Synopsis
The influence of caloric restriction on hepatic glyceraldehyde and glycerol metabolizing enzyme activities of young and old mice were studied. Glycerol kinase and cytoplasmic glycerol-3-phosphate dehydrogenase activities were increased in both young and old CR mice when compared to controls, while triokinase increased only in old CR mice. Aldehyde dehydrogenase and aldehyde reductase activities in both young and old CR were unchanged by CR. Mitochondrial glycerol-3-phosphate dehydrogenase showed a trend towards an increased activity in old CR mice, while a trend towards a decreased activity in alcohol dehydrogenase was observed in both young and old CR mice. Serum glycerol levels decreased in young and old CR mice. Therefore, increases in glycerol kinase and glycerol-3-phosphate dehydrogenase were associated with a decrease in fasting blood glycerol levels in CR animals. A prominent role for triokinase in glyceraldehyde metabolism with CR was also observed. The results indicate that long-term CR induces sustained increases in the capacity for gluconeogenesis from glycerol.
Keywords: Aldehyde dehydrogenase, aldehyde reducatse, glycerol kinase, glycerol-3-phosphate dehydrogenase, triokinase
Introduction
Glycerol and glyceraldehyde are two metabolites of intermediary metabolism, resulting from the breakdown of triglycerides and fructose, respectively. These metabolites occupy important positions by acting as the links between several metabolic pathways, and undergo further metabolism by entering into these pathways. Glycerol is produced as a result of the breakdown of proteins, triglycerides and other glycerolipids, as well as dietary fats [for reviews see 1-4]. It is also formed from de-esterification of triglycerides (TG) in adipose tissues, skeletal muscle, liver, and blood [5], with de-esterification in adipose tissue and blood accounting for the majority of circulating glycerol [6,7]. Glycerol metabolizing enzymes are essentially tissue-specific, with glycerol kinase (GK), glycerol-3-phosphate dehydrogenase (G3PDH) and alcohol dehydrogenase (ADH) found predominately in liver [1,7]. Glycerol is utilized as an important gluconeogenic substrate, mainly in the liver and kidneys. It is converted by phosphorylation to glycerol-3-phosphate (G3P), which is the more physiologically important form of glycerol, and then to dihydroxyacetonephosphate (DHAP), catalyzed by NAD-dependent cG3PDH, therefore, facilitating its entry into the glycolytic pathway. Also, G3P is metabolized by mitochondrial FAD-dependent mG3PDH, part of G3P shuttle, which is found on the outer surface of the inner membrane. cG3PDH and mG3PDH form the glycerol phosphate shuttle, which plays an important role in tissues that oxidize glucose rapidly, such as brain [8], skeletal muscle [9] and the flight muscle of insects [10], regenerating NAD from NADH formed in glycolysis. This shuttle, however, does not play an important role in other mammalian systems, such as liver and kidneys, where the malate-aspartate shuttle is the predominant one [1,4,11]. Glycerol is also metabolized by alcohol dehydrogenase (ADH) to D-glyceraldehyde, as well as undergoing re-esterification to form triglycerides. It has been reported that in humans, under normal conditions, glycerol contribution to gluconeogenesis is less than 5%, but increasing to more than 20% after 2-3 days of starvation and becoming the primary source for gluconeogenesis during prolonged fasting since glycogen stores are depleted within 2 days [6,12]. Under conditions of starvation, glycerol provided almost 80% of new glucose in obese individuals, as opposed to 38% in lean individuals [6,13]. The increased utilization of glycerol for gluconeogenesis during starvation, due to lipolysis, was attributed to increased hepatic GK and G3PDH activities [14]. While there is considerable information about glycerol metabolism during starvation, it is not known if its metabolism is altered with sustained CR.
Glyceraldehyde, on the other hand, is produced from the breakdown of fructose-1-phosphate (F1P), connecting fructose metabolism to that of glycerol and to glycolysis and gluconeogenesis. Therefore, glyceraldehyde can be metabolized via three different routes [15]: first, it can be converted to glyceraldehyde-3-phosphate (GAP) by triokinase (TK), and enters the glycolytic pathway. Second, it can be converted to D-glycerate by aldehyde dehydrogenase (ALDH), which after further conversion by glycerate kinase (GlyK) also enters the glycolytic pathway. Third, it can be converted to glycerol by alcohol dehydrogenase (ADH). Of these three possible routes, the one catalyzed by triokinase is the most dominant one [16,17]. Moreover, the conversion of glyceraldehyde to glycerol can also be achieved by the action of aldehyde reductase [18], which is also known as NADP-dependent alcohol dehydrogenase. While the pathways for glyceraldehyde metabolism have been clearly defined, it is not known if energy intake plays a role in regulating the activity of these pathways.
Caloric restriction (CR), without malnutrition, is the only intervention that has been shown consistently to delay pathophysiological changes and extend maximum life span in a variety of organisms [19,20]. This association between CR-related increases in longevity and lowered age-related pathophysiological changes has been known for over 70 years [21]. Effects of CR appear to be dependent on an overall decrease in energy intake rather than a decrease in any particular nutrient [22]. Therefore, the changes in energy metabolism must clearly play a central role in the actions of CR and the ability of the animal to survive sustained CR. The adaptations in energy metabolism under sustained CR conditions have not been well defined, and it is not known if changes in energy metabolism occur after dynamic weight loss has ceased. In previous studies [17,23-26], we have reported the influence of CR on other major metabolic pathways. The aim of this study was to characterize and establish the effects of long-term CR and aging on the glycerol and glyceraldehyde metabolizing enzymes. The pathways for the metabolism of these two metabolites are interconnected with each other and with previously studied pathways; therefore, it would be of interest to see if these two metabolites do contribute to the provision of alternative energy sources during CR.
Materials and Methods
Materials
All laboratory chemicals and auxiliary enzymes were purchased from Sigma Chemical Company (St. Louis, MO) or Roche diagnostics corporation (Indianapolis, IN). The protein assay kit was from Bio-Rad laboratories (Hercules, CA).
Animals
Male C57Bl/6J mice were purchased from Charles River Laboratories (Wilmington, MA) at one month of age and housed singly, at 23°C and with a 12 h light/12 h dark cycle. Animals were maintained in accordance with the local and federal guidelines governing animal experimentation. The mice were fed ad libitum a non-purified diet, PLI 5001 (Purina Laboratories, St. Louis, MO) for one month and at two months of age they were assigned either to the control or CR group and fed semi-purified diets, as described elsewhere [27]. At the time of sacrifice, young animals were three months of age (one month on either the control or CR diet) and old animals were 30 months of age (28 months on either the control or CR diet). All parameters were measured in six animals for each age and diet group. CR mice were on a 30% restricted diet, and all feeding procedures were as described previously [25].
Tissue harvesting and preparation
After an overnight fast, all mice were sacrificed and their livers harvested between 9:00–10:00am, as described previously [25]. Livers were rapidly freeze-clamped in situ, placed immediately in liquid nitrogen, powdered under liquid nitrogen in a mortar and pestle, and stored in liquid nitrogen for future use. Blood was collected immediately after decapitation, from the severed neck blood vessels and serum prepared as described previously [28]. Body weights for old control and CR mice were 31.13g ± 1.34g and 21.91g ± 0.52g, respectively, and 18.68g ± 0.48g and 15.31g ± 0.59g for young control and CR, respectively. Liver weights for old control and CR mice were 1.73g ± 0.06g and 1.19g ± 0.03g, respectively, and 1.14g ± 0.03g and 0.91g ± 0.04g for young control and CR, respectively. The differences between control and CR body weights, in both young and old mice, were statistically significant, as were the liver weights (P < 0.001).
Measurement of enzyme activities
Liquid N2-stored powders were weighed and homogenized at a 1:10 ratio (w/v) and the supernatants saved for assays. Glycerol and glyceraldehyde metabolizing enzymes that were assayed included glycerol kinase (EC 2.7.1.30) [29], cytosolic glycerol-3-phosphate dehydrogenase (EC 1.1.1.8) [30], mitochondrial glycerol-3-phosphate dehydrogenase (EC 1.1.99.5) [31], NAD-dependent alcohol dehydrogenase (EC 1.1.1.1) [32], aldehyde reductase (also known as NADP-dependent alcohol dehydrogenase, EC1.1.1.2) [33], aldehyde dehydrogenase (EC 1.2.1.3) [34], and triokinase (EC 2.7.1.28) [35]. All assays were performed using a Perkin Elmer Lambda 25 UV/VIS spectrophotometer and activities expressed as μmol/min/mg protein.
Measurement of metabolites
For liver metabolites, liquid N2-stored powders were weighed, homogenized in ice-cold perchloric acid (6%, w/v), centrifuged and supernatants removed and neutralized [23] and DHAP [23] and G3P [36] were determined. Glycerol was determined in serum according to the method of Wieland [37]. Liver metabolites were expressed as μmol/g wet weight, while blood glycerol was expressed as μmol/ml serum.
Other methods
Protein concentrations were measured with a Bio-Rad protein assay kit, using BSA as the standard. Statistical comparisons were performed using Student's t test where values of P ≤ 0.05 were taken as statistically significant.
Results
Glyceraldehyde pathway
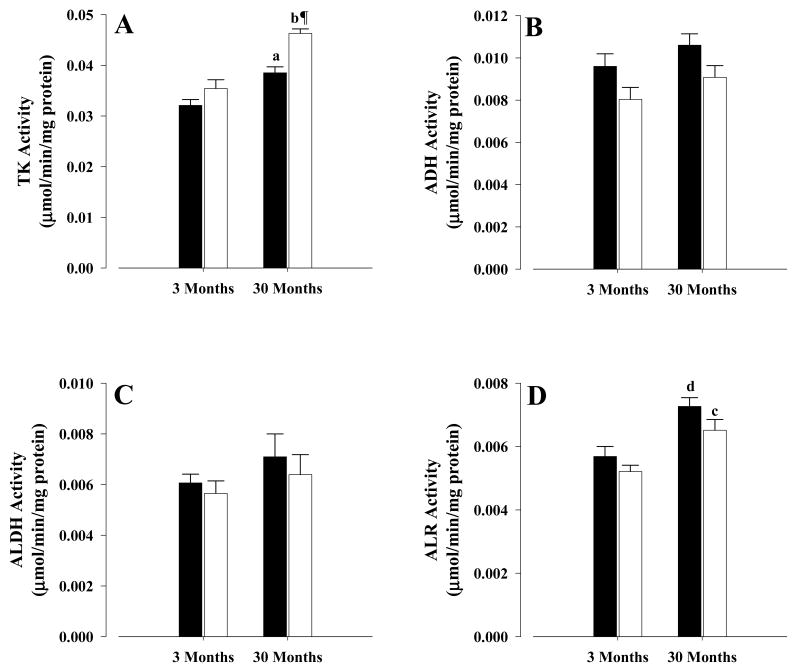
The activities of the glyceraldehyde pathway enzymes are shown in Figure 1. Old CR mice showed a 20% increased (P < 0.001) TK activity (Fig. 1A) and a trend towards a decrease (P = 0.08) in ADH activity (Fig. 1B), when compared with old controls. In the case of ALDH, no significant differences in the activity between old CR and controls were observed (Fig.1C), as was the case for ALR (Fig. 1D). In young mice, on the other hand, no differences were observed between CR and control activities for TK (Fig.1A), ALDH (Fig.1C) and ALR (Fig.1D), however, ADH in young CR (Fig. 1B) showed a trend (P = 0.085) towards a lower activity when compared with young controls.
Figure 1. Activities of glyceraldehyde metabolizing enzymes.
Activities were measured as described in the text. A, triokinase; B, alcohol dehydrogenase; C, aldehyde dehydrogenase; D, aldehyde reductase. All activities were mean ± S.E.M of at least six independent experiments and expressed as μmol/min/mg protein. Controls, solid bars; CR, open bars. Symbols indicate comparisons between CR and control mice: ¶ P < 0.001 old CR vs old control. Letters indicate comparisons between age groups on similar diets: a P < 0.003 old control vs young control; b P < 0.001 old CR vs young CR, c P < 0.01 old CR vs young CR, d P < 0.01 old control vs young control.
As for the effects of age on the activities, TK showed a significantly higher activity in old control (20%, P < 0.003) and old CR (31%, P < 0.001) when compared with their corresponding young mice (Fig.1A), while ADH (Fig.1B) and ALDH (Fig.1C) showed no age-related changes in activity, when young and old were compared. In the case of ALR (Fig.1D), old controls and CR mice were 28% (P < 0.01) and 25% (P < 0.01), respectively, higher in their activities than corresponding young mice.
Glycerol pathway
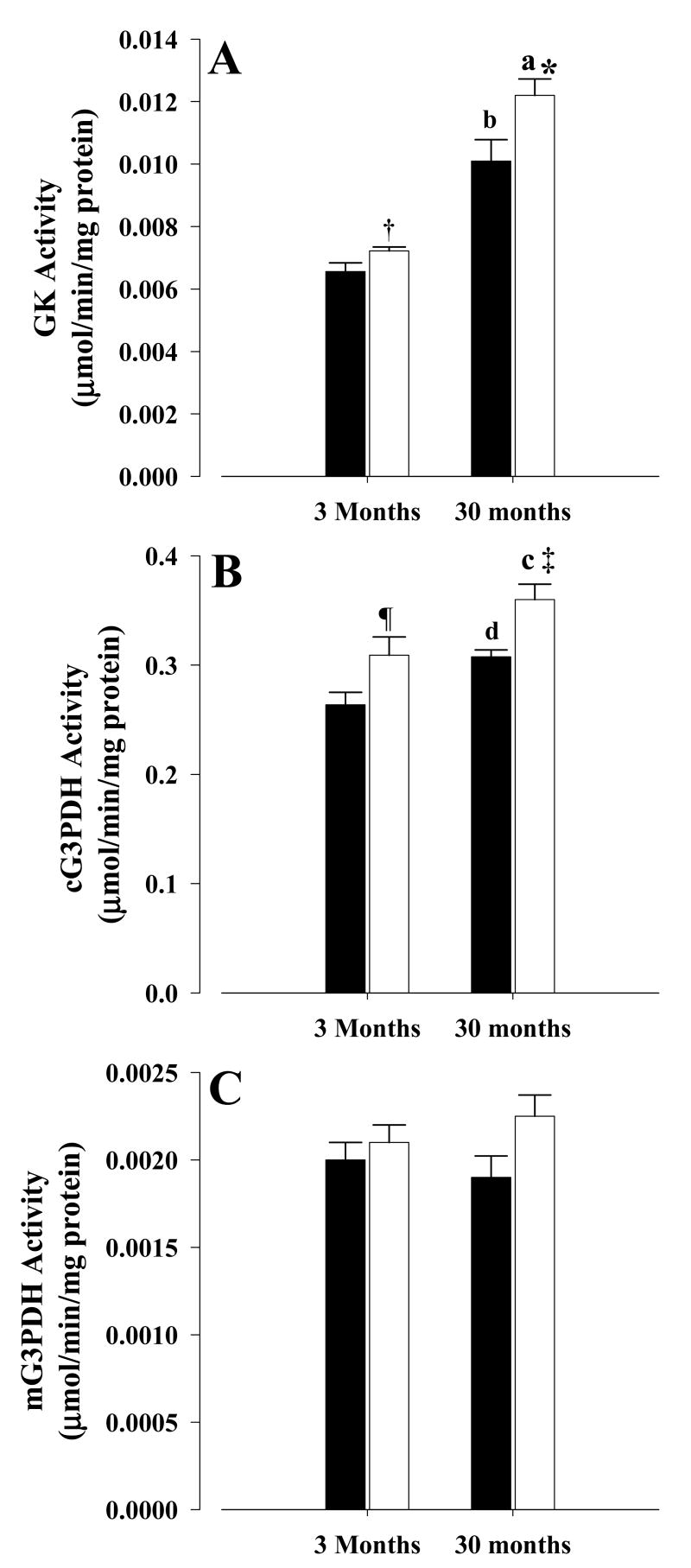
For the glycerol pathway, old CR mice showed a 21% increase (P < 0.03) in GK (Fig. 2A) and 17% increase (P < 0.01) in cG3PDH activities (Fig. 2B) when compared with old controls, while a trend (P = 0.075) toward an increase in mG3PDH was observed in old CR mice when compared with old controls (Fig.2C). In young CR mice, a similar pattern was also observed, with both GK (Fig. 2A) and cG3PDH (Fig. 2B) showing 10% (P < 0.05) and 17% (P < 0.05) increases in their activities, respectively, when compared with young controls while mG3PDH (Fig. 2C) was unchanged between young controls and CR. The other two enzymes involved in glycerol metabolism, namely ADH and ALR, are the same ones involved in the glyceraldehyde metabolism and the results for both old and young CR were the same as those reported in the previous section (Fig. 1B and 1D).
Figure 2. Activities of glycerol metabolizing enzymes.
Activities were measured as described in the text. A, glycerol kinase; B, cytoplasmic glycerol-3-phosphate dehydrogenase; C, mitochondrial glycerol-3-phosphate dehydrogenase. All activities were mean ± S.E.M of at least six independent experiments and expressed as μmol/min/mg protein. Controls, solid bars; CR, open bars. Symbols indicate comparisons between CR and control mice: * P < 0.03 old CR vs old control, † P < 0.05 young CR vs young control, ‡ P < 0.01 old CR vs old control, ¶ P < 0.05 young CR vs young control. Letters indicate comparisons between age groups on similar diets: a P < 0.001 old CR vs young CR, b P < 0.001 old control vs young control, c P < 0.045 old CR vs young CR, d P < 0.01 old control vs young control.
For age-related changes in activities, GK activities (Fig. 2A) were significantly higher (P < 0.001) in both old control and CR mice, when compared with corresponding young mice. This was also the case with cG3PDH (Fig. 2B), where higher activities were observed for old CR (P < 0.045) and old control (P < 0.01), when compared with corresponding young mice. However, no age differences were observed for mG3PDH (Fig. 2C) between young and old mice, while ADH and ALR activities were those previously reported for glyceraldehyde pathway (Fig. 1B & 1D).
Metabolite levels
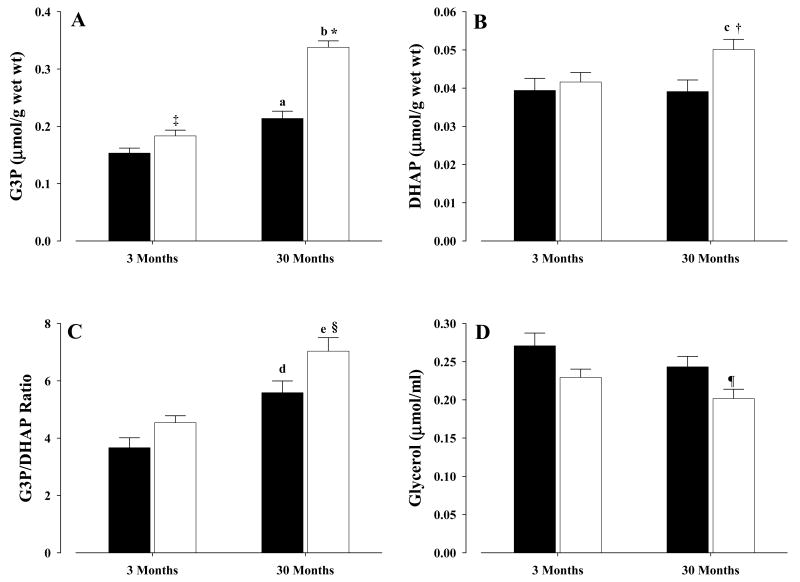
In old CR mice, liver metabolites showed significantly increased levels of G3P (P < 0.001) and DHAP (P < 0.02), as well as an increased G3P/DHAP ratio (P < 0.03), when compared with old controls (Fig. 3A, B, C, respectively). Serum glycerol levels decreased significantly (P < 0.045) in old CR mice when compared with old controls (Fig. 3D). In young CR mice, G3P was increased significantly (P < 0.05), albeit not as dramatically as in the old CR (Fig. 3A), while no differences between young CR and controls were observed for DHAP (Fig. 3B). As for the G3P/DHAP ratio (Fig. 3C), a trend (P = 0.065) towards an increase in young CR was observed when compared with young control. For serum glycerol levels (Fig. 3D), a trend towards a decrease (P = 0.06) was observed in young CR mice when compared with young controls.
Figure 3. Hepatic G3P, DHAP, G3P/DHAP ratio and serum glycerol levels form mice.
Hepatic metabolites and serum glycerol levels were determined as described in the text. A, glycerol-3-phosphate; B, dihydroxyacetonephosphate; C, glycerol-3-phosphate/dihydroxyacetonephosphate ratio; D, serum glycerol. All values were mean ± S.E.M of at least six independent experiments. Controls, solid bars; CR, open bars. Symbols indicate comparisons between CR and control mice: * P < 0.001 old CR vs old control, ‡ P < 0.05 young CR vs young control, † P < 0.02 old CR vs old control, § P < 0.03 old CR vs old control, ¶ P < 0.045 old CR vs old control. Letters indicate comparisons between age groups on similar diets: a P < 0.003 old control vs young control, b P < 0.0001 old CR vs young CR, c P < 0.04 old CR vs young CR, d P < 0.004 old control vs young control, e P < 0.04 old CR vs young CR.
Influence of age was also determined, with significantly higher levels of G3P in old control (P < 0.003) and old CR (P < 0.0001) mice than in the young (Fig. 3A). In the case of DHAP, old CR mice showed significantly higher levels (P < 0.04) than the young CR, while no differences were observed between old and young controls (Fig. 3B). The G3P/DHAP ratio also increased significantly with age, with old controls and old CR mice having higher ratios than the corresponding young mice (P < 0.004 and P < 0.04, respectively) (Fig. 3C). Differences in serum glycerol levels were insignificant between young and old controls and between young and old CR mice (Fig. 3D).
Discussion
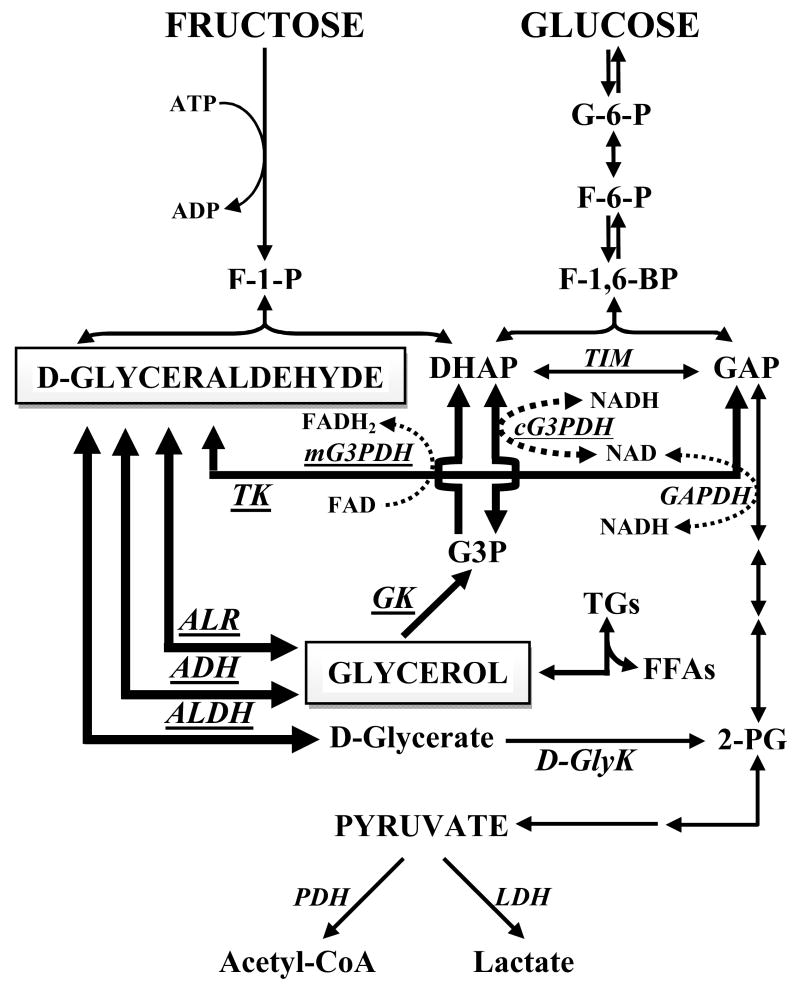
Glyceraldehyde and glycerol are at the crossroads of several inter-connecting pathways (Fig. 4). To our knowledge, there are no previous reports concerning the effects of CR on the enzymes investigated in the current study, except for one previous study of TK under control and CR conditions [17]. The current work is the first to describe the activities of the enzymes involved in the metabolism of these two metabolites under CR conditions.
Figure 4.
Glyceraldehyde and glycerol metabolism and their inter-connection to other metabolic pathways. Enzymes assayed in this study are indicated in underlined-italicized-block uppercase letters. Metabolites assayed were DHAP, G3P and glycerol. Bold arrows indicate the pathways studies.
Of the four possible pathways for glyceraldehyde metabolism, the TK-catalyzed one is the predominant pathway since TK enzyme has the lowest Km of all other enzymes involved in glyceraldehyde metabolism [16]. Our current results indicate that CR significantly influenced TK activity only in old mice (Fig. 1A), which is in agreement with a previous observation [17]. TK is an important enzyme since it converts glyceraldehyde to GAP, therefore, completing the entry of fructose into the glycolytic pathway (Fig. 4). Previous reports have shown increased TK activity in the presence of high dietary sucrose [38,39] or fructose [40], implying activity modulation by these sugars. These findings indicate that glucose supplementation alone did not change activity, and that the fructose moiety was the cause of the increased activity [38]. In contrast, one short-term (3 days) fructose feeding study has reported no increased activity [41]. A wealth of information on ALDH concerning subjects as diverse as cellular distribution, developmental expression, carcinogenesis and others is already available [34,42,43]. However, to the best of our knowledge, this information does not extend to the influence of CR on the enzyme's activity. The lack of activity changes by CR in both young and old mice (Fig.1C) indicate that this pathway is not responsive to calorie intake and may not be important for the metabolism of glyceraldehyde. Moreover, no significant age differences were observed when young controls and CR were compared with their old counterparts. This lack of age difference is in agreement with previous findings from rat liver, where activities between 3 month old and 23-26 month old [44], and 3 months old and adult [45] were similar.
Similar to ALDH, a vast amount of information is also available in the literature concerning ADH, however, none of this covers the influence of CR on the enzyme's activity. ADH catalyzes the reversible conversion of alcohols to their corresponding aldehydes and ketones. The trend towards a decrease (P = 0.08) in our results (Fig. 1B) between CR and control mice in both young and old groups could indicate a role for ADH in the glyceraldehyde to glycerol inter-conversion. On the other hand, the lack of age-related differences between young and old mice, in both control and CR groups (Fig. 1B) is in agreement with previous work [46]. Previous studies reported decreased ADH activity due to decreased dietary protein content (dietary protein restriction) rather than decreased calories [47-50], however, increased dietary protein did not result in increased ADH activity [47]. This is of interest since the diet used in our study to feed the CR group has a higher protein content than the diet of the control group [27], yet no increased ADH activity was observed in the CR mice.
ALR on the other hand, is one of several enzymes that form the aldo-keto reductase superfamily of enzymes, characterized by the NADPH-dependent reduction of a vast array of aldehydes [51-54]. To our knowledge, no studies could be found on the enzyme's activity under various nutritional conditions or concerning the effects of CR on its activity. However, there are several studies available concerning the characteristics of the enzyme from various sources and its substrate specificities. The lack of significant differences in activity between CR and control mice in both young and old groups (Fig. 1D) indicates that the pathway is not responsive to changes in calorie intake and may not be important for the metabolism of glyceraldehyde. Significant age differences between old and young mice, on both diets, are in agreement with the pattern found in rat liver [55].
The metabolism of glycerol is also of great interest since, as a junctional metabolite, it occupies an important inter-connecting position in metabolism. Also, in several species, it is an important substrate for energy metabolism and biomass synthesis [6]. Similar to the enzymes of glyceraldehyde metabolism, information concerning the effects of CR on glycerol metabolizing enzymes is lacking, although the effects of fasting for up to 24 hours have been reported [56]. The significantly increased activities of GK (Fig. 2A) and cG3PDH (Fig.2B) in both old and young CR mice is interesting since glycerol is a gluconeogenic substrate [2,3] and CR induces sustained increases in gluconeogenesis [24,57], therefore, allowing glycerol to be utilized and converted to glucose. It has been shown that during starvation, adipose tissue lipolysis gives rise to the release of glycerol and fatty acids into the blood, with the subsequent transport of glycerol to liver for further metabolism and entry into glycolytic pathway [56]. Lipolysis occurs with CR [58,59], therefore, making glycerol available for metabolism in the liver by the increased activities of GK and cG3PDH. One striking phenotype observed in animals on CR diet is the loss of fat mass [60] that results in lean animals in which the liver plays a dominant role, as evidenced by increased gluconeogenesis and decreased glycolysis [23,24,57,61]. Additionally, increased levels of ketone bodies and transamination are also observed under CR conditions [23,24], indicating sustained capacities for utilization of fat and proteins, respectively. It is interesting that increased gluconeogenic capacity with CR is maintained even after much of the fat mass has disappeared and the animals are no longer undergoing dynamic weight loss.
The activity of mG3PDH in young and old CR mice was not significantly different from young and old controls (Fig. 2C). Similar to cG3PDH, information concerning the effects of CR on mG3PDH activity is also lacking, and previous reports have also shown mG3PDH, but not cG3PDH, to be regulated by thyroid hormones [9,62]. It is known that thyroid hormone levels are decreased with CR [19,63], however, our results suggest that this had no significant effect on mG3PDH activity in liver. It is possible to speculate that mG3PDH expression and activity in liver are very low, and although mG3PDH with cG3PDH are part of the G3P shuttle, the malate-aspartate shuttle is the predominant shuttle in liver [1,4,11,64] and only cG3PDH is needed for the G3P to DHAP conversion. Therefore, the need for a highly active mG3PDH does not exist is this tissue, unlike its role in brain [8,64], skeletal muscle [9,64] and the flight muscle of insects [10,64].
Recently, hepatic glycerol metabolism in mice has been reported to be regulated by PPARα, whose expression is increased by fasting (2.5 – 24hr) and leads to increased expression of glycerol metabolism genes such as GK, cG3PDH, mG3PDH, and glycerol transporters aquaporin 3 and 9, with cG3PDH being the direct target for PPARα [56]. In the adipose tissue, on the other hand, glycerol metabolism is regulated by PPARγ [56], the nuclear receptor that promotes adipogenesis. In mammals under CR conditions, Sirt1 is induced and promotes lipolysis and fat mobilization from adipose tissues into the blood, and represses PPARγ and adipogenesis [59,65,66], therefore, glycerol becomes available for utilization as a source for glucose synthesis under CR. It has also been shown that expression levels of PPARα and PPARγ were influenced by aging [67-69] and by CR [69]. It is possible that in our study, the increased activities of GK and cG3PDH in both old and young CR mice were due to increased PPARα expression. Moreover, PPARα has also been reported to stimulate hepatic ketogenesis and β-oxidation [56,70], which is of interest since we have shown previously [23] increased ketone body levels under CR conditions.
Plasma glycerol levels (Fig. 3D) in both old and young CR mice were decreased, significantly in old CR (P < 0.045) and a trend in young CR (P = 0.06). This decreased level of serum glycerol is likely due to increased activities of GK and cG3PDH. The breakdown of triglycerides during lipolysis results in glycerol generation, which is transported by blood to the liver where it is taken up for glucose synthesis through gluconeogenesis. Previously, it was shown that glycerol levels in fasted mice were lower even though there was increased glycerol release from adipose tissue and this was attributed to the fact that PPARα activation resulted in increased expression of genes involved in hepatic gluconeogenesis (GK, cG3PDH, etc) from glycerol [56]. In this study we have seen decreased glycerol levels in CR mice and increased GK and cG3PDH activities, therefore, it is possible that a similar mechanism is in operation.
Of the two hepatic metabolites (G3P and DHAP) measured (Fig. 3A & B), G3P showed an increased pattern in both old and young CR mice (Fig. 3A) matching that of GK (Fig. 2A), indicating the increase was likely due to the increased GK activity. In the case of DHAP levels, old CR mice showed a pattern of increase which matched that of cG3PDH, however, this was not the case for young CR where no change in DHAP level was observed between young CR and controls. This could likely be due to the fact that the enzymes of gluconeogenic pathway upstream of DHAP-GAP point (Fig. 4) were unaffected by CR in young mice [23,24]. The two metabolites were used to determine the G3P/DHAP ratio, which reflects the cytoplasmic NADH/NAD ratio, in a manner similar to lactate/pyruvate ratio. Raised NADH/NAD ratios were previously reported from animals under different dietary restriction conditions [23,71], indicating a more reduced state. Higher G3P/DHAP ratios in CR animals (Fig. 3C) indicate a more reduced state, which is in agreement with a previous report [23]. Also, increased G3P levels are associated with decreased 6-phosphofructo-1-kinase (PFK-1) activity since the metabolite is an allosteric inhibitor of PFK-1 [72]. Moreover, G3P inhibits 6-phosphofructo-2-kinase (PFK-2), which is responsible for fructose-2,6-bisphosphate (F-2,6-BP) synthesis, hence decreasing the intracellular levels of F-2,6-BP [72]. F-2,6-BP is a potent activator of PFK-1 and inhibitor of fructose-1,6-bisphosphatase (F-1,6-BPase) [73]. This is interesting because our previous results have shown that under CR conditions, decreased PFK-1 activity and F-2,6-BP levels [23] and increased F-1,6-BPase activity [24] were observed.
The results from our study have shown that glycerol and glyceraldehyde metabolisms are influenced by sustained CR. Information concerning the effects of CR on glyceraldehyde metabolism is almost non-existent and with the exception of TK, none of the other enzymes showed any major changes under CR condition, except for ADH which showed a trend towards decreased activity. Glycerol metabolism showed a much different pattern in that GK and cG3PDH were influenced by CR, while mG3PDH was not. The results indicate that long-term CR induces sustained increases in the capacity for gluconeogenesis from glycerol. We have discussed the role of PPAR in this regulation and future studies are required to elucidate the mechanisms involved in the regulation of glyceraldehyde and glycerol metabolism under sustained CR conditions.
Acknowledgments
The work was supported by NIH grants PO1 AG11915 and RO1 AG28125.
Abbreviations used
- ADH
alcohol dehydrogenase
- ALDH
aldehyde dehydrogenase
- ALR
aldehyde reductase
- DHAP
dihydroxyacetonephosphate
- GK
glycerol kinase
- G3P
glycerol-3-phosphate
- cG3PDH and mG3PDH
cytosolic and mitochondrial glycerol-3-phosphate dehydrogenase, respectively
- TK
triokinase
References
- 1.Lin EC. Glycerol utilization and its regulation in mammals. Annu Rev Biochem. 1977;46:765–795. doi: 10.1146/annurev.bi.46.070177.004001. [DOI] [PubMed] [Google Scholar]
- 2.Frank MS, Nahata MC, Hilty MD. Glycerol: a review of its pharmacology, pharmacokinetics, adverse reactions, and clinical use. Pharmacotherapy. 1981;1:147–160. doi: 10.1002/j.1875-9114.1981.tb03562.x. [DOI] [PubMed] [Google Scholar]
- 3.Robergs RA, Griffin SE. Glycerol. Biochemistry, pharmacokinetics and clinical and practical applications. Sports Med. 1998;26:145–167. doi: 10.2165/00007256-199826030-00002. [DOI] [PubMed] [Google Scholar]
- 4.Brisson D, Vohl MC, St-Pierre J, Hudson TJ, Gaudet D. Glycerol: a neglected variable in metabolic processes? BioEssays. 2001;23:534–542. doi: 10.1002/bies.1073. [DOI] [PubMed] [Google Scholar]
- 5.Oscai LB, Essig DA, Palmer WK. Lipase regulation of muscle triglyceride hydrolysis. J Appl Physiol. 1990;69:1571–1577. doi: 10.1152/jappl.1990.69.5.1571. [DOI] [PubMed] [Google Scholar]
- 6.Bortz W, Paul P, Haff AC, Holmes WL. Glycerol turnover and oxidation in man. J Clin Invest. 1972;51:1537–1546. doi: 10.1172/JCI106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elia M, Khan K, Calder G, Kurpad A. Glycerol exchange across the human forearm assessed by combination of tracer and arteriovenus exchange techniques. Clin Sci. 1993;84:99–104. doi: 10.1042/cs0840099. [DOI] [PubMed] [Google Scholar]
- 8.Ringler RL, Singer TP. Studies of the mitochondrial α-glycerophosphate dehydrogenase. J Biol Chem. 1959;234:2211–2218. [PubMed] [Google Scholar]
- 9.Lee YP, Lardy HA. Influence of thyroid hormones on 1-α-glycerophosphate dehydrogenase and other dehydrogenases in various organs of the rat. J Biol Chem. 1965;240:1427–1436. [PubMed] [Google Scholar]
- 10.Estabrook RW, Sacktor B. A-glycerophosphate oxidase of flight muscle mitochondria. J Biol Chem. 1958;233:1014–1019. [PubMed] [Google Scholar]
- 11.Barron JT, Gu L, Parrillo JE. Malate aspartate shuttle, cytoplasmic NADH redox potential and energetic in vascular smooth muscle. J Mol Cell Cardiol. 1998;30:1571–1579. doi: 10.1006/jmcc.1998.0722. [DOI] [PubMed] [Google Scholar]
- 12.Baba H, Zhang XJ, Wolfe RR. Glycerol gluconeogenesis in fasting humans. Nutrition. 1995;11:149–153. [PubMed] [Google Scholar]
- 13.Owen DE, Felig P, Morgan AP, Wahren J, Cahill GF. Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969;48:574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding JW, Pyeritz EA, Copeland ES, White HB. Role of glycerol-3-phosphate dehydrogenase in glyceride metabolism. Biochem J. 1975;146:223–229. doi: 10.1042/bj1460223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kattermann R, Dold U, Holzer H. D-Glycerat beim fructoseabbau in der leber. Biochem Z. 1961;334:218–226. [PubMed] [Google Scholar]
- 16.Sillero MAG, Sillero A, Sols A. Enzymes involved in fructose metabolism in liver and in glyceraldehydes metabolic crossroads. Eur J Biochem. 1969;10:345–350. doi: 10.1111/j.1432-1033.1969.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 17.Hagopian K, Ramsey JJ, Weindruch R. Fructose metabolizing enzymes from mouse liver: influence of age and caloric restriction. Biochim Biophys Acta. 2005;1721:37–43. doi: 10.1016/j.bbagen.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Jeffery J, Jörnval H. Enzyme relationships in a sorbitol pathway that bypasses glycolysis and pentose phosphates in glucose metabolism. Proc Nat Acad Sci USA. 1983;80:901–905. doi: 10.1073/pnas.80.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Charles C. Thomas Publisher; Springfield: 1988. [Google Scholar]
- 20.Sohal RS, Weindruch R. Oxidative stress, caloric restriction and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 22.Masoro EJ. Caloric restriction and aging: controversial issues. J Gerontol. 2006;61A:14–19. doi: 10.1093/gerona/61.1.14. [DOI] [PubMed] [Google Scholar]
- 23.Hagopian K, Ramsey JJ, Weindruch R. Influence of age and caloric restriction on liver glycolytic enzyme activities and metabolite concentrations in mice. Exp Gerontol. 2003;38:253–266. doi: 10.1016/s0531-5565(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 24.Hagopian K, Ramsey JJ, Weindruch R. Caloric restriction increases gluconoegenic and transaminase enzyme activities in mouse liver. Exp Gerontol. 2003;38:267–278. doi: 10.1016/s0531-5565(02)00202-4. [DOI] [PubMed] [Google Scholar]
- 25.Hagopian K, Ramsey JJ, Weindruch R. Krebs cycle enzymes from livers of old mice are differentially regulated by caloric restriction. Exp Gerontol. 2004;39:1145–1154. doi: 10.1016/j.exger.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Hagopian K, Ramsey JJ, Weindruch R. Serine utilization in mouse liver: influence of caloric restriction and aging. FEBS Lett. 2005;579:2009–2013. doi: 10.1016/j.febslet.2005.02.062. [DOI] [PubMed] [Google Scholar]
- 27.Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20:157–165. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 28.Liepa GU, Masoro EJ, Bertrand HA, Yu BP. Food restriction as a modulator of age-related changes in serum lipids. Am J Physiol. 1980;238:E253–E257. doi: 10.1152/ajpendo.1980.238.3.E253. [DOI] [PubMed] [Google Scholar]
- 29.Bergmeyer HU, Grassl M, Walter HE. Glycerokinase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis Verlag-Chemie. II. Weinheim, Germany: 1983. pp. 216–217. [Google Scholar]
- 30.Bergmeyer HU, Grassl M, Walter HE. Glycerol-3-phosphate dehydrogenase. In: Bergmeyer HE, editor. Methods of Enzymatic Analysis Verlag-Chemie. II. Weinheim, Germany: 1983. pp. 215–216. [Google Scholar]
- 31.MacDonald MJ, Marshall LK. Mouse lacking NAD+-linked glycerol phosphate dehydrogenase has pancreatic beta cell function but abnormal metabolic pattern in skeletal muscle. Anal Biochem. 2000;384:143–153. doi: 10.1006/abbi.2000.2107. [DOI] [PubMed] [Google Scholar]
- 32.Algar EM, Seeley TL, Holmes RS. Purification and molecular properties of mouse alcohol dehydrogenase isozymes. Eur J Biochem. 1983;137:139–147. doi: 10.1111/j.1432-1033.1983.tb07807.x. [DOI] [PubMed] [Google Scholar]
- 33.Turner AJ, Hryszko J. Isolation and characterization of rat liver aldehyde reductase. Biochim Biophys Acta. 1980;613:256–265. doi: 10.1016/0005-2744(80)90081-9. [DOI] [PubMed] [Google Scholar]
- 34.Smolen A, Wayman AL, Smolen TN, Petersen DR, Collins AC. Subcellular distribution of hepatic aldehyde dehydrogenase activity in four inbred mouse strains. Comp Biochem Physiol. 1981;69C:199–204. doi: 10.1016/0306-4492(81)90129-5. [DOI] [PubMed] [Google Scholar]
- 35.Bismut H, Hers HE, van Schaftingen E. Conversion of fructose to glucose in the rabbit small intestine. A reappraisal of the direct pathway. Eur J Biochem. 1993;213:721–726. doi: 10.1111/j.1432-1033.1993.tb17812.x. [DOI] [PubMed] [Google Scholar]
- 36.Lang G. L-(-)-Glycerol-3-phosphate. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis Verlag-Chemie. VI. Weinheim, Germany: 1984. pp. 525–531. [Google Scholar]
- 37.Wieland OH. Glycerol: UV method. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis Verlag-Chemie. VI. Weinheim, Germany: 1984. pp. 504–510. [Google Scholar]
- 38.Pridham JB, Davies DR. The effect of dietary sucrose on enzyme and metabolite levels in male and female rats. In: Birch GG, Parker KJ, editors. Sugar: Science and Technology. Applied Science Publishers; London: 1979. pp. 437–456. [Google Scholar]
- 39.Shafrir E, Orevi M. Response of hepatic fructokinase to long-term sucrose diets and diabetes in spiny mice, albino mice and rats. Comp Biochem Physiol. 1984;78B:493–498. doi: 10.1016/0305-0491(84)90064-6. [DOI] [PubMed] [Google Scholar]
- 40.Veneziale CM. Regulation of D-triokinase and NAD-linked glycerol dehydrogenase activities in rat liver. Eur J Biochem. 1972;31:59–62. doi: 10.1111/j.1432-1033.1972.tb02500.x. [DOI] [PubMed] [Google Scholar]
- 41.Korieh A, Crouzoulon G. Dietary regulation of fructose metabolism in the intestine and liver of the rat. Duration of the effects of high fructose after the return of the standard diet. Arch Biochem Biophys. 1991;99:455–460. [PubMed] [Google Scholar]
- 42.Lindahl R. Aldehyde dehydrogenases and their role in cvarcinogenesis. Crit Rev Biochem Mol Biol. 1992;27:283–335. doi: 10.3109/10409239209082565. [DOI] [PubMed] [Google Scholar]
- 43.Yoon M, Madden MC, Barton HA. Developmental expression of aldehyde dehydrogenase in rat: a comparison of liver and lung development. Toxicol Sci. 2006;89:386–398. doi: 10.1093/toxsci/kfj045. [DOI] [PubMed] [Google Scholar]
- 44.Danh HC, Benedetti MS, Dostert P. Age-related changes in aldehyde dehydrogenase activity of rat brain, liver, and heart. J Neurochem. 1983;41:618–622. doi: 10.1111/j.1471-4159.1983.tb04786.x. [DOI] [PubMed] [Google Scholar]
- 45.Horton AA, Mills DJ. Developmental patterns of alcohol dehydrogenase and aldehyde dehydrogenases in homogenated and subcellular fractions of rat liver. Mech Ageing Dev. 1979;11:363–370. doi: 10.1016/0047-6374(79)90011-3. [DOI] [PubMed] [Google Scholar]
- 46.Balak KJ, Keith RH, Felder MR. Genetic and developmental regulation of mouse liver alcohol dehydrogenase. J Biol Chem. 1982;257:15000–15007. [PubMed] [Google Scholar]
- 47.Lindros KO, Pakkanen L, Koivula T. Enzymatic and metabolic modification of hepatic ethanol and acetaldehyde oxidation by dietary protein level. Biochem Pharmacol. 1979;28:2313–2320. [PubMed] [Google Scholar]
- 48.Lumeng L, Bosron WF, Li TK. Quantitative correlation of ethanol elimination rates in vivo wirh liver alcohol dehydrogenase activities in fed, fasted and food-restricted rats. Biochem Pharmacol. 1979;28:1547–1551. doi: 10.1016/0006-2952(79)90471-4. [DOI] [PubMed] [Google Scholar]
- 49.Braggins TJ, Crow KE. The effects of high ethanol doses on rates of ethanol oxidation in rats. A reassessment of factors controlling rates of ethanol oxidation in vivo. Eur J Biochem. 1981;119:633–640. doi: 10.1111/j.1432-1033.1981.tb05654.x. [DOI] [PubMed] [Google Scholar]
- 50.Bosron WF, Crabb DW, Housinger TA, Li TK. Effects of fasting on the activity and turnover of rat liver alcohol dehydrogenase. Alcohol Clin Exp Res. 1984;8:196–200. doi: 10.1111/j.1530-0277.1984.tb05837.x. [DOI] [PubMed] [Google Scholar]
- 51.Flynn TG. Aldehyde reductases: monomeric NADPH-dependent oxidoreductases with multifunctional potential. Biochem Pharmacol. 1982;31:2705–2712. doi: 10.1016/0006-2952(82)90123-x. [DOI] [PubMed] [Google Scholar]
- 52.Bohren KM, Bullock B, Wermuth B, Gabbay KH. The aldo-keto reductase superfamily. cDNA and deduced amino acid sequences of human aldehyde and aldose reductases. J Biol Chem. 1989;264:9547–9551. [PubMed] [Google Scholar]
- 53.Jez JM, Bennett MJ, Schlegel BP, Lewis M, Penning TM. Comparative anatomy of aldo-ket reductase superfamily. Biochem J. 1997;326:625–636. doi: 10.1042/bj3260625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jez JM, Flynn TG, Penning TM. A new nomenclature for aldo-keto reductase superfamily. Biochem Pharmacol. 1997;54:639–647. doi: 10.1016/s0006-2952(97)84253-0. [DOI] [PubMed] [Google Scholar]
- 55.Danh HC, Benedetti MS, Dostert P. Age-related changes in aldehyde reductase activity of rat brain, liver, and heart. Gerontology. 1980;30:159–166. doi: 10.1159/000212624. [DOI] [PubMed] [Google Scholar]
- 56.Patsouris D, Mandard S, Voshol PJ, Escher P, Tan NS, Havekes LM, Koeing W, März W, Tafuri S, Wahli W, Müller M, Kersten S. PPARα governs glycerol metabolism. J Clin Invest. 2004;114:94–103. doi: 10.1172/JCI20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dhahbi JM, Mote PL, Wingo J, Tillman JB, Walford RL, Spindler SR. Calories and aging alter gene expression for gluconeogenic, glycolytic and nitrogen-metabolizing enzymes. Am J Physiol. 1999;277:E352–E360. doi: 10.1152/ajpendo.1999.277.2.E352. [DOI] [PubMed] [Google Scholar]
- 58.Guarente L, Picard F. Caloric restriction - the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 59.Wolf G. Calorie restriction increases life span: a molecular mechanism. Nutr Rev. 2006;64:89–92. doi: 10.1301/nr.2006.feb.89-92. [DOI] [PubMed] [Google Scholar]
- 60.Barzilai N, Gabriely I. The role of fat depletion in the biological benefits of caloric restriction. J Nutr. 2001;131:903S–906S. doi: 10.1093/jn/131.3.903S. [DOI] [PubMed] [Google Scholar]
- 61.Dhahbi JM, Mote PL, Wingo J, Rowley BC, Cao SX, Walford RL, Spindler SR. Caloric restriction alters the feeding response of key metabolic enzyme genes. Mech Ageing Dev. 2001;122:1033–1048. doi: 10.1016/s0047-6374(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 62.Müller S, Seitz HA. Cloning of a cDNA for the FAD-linked glycerol-3-phosphate dehydrogenase from rat liver and its regulation by thyroid hormones. Proc Natl Acad Sci USA. 1994;91:10581–10585. doi: 10.1073/pnas.91.22.10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herlihy JT, Stacy C, Bertrand HA. Long-term food restriction depresses serum thyroid hormone concentrations in the rat. Mech Ageing Dev. 1990;53:9–16. doi: 10.1016/0047-6374(90)90030-j. [DOI] [PubMed] [Google Scholar]
- 64.Lehninger AL, Nelson DL, Cox MM. Principles of Biochemistry. 2nd. Worth Publishers; New York: 1993. [Google Scholar]
- 65.Guarente L. Calorie restricition and SIR2 genes – Towards a mechanism. Mech Ageing Dev. 2005;126:923–928. doi: 10.1016/j.mad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 66.Bordone L, Guarente L. Calorie restriction, Sirt1 and metabolism: understanding longevity. Nature Rev. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 67.Poynter ME, Daynes RA. Peroxisome prolifirator-activated receptor α activation modulates cellular redox status, represses nuclear factor-κB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem. 1998;273:32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- 68.Iemitsu M, Miyauchi T, Maeda S, Tanabe T, Takanashi M, Irukayama-Tomobe Y, Sakai S, Ohmori H, Matsuda M, Yamaguchi I. Aging-induced decrease in the PPAR-alpha level in hearts is improved by exercise training. Am J Physiol. 2002;283:H1750–H1760. doi: 10.1152/ajpheart.01051.2001. [DOI] [PubMed] [Google Scholar]
- 69.Sung B, Park S, Yu BP, Chung HY. Modulation of PPAR in aging, inflammation, and calorie restriction. J Gerontol. 2004;59A:997–1006. doi: 10.1093/gerona/59.10.b997. [DOI] [PubMed] [Google Scholar]
- 70.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 71.Henley KS. Glycolytic substrates in cirrhosis and in quantitative undernutrition in the rat. Lab Clin Med Clin Exp. 1968;71:183–191. [PubMed] [Google Scholar]
- 72.Claus TH, Schlumfp JR, El-Maghrabi R, Pilkis SJ. Regulation of the phosphorylation and activity of 6-phosphofructo-1-kinase in isolated hepatocytes by α-glycerophosphate and fructose-2,6-bisphosphate. J Biol Chem. 1982;257:7541–7548. [PubMed] [Google Scholar]
- 73.Pilkis SJ, Claus TH, Kurland LJ, Lange AJ. 6-phosphofructoi-2-kinase/fructose-2,6-bisphosphatase: a metabolic signaling enzyme. Annu Rev Biochem. 1995;64:799–835. doi: 10.1146/annurev.bi.64.070195.004055. [DOI] [PubMed] [Google Scholar]