylimidazo[4,5-f]quinoxaline; ESI, electrospray ionization; HCAs, heterocyclic amines; 2-OH-MeIQx, 2-hydroxy-3,8-dimethylimidazo[4,5-f]quinoxaline; N-OH-MeIQx; N-hydroxy-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline; iNOS, inducible nitric oxide synthase; MS, mass spectrometry; dG-C8-MeIQx, N-(deoxyguanosin-8-yl)-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline; N-NO-IQ, 2-nitrosoamino-3-methylimidazo[4,5-f]quinoline; N-NO-MeIQx, 2-nitrosoamino-3,8-dimethylimidazo[4,5-f]quinoxaline; RNOS, reactive nitrogen oxygen species, RAL, Relative Adduct Labeling
References
- 1.Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 2.Pais P, Salmon CP, Knize MG, Felton JS. Formation of mutagenic/carcinogenic heterocyclic amines in dry-heated model systems, meats, and meat drippings. J Agric Food Chem. 1999;47:1098–1108. doi: 10.1021/jf980644e. [DOI] [PubMed] [Google Scholar]
- 3.NTP. Report on carcinogens background document for heterocyclic amines: MeIQ, MeIQx, IQ, and PhIP. National Toxicology Program. 2005 http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s092vhca.pdf.
- 4.Ushiyama H, Wakabayashi K, Hirose M, Itoh H, Sugimura T, Nagao M. Presence of carcinogenic heterocyclic amines in urine of healthy volunteers eating normal diet, but not of inpatients receiving parenteral alimentation. Carcinogenesis. 1991;12:1417–1422. doi: 10.1093/carcin/12.8.1417. [DOI] [PubMed] [Google Scholar]
- 5.Thompson LH, Tucker JD, Stewart SA, Christensen ML, Salazar EP, Carrano AV, Felton JS. Genotoxicity of compounds from cooked beef in repair-deficient CHO cells versus Salmonella mutagenicity. Mutagenesis. 1987;2:483–487. doi: 10.1093/mutage/2.6.483. [DOI] [PubMed] [Google Scholar]
- 6.Ohgaki H, Hasegawa H, Suenaga M, Sato S, Takayama S, Sugimura T. Carcinogenicity in mice of a mutagenic compound, 2-amino-3,8- dimethylimidazo[4,5-f]quinoxaline (MeIQx) from cooked foods. Carcinogenesis. 1987;8:665–668. doi: 10.1093/carcin/8.5.665. [DOI] [PubMed] [Google Scholar]
- 7.Hoshi M, Morimura K, Wanibuchi H, Wei M, Okochi E, Ushijima T, Takaoka K, Fukushima S. No-observed effect levels for carcinogenicity and for in vivo mutagenicity of a genotoxic carcinogen. Toxicol Sci. 2004;81:273–279. doi: 10.1093/toxsci/kfh241. [DOI] [PubMed] [Google Scholar]
- 8.Tanakamaru Z, Mori I, Nishikawa A, Furukawa F, Takahashi M, Mori H. Essential similarities between spontaneous and MeIQx-promoted aberrant crypt foci in the F344 rat colon. Cancer Lett. 2001;172:143–149. doi: 10.1016/s0304-3835(01)00636-x. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Stampfer MJ, Hough HL, Garcia-Closas M, Willett WC, Hennekens CH, Kelsey KT, Hunter DJ. A prospective study of N-Acetyltransferase genotype, red meat intake, and risk of colorectal cancer. Cancer Res. 1998;58:3307–3311. [PubMed] [Google Scholar]
- 10.Sinha R, Chow WH, Kulldorff M, Denobile J, Butler J, Garcia-Closas M, Weil R, Hoover RN, Rothman N. Well-done, grilled red meat increases the risk of colorectal adenomas. Cancer Res. 1999;59:4320–4324. [PubMed] [Google Scholar]
- 11.Felton JS, Knize MG. Heterocyclic-amine mutagens/carcinogens in foods. In: Cooper CS, Grover PL, editors. Handbook of Experimental Pharmacology. Springer-Verlag; Berlin: 1990. pp. 471–502. [Google Scholar]
- 12.Sinha R, Kulldorff M, Chow WH, Denobile J, Rothman N. Dietary intake of heterocyclic amines, meat-derived mutagenic activity, and risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2001;10:559–562. [PubMed] [Google Scholar]
- 13.Turesky RJ, Markovic J, Bracco-Hammer I, Fay LB. The effect of dose and cytochrome P450 induction on the metabolism and disposition of the food-borne carcinogene 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) in the rat. Carcinogenesis. 1991;12:1847–1855. doi: 10.1093/carcin/12.10.1847. [DOI] [PubMed] [Google Scholar]
- 14.Turesky RJ, Constable A, Richoz J, Varga N, Markovic J, Martin MV, Guengerich FP. Activation of heterocyclic aromatic amines by rat and human liver microsomes and by purified rat and human cytochrome P450 1A2. Chem Res Toxicol. 1998;11:925–936. doi: 10.1021/tx980022n. [DOI] [PubMed] [Google Scholar]
- 15.Turesky RJ, Rossi SC, Welti DH, Lay JO, Jr, Kadlubar FF. Characterization of DNA adducts formed in vitro by reaction of N-hydroxy-2-amino-3-methylimidazo[4,5-f]quinoline and N-hydroxy-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline at the C-8 and N2 atoms of guanine. Chem Res Toxicol. 1992;5:479–490. doi: 10.1021/tx00028a005. [DOI] [PubMed] [Google Scholar]
- 16.Snyderwine EG, Davis CD, Nouso K, Roller PP, Schut HA. 32P-postlabeling analysis of IQ, MeIQx and PhIP adducts formed in vitro in DNA and polynucleotides and found in vivo in hepatic DNA from IQ-, MeIQx- and PhIP-treated monkeys. Carcinogenesis. 1993;14:1389–1395. doi: 10.1093/carcin/14.7.1389. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita K, Adachi M, Kato S, Nakagama H, Ochiai M, Wakabayashi K, Sato S, Nagao M, Sugimura T. DNA adducts formed by 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in rat liver: dose-response on chronic administration. Jpn J Cancer Res. 1990;81:470–476. doi: 10.1111/j.1349-7006.1990.tb02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turteltaub KW, Felton JS, Gledhill BL, Vogel JS, Southon JR, Caffee MW, Finkel RC, Nelson DE, Proctor ID, Davis JC. Accelerator Mass Spectrometry in Biomedical Dosimetry: Relationship Between Low-Level Exposure and Covalent Binding of Heterocyclic Amine Carcinogens to DNA. Proc Natl Acad Sci. 1990;87:5288–5292. doi: 10.1073/pnas.87.14.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schut HA, Snyderwine EG. DNA adducts of heterocyclic amine food mutagens: implications for mutagenesis and carcinogenesis. Carcinogenesis. 1999;20:353–368. doi: 10.1093/carcin/20.3.353. [DOI] [PubMed] [Google Scholar]
- 20.Solomon MS, Morgenthaler PML, Turesky RJ, Essigmann JM. Mutational and DNA Binding Specificity of the Carcinogen 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline. J Biol Chem. 1996;271:18368–18374. doi: 10.1074/jbc.271.31.18368. [DOI] [PubMed] [Google Scholar]
- 21.Kudo M, Ogura T, Esumi H, Sugimura T. Mutational activation of c-Ha-ras gene in squamous cell carcinomas of rat Zymbal gland induced by carcinogenic heterocyclic amines. Mol Carcinog. 1991;4:36–42. doi: 10.1002/mc.2940040107. [DOI] [PubMed] [Google Scholar]
- 22.Parsonnet J. Infection as a Cause of Human Cancer. Oxford University Press; New York: 1999. Microbes and Malignancy. [Google Scholar]
- 23.Harpaz N, Talbot IC. Colorectal cancer in idiopathic inflammation bowel disease. Semin Diagn Pathol. 1996;13:339–357. [PubMed] [Google Scholar]
- 24.Grisham MB, Jourd’Heuil D, Wink DA. Nitric oxide - I. Physiological chemistry of nitric oxide and its metabolites: implications in inflammation. Am J Physiol. 1999;39:G315–G321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- 25.Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, Shields PG, Ham AJ, Swenberg JA, Marrogi AJ, Harris CC. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: A cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- 26.Singer II, Kawka DW, Scott S, Weidner JR, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- 27.Dijkstra G, Moshage H, Van Dullemen HM, De Jager-Krikken A, Tiebosch ATMG, Kleibeuker JH, Jansen PLM, Van Goor H. Expression of nitric oxide synthases and formation of nitrotyrosine and reactive oxygen species in inflammatory bowel disease. J Pathol. 1998;186:416–421. doi: 10.1002/(SICI)1096-9896(199812)186:4<416::AID-PATH201>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 28.Ambs S, Bennett WP, Merriam WG, Ogunfusika MO, Oser SM, Harrington AM, Shields PG, Felley-Bosco E, Hussain SP, Harris CC. Relationship between p53 mutations and inducible nitric oxide synthase expression in human colorectal cancer. J Natl Cancer Inst. 1999;91:86–88. doi: 10.1093/jnci/91.1.86. [DOI] [PubMed] [Google Scholar]
- 29.Jaiswal M, LaRusso NF, Nishioka N, Nakabeppu Y, Gores GJ. Human Ogg1, a protein involved in the repair of 8-oxoguanine, is inhibited by nitric oxide. Cancer Res. 2001;61:6388–6393. [PubMed] [Google Scholar]
- 30.Liu L, Xu-Welliver M, Kanugula S, Pegg AE. Inactivation and degradation of O6-alkylguanine-DNA alkyltransferase after reaction with nitric oxide. Cancer Res. 2002;62:3037–3043. [PubMed] [Google Scholar]
- 31.Srivatanakul P, Ohshima H, Khlat M, Parkin M, Sukaryodhin S, Brouet I, Bartsch H. Opisthorchis viverrini infestation and endogenous nitrosamines as risk factors for cholangiocarcinoma in Thailand. Int J Cancer. 1991;48:821–825. doi: 10.1002/ijc.2910480606. [DOI] [PubMed] [Google Scholar]
- 32.Satarug S, Haswell-Elkins MR, Tsuda M, Mairiang P, Sithithaworn P, Mairiang E, Esumi H, Sukprasert S, Yongvanit P, Elkins DB. Thiocyanate-independent nitrosation in humans with carcinogenic parasite infection. Carcinogenesis. 1996;17:1075–1081. doi: 10.1093/carcin/17.5.1075. [DOI] [PubMed] [Google Scholar]
- 33.Tricker AR, Mostafa MH, Spiegelhalder B, Preussmann R. Urinary excretion of nitrate, nitrite and N-nitroso compounds in Schistosomiasis and bilharzia bladder cancer patients. Carcinogenesis. 1989;10:547–552. doi: 10.1093/carcin/10.3.547. [DOI] [PubMed] [Google Scholar]
- 34.Morgan ET. Regulation of cytochromes P450 during inflammation and infection. Drug Metab Rev. 2003;29:1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- 35.Lakshmi VM, Hsu FF, Zenser TV. Nitric oxide-mediated nitrosation of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline potentiated by hemin and myeloperoxidase. Chem Res Toxicol. 2005;18:1038–1047. doi: 10.1021/tx0500070. [DOI] [PubMed] [Google Scholar]
- 36.Kettle AJ, Winterbourn CC. Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep. 1997;3:3–15. doi: 10.1080/13510002.1997.11747085. [DOI] [PubMed] [Google Scholar]
- 37.Dubos RJ. The micro-environment of inflammation or Metchnikoff revisited. Lancet. 1955;269:1–5. doi: 10.1016/s0140-6736(55)93374-2. [DOI] [PubMed] [Google Scholar]
- 38.Bryant RE, Rashad AL, Mazza JA, Hammond D. β-Lactamase activity in human pus. J Infect Dis. 1980;142:594–601. doi: 10.1093/infdis/142.4.594. [DOI] [PubMed] [Google Scholar]
- 39.NIH Guidelines for the Laboratory Use of Chemical Carcinogens. NIH Publication 81–2385. U.S. Government Printing Office; Washington, DC: 1981. [Google Scholar]
- 40.Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, Bove AA, Isaac L, Hrabie JA, Keefer LK. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant Effects. J Med Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 41.Cummings DA, Schut HAJ. Removal of DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the male Fischer-344 rat. Carcinogenesis. 1994;15:2623–2628. doi: 10.1093/carcin/15.11.2623. [DOI] [PubMed] [Google Scholar]
- 42.Lakshmi VM, Hsu FF, Zenser TV. Nitrosation and nitration of 2-amino-3-methylimidazo[4,5-f]quinoline by reactive nitrogen oxygen species. Chem Res Toxicol. 2002;15:1059–1068. doi: 10.1021/tx020008h. [DOI] [PubMed] [Google Scholar]
- 43.Lakshmi VM, Zenser TV, Goldman HD, Spencer GG, Gupta RC, Hsu FF, Davis BB. The role of acetylation in benzidine metabolism and DNA adduct formation in dog and rat liver. Chem Res Toxicol. 1995;8:711–720. doi: 10.1021/tx00047a011. [DOI] [PubMed] [Google Scholar]
- 44.Gupta RC, Reddy MV, Randerath K. 32P-Postlabeling analysis of non-radioactive aromatic carcinogen-DNA adducts. Carcinogenesis (Lond) 1982;3:1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- 45.Schut HAJ, Herzog CR. Formation of DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in male Fischer-344 rats. Cancer Lett. 1992;67:117–124. doi: 10.1016/0304-3835(92)90134-h. [DOI] [PubMed] [Google Scholar]
- 46.Kirsch M, Fuchs A, de Groot H. Regiospecific nitrosation of N-(terminal) blocked tryptophan derivatives by N2O3 at physiological pH. J Biol Chem. 2003;278:11931–11936. doi: 10.1074/jbc.M300237200. [DOI] [PubMed] [Google Scholar]
- 47.Novak M, Kahley MJ, Eiger E, Helmick JS, Peters HE. Reactivity and selectivity of nitrenium ions derived from ester derivatives of carcinogenic N-(4-biphenylyl)hydroxylamine and the corresponding hydroxamic acid. J Am Chem Soc. 1993;115:9453–9460. [Google Scholar]
- 48.Novak M, Toth K, Rajagopal S, Brooks M, Hott LL, Moslener M. Reactivity and selectivity of the N-acetyl-Glu-P-1, N-acetyl-Glu-P-2, N-acetyl-MeIQx, and N-acetyl-IQx nitrenium ions: comparison to carbocyclic N-arylnitrenium ions. J Am Chem Soc. 2002;124:7972–7981. doi: 10.1021/ja0121944. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki T, Nakamura T, Yamada M, Ide H, Kanaori K, Tajima K, Morii T, Makino K. Isolation and characterization of diazoate intermediate upon nitrous acid and nitric oxide treatment of 2′-deoxycytidine. Biochemistry. 1999;38:7151–7158. doi: 10.1021/bi982803t. [DOI] [PubMed] [Google Scholar]
- 50.Bunton CA, Wolfe BB. Decomposition of pyridine-2- and -4-diazoates. J Am Chem Soc. 1974;96:3267–3275. [Google Scholar]
- 51.Zollinger H. Diazo Chemistry I: Aromatic and Heteroaromatic Compounds. VCH, Weinheim; Germany: 1994. [Google Scholar]
- 52.Lakshmi VM, Hsu FF, Zenser TV. 2-Nitrosoamino-3-methylimidazo[4,5-f]quinoline stability and reactivity. Chem Res Toxicol. 2004;17:709–716. doi: 10.1021/tx030042b. [DOI] [PubMed] [Google Scholar]
- 53.Zenser TV, Lakshmi VM, Zhou H, Josephy PD, Schut HAJ. Genotoxicity of 2-Nitrosoamino-3-Methylimidazo[4,5-f]Quinoline. Proc Am Assoc Cancer Res. 2004;45:4863. [Google Scholar]
- 54.Lakshmi VM, DeHaven P, Hsu HH, Schut HAJ, Zenser TV. Reactivity and genotoxicity of 2-nitrosoamino-3,8-dimethylimidazo[4,5-f]quinoxaline. Proc Am Assoc Cancer Res. 2005;46:3060. [Google Scholar]
- 55.Totsuka Y, Fukutome K, Takahashi M, Takahashi S, Tada A, Sugimura T, Wakabayashi K. Presence of N2-(deoxyguanosin-8-yl)-2-amino-3,8-dimethylimidazo[4,5- f]quinoxaline (dG-C8-MeIQx) in human tissues. Carcinogenesis. 1996;17:1029–1034. doi: 10.1093/carcin/17.5.1029. [DOI] [PubMed] [Google Scholar]
- 56.Mauthe RJ, Dingley KH, Leveson SH, Freeman SP, Turesky RJ, Garner RC, Turteltaub KW. Comparison of DNA-adduct and tissue-available dose levels of MeIQx in human and rodent colon following administration of a very low dose. Int J Cancer. 1999;80:539–545. doi: 10.1002/(sici)1097-0215(19990209)80:4<539::aid-ijc10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]