Abstract
Background and purpose:
Recent studies have demonstrated that the naturally occurring isoflavone compounds genistein and daidzein inhibit the hydrolysis of anandamide by fatty acid amide hydrolase (FAAH) in the low micromolar concentration range. The purpose of the present study was to determine whether this property is shared by flavonoids.
Experimental approach:
The hydrolysis of anandamide in homogenates and intact cells was measured using the substrate labelled in the ethanolamine part of the molecule.
Key results:
Twenty compounds were tested. Among the commonly occurring flavonoids, kaempferol was the most potent, inhibiting FAAH in a competitive manner with a Ki value of 5 μM. Among flavonoids with a more restricted distribution in nature, the two most active toward FAAH were 7-hydroxyflavone (IC50 value of 0.5–1 μM depending on the solvent used) and 3,7-dihydroxyflavone (IC50 value 2.2 μM). All three compounds reduced the FAAH-dependent uptake of anandamide and its metabolism by intact RBL2H3 basophilic leukaemia cells.
Conclusions and implications:
Inhibition of FAAH is an additional in vitro biochemical property of flavonoids. Kaempferol, 7-hydroxyflavone and 3,7-dihydroxyflavone may be useful as templates for the synthesis of novel compounds, which target several systems that are involved in the control of inflammation and cancer.
Keywords: anandamide, cannabinoid, fatty acid amide hydrolase, flavonoids, kaempferol, apigenin
Introduction
Flavonoids are a family of naturally occurring compounds that are currently of considerable interest in view of their possible preventative effects in diseases such as cancer and stroke (Keli et al., 1996; Havsteen, 2002; Gates et al., 2007). In addition to the well-known effects of the flavonoids, such as their antioxidant (in micromolar concentrations) and phytoestrogen actions (in nanomolar concentrations) (Kuiper et al., 1998; Furusawa et al., 2005), the compounds affect a variety of cellular processes (see Havsteen, 2002). Thus, for example, the flavone apigenin (structure shown in Figure 1a), which is present in many food sources, such as parsley, celery and teas, and which has been shown to act as a chemopreventative agent both in vitro and in vivo in experimental models (Patel et al., 2007; Shukla et al., 2007), also inhibits, when used in micromolar concentrations, a variety of enzymes, including fatty acid synthase (Brusselmans et al., 2005), PDE 1–3 (Ko et al., 2004), PI3-kinaseα (Agullo et al., 1997) and COX-2, the latter secondary to its ability to activate peroxisome proliferator activated receptor γ (PPARγ) (Liang et al., 2001). Similarly, the flavonole kaempferol (structure given in Figure 1a), which is found, for example, in broccoli and endives and whose intake may also be beneficial in certain types of cancer (Nöthlings et al., 2007), has a myriad of biological effects when used at micromolar concentrations, including activation of PPARγ (Liang et al., 2001) and inhibition of interleukin-4-induced STAT6 activation (Cortes et al., 2007).
Figure 1.
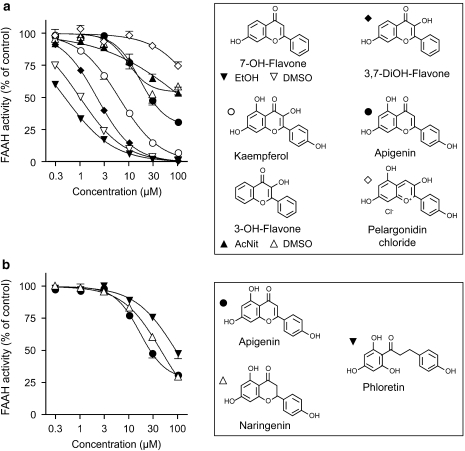
Inhibition by flavonoids and related compounds of the hydrolysis of 0.5 μM [3H]-AEA by rat brain homogenates. In panel a, example compounds related to kaempferol are shown. In panel b, saturated and ring-opened analogues of apigenin are shown. Shown are means±s.e.mean (when not enclosed by the symbols), n=3, except for the dimethylsulphoxide samples for 7-hydroxyflavone (n=6). The dose–response curve for apigenin is the same in both panels. AcNit, acetonitrile; AEA, anandamide; DiOH, dihydroxy; FAAH, fatty acid amide hydrolase; OH, hydroxy.
The finding that both apigenin and kaempferol activate PPARγ is worthy of comment. PPARγ is a ligand-activated transcription factor that, in addition to its role in adipocyte differentiation, fatty acid and lipid metabolism, and insulin sensitivity, also has potentially important roles in inflammation and cancer (Moraes et al., 2006; Wang et al., 2006). There appears to be an overlap between this system and the endogenous cannabinoid (endocannabinoid) system (itself important in inflammation and cancer) (Klein, 2005; Bifulco et al., 2006), as the endocannabinoid ligands anandamide (arachidonoylethanolamide, AEA) and 2-arachidonoylglycerol interact with PPARγ (O'Sullivan, 2007). The reverse is also true, as several compounds that activate PPARγ are also capable of inhibiting fatty acid amide hydrolase (FAAH) (Lenman and Fowler, 2007), the enzyme primarily responsible for the hydrolysis of AEA (Deutsch and Chin, 1993) and a current target for drug development for disorders such as inflammation, inflammatory pain and possibly cancer chemotherapy (Bifulco et al., 2004; Holt et al., 2005; D'Argenio et al., 2006; Jayamanne et al., 2006; Izzo et al., 2008). This overlap also includes the phytoestrogen genistein, the isoflavone analogue of apigenin, which activates PPARγ (at micromolar concentrations; Dang et al., 2003) and is a potent competitive inhibitor of FAAH (Ki value 2.8 μM) (Thors et al., 2007a). The related isoflavone compound daidzein (the isoflavone analogue of 4′,7′-dihydroxyflavone, a flavonoid found in alfalfa roots; Maxwell et al., 1989) also inhibits rat brain FAAH in a competitive manner, with a Ki value of 1.7 μM (Thors et al., 2007b) and activates PPARγ (Chacko et al., 2007). Taken together, these findings raise the possibility that flavonoids, such as apigenin and kaempferol, inhibit FAAH. This possibility has been investigated in the present study. In particular, two questions have been asked. Firstly, do the commonly occurring flavonoids inhibit FAAH at concentrations that can be attained after dietary ingestion? Secondly, what are the structural requirements for inhibition of FAAH by flavonoids?
Methods
Assay of FAAH activity in rat brain homogenates and RBL2H3 cells
Frozen brains (without cerebella) from adult Wistar or Sprague–Dawley rats were thawed and homogenized in 20 mM HEPES, 1 mM MgCl2 (pH 7.0), and thereafter centrifuged at ∼35 000 g for 20 min (4 °C). After resuspension in buffer, recentrifugation and a second resuspension in buffer, the pellets were incubated at 37 °C for 15 min in order to hydrolyse all endogenous FAAH substrates. Following recentrifugation, the pellets were resuspended in 50 mM Tris-HCl buffer (pH 7.4) containing 1 mM EDTA and 3 mM MgCl2 and frozen at −80 °C in aliquots until used for assay. FAAH was assayed by the method described by Boldrup et al. (2004) using 0.5 μM (unless otherwise stated) [3H]-AEA, labelled in the ethanolamine part of the molecule. Blank values were obtained by the use of buffer rather than homogenate.
For experiments using intact RBL2H3 cells, the cells were cultured in minimum essential medium with Earl's salts, 15% foetal bovine serum and 100 U mL−1 penicillin+100 μg mL−1 streptomycin (termed ‘medium' below), and aliquots (containing 2 × 105 cells) were added to 24-well plates and allowed to affix to the wells overnight. [3H]-AEA hydrolysis by the cells was measured as described previously (Paylor et al., 2006), using a preincubation time of 10 min, an incubation time of 20 min and an assay substrate concentration of 100 nM. Blank values were obtained from wells on the same culture plates that had not been seeded with cells. The final concentration of solvent in these experiments did not exceed 0.5% (vol/vol).
Assay of AEA uptake by adherent RBL2H3 and PC3 cells
The assays for RBL2H3 cells (cultured as described above) and for PC3 prostate cancer cells (cultured in Hams F-10, 2 mM L-glutamine, 10% foetal bovine serum and 100 U mL−1 penicillin +100 μg mL−1 streptomycin) were carried out as described by Thors et al. (2007b), using a preincubation time of 10 min and an incubation time of 5 min with 100 nM [3H]-AEA, labelled in the arachidonoyl part of the molecule. The final concentration of solvent in these experiments did not exceed 0.5% (vol/vol).
Assay of URB597-sensitive accumulation of tritium in RBL2H3 cell membranes following incubation of cells in suspension with [3H]-AEA
RBL2H3 cells were pelleted by centrifugation and resuspended in medium in Eppendorf tubes to a concentration of 2 × 105 cells per tube (5 × 105 cells mL−1). The cells were then preincubated with either the test flavonoids or URB597 for 10 min at 37 °C before the addition of [3H]-AEA (assay concentration 100 nM, substrate labelled in the arachidonoyl part of the molecule) in medium and incubation for a further 10 min (final assay volume: 500 μL). After incubation, the cells were sedimented using a microcentrifuge (1 min, 1000g) and washed twice with ice-cold medium. Aliquots (200 μL) of the suspensions were placed in 96-well plates and the radioactivity retained by the cells was separated from that in the medium by filtration through polyethylenimine-coated FilterMAT filters (Skatron Instruments Inc., Sterling, VA, USA) using a Micro cell harvester (Skatron Instruments Inc.) and a 30 s period of washing with deionized water (setting 4), which ruptures the cells. The URB597-sensitive accumulation of tritium was defined as the radioactivity recovered on the filter papers minus the corresponding radioactivity for the cells treated with 100 nM URB597.
Statistical analyses
The values of pI50, IC50, Hill slope (nH, given here as the positive rather than the negative values), Km and Vmax (nonlinear regression analyses), linear regressions and confidence limits were determined using the GraphPad Prism computer programme (GraphPad Software Inc., San Diego, CA, USA). The pI50, nH and IC50 values were calculated using the built-in programme ‘sigmoidal dose–response (variable slope)' from the data expressed as percentage of control, using top (that is, uninhibited) values of 100% and bottom (residual activity) values that were either set to zero or allowed to float. The two curves were compared using Akaike's Informative Criteria. When this statistical analysis suggested that the simple 0% residual inhibition model fitted the curve more appropriately, this alone was presented in the results. In the other three cases, both models are presented. When Km and Vmax values were calculated by the other analyses (direct linear plot, Hanes–Woolf, Lineweaver–Burk, Eadie–Hofstee and Johansen–Lumry plots), the Enzyme Kinetics v1.4 computer programme for Macintosh (Trinity Software, Campton, NH, USA) was used.
Compounds
Anandamide (ethanolamine-1-3H; specific activity: 2.22 TBq mmol−1 for the FAAH experiments) and anandamide (arachidonoyl 5,6,8,9,11,12,14,15-3H; specific activity: 7.4 TBq mmol−1 for the uptake and membrane tritium accumulation experiments) were purchased from American Radiolabeled Chemicals Inc. (St Louis, MO, USA). All of the flavonoid compounds used in the present study were obtained from Sigma-Aldrich (St Louis, MO, USA), with the exception of 7,4′-dihydroxyflavone and 5-deoxykaempferol, which were obtained from Extrasynthese (Genay, France). Non-radioactive AEA and URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexyl-carbamate) were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). OMDM-1 ((9Z)-N-(1-((S)-4-hydroxybenzyl)-2-hydroxyethyl)-9-octadecenamide) was obtained from Tocris Bioscience (Ellisville, MO, USA). RBL2H3 cells (passage range 23–36) were obtained from the American Type Culture Collection (Manassas, VA, USA). PC3 cells (passage range 27–34) were obtained from Professor Anders Bergh (Department of Medical Biosciences, Umeå University, Umeå, Sweden).
Results
Structure–activity relationships for the inhibition of FAAH by flavonoids
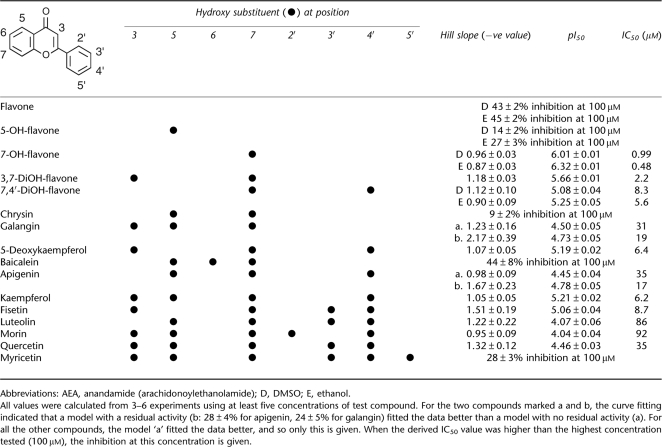
A total of 20 flavonoids and related compounds were tested for their ability to inhibit 0.5 μM [3H]-AEA hydrolysis by rat brain homogenates. Unless otherwise stated, the compounds were initially dissolved in dimethylsulphoxide (DMSO). The data for flavonoids, with the exception of 3-hydroxyflavone, are shown in Table 1, and selected examples are given in Figure 1a. In most cases, data were best fitted by a curve with no residual activity, that is, the maximum attainable inhibition was 100%, and the pI50, and hence IC50, values can be considered to be robust. For apigenin and galangin, there was an apparent residual activity of ∼25%, which may reflect a solubility issue at the highest concentration tested (100 μM) (see Figure 1 for apigenin). Certainly, the Hill slopes were nearer to unity when the data were calculated assuming no residual inhibition (Table 1). 3-Hydroxyflavone (shown in Figure 1a) was an extreme example of the difficulties associated with analysing compounds with residual activities. When the compound was dissolved in DMSO, the results were better fitted with a curve with a residual activity of 46±5% (−nH value 1.98±0.98, pI50 value 5.03±0.11, IC50 value 0.94 μM) than with a curve assuming 100% inhibition (pI50 value <4). However, when the compound was dissolved in acetonitrile, the curve of best fit was that for 100 % inhibition (pI50 value <4). The raw data for the two solvents are not that dissimilar (see Figure 1a), illustrating the need to be cautious in interpreting curve-fitting data. In consequence, we have not assigned an IC50 value for 3-hydroxyflavone.
Table 1.
Inhibition of rat brain AEA hydrolysis by flavonoids
At the extremes, flavone, with no hydroxy groups, and myricetein, with six hydroxy substituents, did not inhibit FAAH to any great extent (Table 1). A single hydroxy substituent at position 5 did not produce an active compound, whereas 7-hydroxyflavone, with an IC50 value of 0.99 μM (in DMSO) and 0.48 μM (in ethanol (EtOH)) was the most active toward FAAH among all the compounds in the series. Addition of a second hydroxy group to this compound either reduced (3,7-dihydroxyflavone, IC50 value 2.2 μM; 7,4′-dihydroxyflavone, IC50 value 5.6–8.3 μM) or obliterated (5,7-dihydroxyflavone (chrysin)) the activity. For the trihydroxy compounds, 5-deoxykaempferol (3,7,4′-trihydroxyflavone) was the most active toward FAAH (IC50 value 6.4 μM), followed by the 5,7,4′- (apigenin) and 3,5,7- (galangin) derivatives (IC50 values ∼30 μM), whereas the 5,6,7-derivative (baicalein) was a poor inhibitor of FAAH. Additional hydroxy substituents did not improve the ability of the compounds to inhibit FAAH; for the three compounds with four hydroxy substituents, kaempferol (IC50 value 6.2 μM) had the same potency as 5-deoxykaempferol, fisetin (3,7,3′4′-derivative, IC50 value 8.7 μM) was equipotent with the 7,4′- and 3,7,4′- (5-deoxykaempferol) derivatives, whereas luteolin (IC50 value 86 μM) was a poor inhibitor. Replacement of the benzopyran-4-one ring of kaempferol with a flavylium chloride structure (pelargonidin chloride) led to a loss of activity (Figure 1a). Morin and quercetin, with five hydroxy substituents, were weak FAAH inhibitors, with IC50 values of 92 and 35 μM, respectively (Table 1).
Flavone compounds are often strongly coloured (flavonoids are found in petal pigments; Havsteen, 2002), a case in point being 7-hydroxyflavone in DMSO. However, addition of 1, 10 or 100 μM of 7-hydroxyflavone (in either DMSO or EtOH) to homogenates first after the incubation with [3H]-AEA and placing of the samples on ice had no effect on the observed rate of hydrolysis (data not shown), indicating that the colouration did not affect the assay results.
The effects of ring saturation and ring opening of apigenin were investigated. Naringenin, the flavanone analogue of apigenin, was roughly equipotent, with a pI50 value of 4.34±0.02 (IC50 value 45 μM, −nH value 1.10±0.05) (Figure 1b). Ring opening of apigenin to give phloretin resulted in a reduction of activity toward FAAH (pI50, 4.04±0.04; IC50, 92 μM; −nH, 1.03±0.10) (Figure 1b).
Mode of inhibition of FAAH by kaempferol and 7-hydroxyflavone
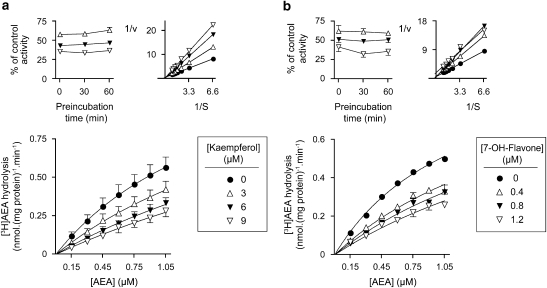
The modes of inhibition of [3H]-AEA hydrolysis by kaempferol and 7-hydroxyflavone are shown in Figure 2. Neither compound showed a time-dependent inhibition of AEA hydrolysis (left insets to Figures 2a and b). In the experiments with kaempferol, the data were clear-cut. In the absence of kaempferol, hydrolysis was saturable, and the Km and Bmax values obtained using nonlinear regression analysis of the data were 1.74 μM and 1.51 nmol mg−1 protein min−1, respectively. Almost identical values were seen when the mean values were re-analysed using other plots (direct linear plot, Hanes–Woolf, Lineweaver–Burk, Eadie–Hofstee, Johansen–Lumry; data not shown). For 3 μM of kaempferol, the Km and Bmax values obtained using nonlinear regression analysis of the data were 2.78 μM and 1.53 nmol mg−1 protein min−1, respectively, and once again very similar values were found with the other plots. These values indicate a competitive mode of interaction, from which a Ki value of 5.0 μM could be calculated. At the 6 μM and particularly 9 μM concentrations of kaempferol, the apparent Km and Vmax values varied more between the analysis methods, which is to be expected for a situation where a Km value is greater than the highest assay concentration used. However, visual inspection of the Lineweaver–Burk plot (shown as the right inset of Figure 2a) confirmed the competitive nature of the interaction between kaempferol and FAAH. Indeed, when the data were assessed using a fixed Bmax value of 1.512 nmol mg−1 protein min−1 for all concentrations of kaempferol, the theoretical curves fitted the observed data very well (main graph, Figure 2a). The Kmapp values using this constrained model were 1.74, 2.73, 3.82 and 4.73 μM for 0, 3, 6 and 9 μM of kaempferol, respectively, and from these values, Ki was calculated to be 5.2 μM. These Km and Ki values would give an IC50 value of 6.7 μM at an assay AEA concentration of 0.5 μM according to the Cheng–Prusoff relation, which is in good agreement with the value of 6.2 μM found for kaempferol in the initial experiments (Table 1).
Figure 2.
Inhibition of AEA hydrolysis by (a) kaempferol and (b) 7-hydroxyflavone. In the main graphs, the curves are those of best fit from analyses where Vmax values were fixed at 1.512 and 1.112 nmol mg−1 protein min−1 for a and b, respectively. In the insets to the left of each panel, the values are percentage control for 0.5 μM AEA hydrolysis, following preincubation periods for the times shown. In the insets on the right, the mean data from the main graphs are replotted as double-reciprocal plots to show the competitive nature of the inhibition. Results are means (±s.e.mean, where appropriate and when not enclosed by the symbols), n=3. AEA, anandamide.
In the case of 7-hydroxyflavone (using EtOH as solvent), inhibition by 0.4 and 0.8 μM again appeared to be competitive in nature, whereas the situation for 1.2 μM was less clear. However, constraining the Bmax value to that seen in the absence of 7-hydroxyflavone (1.112 nmol mg−1 protein min−1) once again fitted the data points well at all concentrations of this compound (Figure 2b), suggesting that a competitive mode of inhibition is a reasonable interpretation of the data. The Ki value determined from the Kmapp values calculated from these constrained plots (1.22, 2.13, 2.53 and 3.09 μM at 0, 0.4, 0.8 and 1.2 μM of 7-hydroxyflavone, respectively) was 0.9 μM.
Effects of flavonoids on the cellular uptake and hydrolysis of AEA by adherent cells
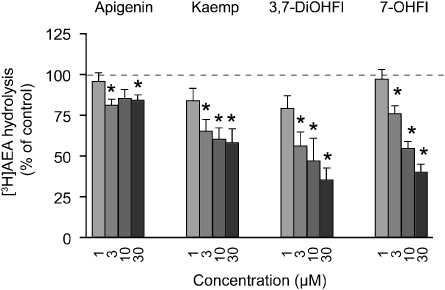
The ability of adherent RBL2H3 cells to hydrolyse AEA was measured by quantification of the water-soluble products following incubation with 100 nM [3H]-AEA, labelled in the ethanolamine part of the molecule (Figure 3). Kaempferol, 3,7-dihydroxyflavone and 7-hydroxyflavone at concentrations ⩾3 μM significantly inhibited the hydrolysis of AEA by the RBL2H3 cells, whereas apigenin had only minor effects (Figure 3).
Figure 3.
Inhibition by apigenin, kaempferol (kaemp), 3,7-dihydroxyflavone (3,7-DiOHFl) and 7-hydroxyflavone (7-OHFl) of the hydrolysis of 100 nM AEA by adherent RBL2H3 cells. The compounds were dissolved in dimethylsulphoxide with the exception of 7-hydroxyflavone (ethanol). Shown are means and s.e.mean, n=4–5. *Indicates values where both lower and higher confidence limits were <100% (in the other cases, the values straddled 100%). AEA, anandamide.
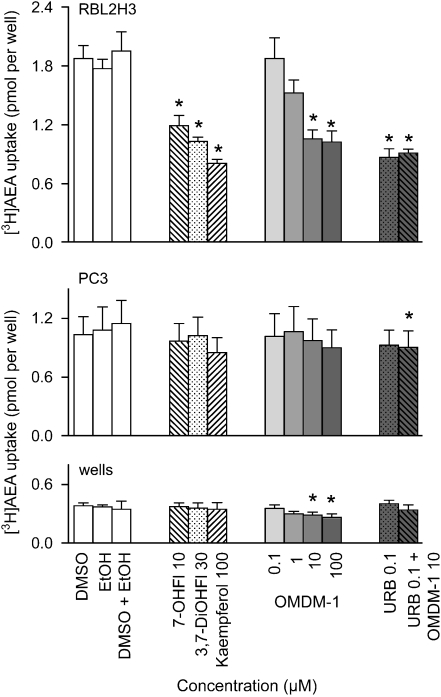
The reduction of the rate of hydrolysis by the flavones can, in theory, be due to the inhibition of the cellular accumulation of the compounds, a direct action on FAAH following uptake, or an effect on both these components. In order to investigate this further, the effects of 7-hydroxyflavone, 3,7-dihydroxyflavone and kaempferol on the uptake of 100 nM [3H]-AEA (labelled in the arachidonate part of the molecule) were assessed in adherent RBL2H3 basophilic leukaemia cells (where the uptake is ‘driven' to a large extent by FAAH) and PC3 prostate carcinoma cells (where FAAH does not contribute to the uptake) (Day et al., 2001; Ruiz-Llorente et al., 2004; Kaczocha et al., 2006; Thors et al., 2007a, 2007b). For comparative purposes, OMDM-1, which inhibits the cellular accumulation of AEA (Ortar et al., 2003), was also tested. In the RBL2H3 cells, all four compounds reduced the uptake (Figure 4). The reduction in uptake was in no case greater than that seen with the FAAH inhibitor URB597 (0.1 μM), and the combination of OMDM-1 and URB597 did not produce a greater inhibition than seen with either compound per se. Given that different authors find different relative potencies of uptake inhibitors relative to their direct effects on FAAH (see Ortar et al., 2003; Fowler et al., 2004; Hillard et al., 2007), the precise point(s) of inhibition of AEA uptake produced by OMDM-1 in the RBL2H3 cells cannot be pinpointed, other than being along the uptake–hydrolysis pathway. In the PC3 cells, on the other hand, none of the compounds produced a significant reduction in AEA uptake, although a very small decrease was seen with the combination of URB597 and OMDM-1 (Figure 4). OMDM-1 also produced a small, but significant, reduction in the retention of AEA by wells alone, consistent with previous data (Fowler et al., 2004). The simplest explanation for these data is that the effects of the flavonoids on the accumulation of AEA are a result of their effects on FAAH rather than on the uptake process itself.
Figure 4.
Uptake of 100 nM [3H]-AEA by RBL2H3 cells, PC3 cells and retention by wells alone. Test compounds (in dimethylsulphoxide except for OMDM-1, which was dissolved in ethanol before dilution with assay buffer) were preincubated with the cells (or wells) for 10 min at 37 °C before the addition of AEA and incubation for a further 5 min. Shown are means±s.e.mean, n=4. *P<0.05 vs the corresponding vehicle treatment, two-tailed paired t-test (flavonoids, URB597) or Dunnett's multiple comparison test following significant one-way ANOVA for repeated measures (OMDM-1). AEA, anandamide.
Effects of flavonoids on the processing of AEA metabolites
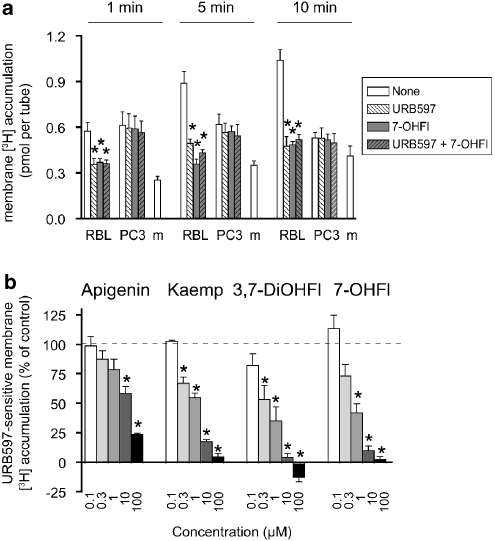
McFarland et al. (2004) reported that in RBL2H3 cells incubated with AEA, the products of FAAH catalysis (arachidonic acid and ethanolamine) were enriched in the lipid raft regions of the cell membrane. By measuring the accumulation of tritium label in RBL2H3 membrane fractions after incubation of intact cells in suspension with 100 nM [3H]-AEA (labelled in the arachidonate part of the molecule), a simple indirect measure of FAAH activity in the cells can be obtained. Consistent with this contention, the accumulation of tritium by the RBL2H3 cells was time-dependent and was greatly inhibited by URB597 (Figure 5a), but was not significantly affected by the CB1 and CB2 receptor inverse agonists AM251 (1 μM) and AM630 (1 μM), respectively (data not shown). PC3 cells, with low expression of FAAH, showed no time-dependent accumulation, and URB597 was without effect (Figure 5a). Thus, URB597-sensitive tritium accumulation by the membranes is a measure of the FAAH-dependent processing of AEA by the cells. In Figure 5b, the effects of apigenin, kaempferol, 3,7-dihydroxyflavone and 7-hydroxyflavone on URB597-sensitive accumulation of tritium in RBL2H3 cell membranes are shown following incubation of the intact cells with 100 nM [3H]-AEA. All four compounds inhibited URB597-sensitive tritium accumulation. The IC50 values calculated from the data shown in Figure 5b were 15, 1.3, 0.8 and 0.4 μM for apigenin, kaempferol, 3,7-dihydroxyflavone and 7-hydroxyflavone, respectively. The effects of 7-hydroxyflavone and URB597 on the total accumulation of tritium were not additive (Figure 5a). It can be noted that the rank order of potency of the compounds was the same as for the cell-free homogenates. Thus, it can be concluded that kaempferol, 3,7-dihydroxyflavone and 7-hydroxyflavone inhibit FAAH in both intact cells and cell-free preparations at low micromolar concentrations, whereas apigenin is less active in this regard.
Figure 5.
Accumulation of tritium label in RBL2H3 (‘RBL') and PC3 cell membranes following incubation of 100 nM [3H]-AEA with intact cells in suspension. (a) Effects of URB597 (1 μM), 7-hydroxyflavone (7-OHFl, 30 μM) and the combination of the two compounds on membrane tritium labelling. The cells were preincubated with the compounds or vehicle, as appropriate, for 10 min at 37 °C before the addition of AEA and incubated for the times shown; ‘m' indicates data for assay medium alone in the absence of cells. Shown are means±s.e.mean, n=4. *P<0.05 vs the corresponding vehicle treatment, Tukey's multiple comparisons test following significant one-way ANOVA for repeated measures performed for the incubation time point and cell line shown. In no case were the 7-hydroxyflavone, URB597 and the 7-hydroxyflavone+URB597 values significantly different from each other. (b) Inhibition by apigenin, kaempferol (kaemp), 3,7-dihydroxyflavone (3,7-DiOHFl) and 7-OHFl of the URB597 (100 nM)-sensitive labelling of RBL2H3 cell membranes. The compounds were dissolved in dimethylsulphoxide, with the exception of 7-hydroxyflavone (ethanol), before dilution with assay buffer. Shown are means and s.e.mean, n=4–5. *Indicates values where both lower and higher confidence limits were <100% (in the other cases, the values straddled 100%).
Discussion
The present study was instigated by the finding that the isoflavones genistein and daidzein were quite potent competitive inhibitors of FAAH. The finding that this property is shared by several flavonoids adds to the multitude of biological effects that these natural products possess (Havsteen, 2002). The concentrations required for inhibition of FAAH are generally in the micromolar range, which is, by any standard, modest, particularly when compared either with the most potent FAAH inhibitors available, or with the potencies of the flavones toward oestrogen receptors (Kuiper et al., 1998). However, the potencies are well in line with those required for other in vitro flavonoid biological actions. Thus, the concentrations of kaempferol required to inhibit AEA hydrolysis in both homogenates and intact cells are similar to those required for antioxidant effects (Furusawa et al., 2005), inhibition of EGF-receptor intrinsic tyrosine kinase and PKC (Agullo et al., 1997), inhibition of 20α-hydroxysteroid dehydrogenase (Brožic et al., 2006), inhibition of interleukin-4-induced STAT6 activation (Cortes et al., 2007) and activation of COX-2 (Liang et al., 2001). Thus, FAAH inhibition should be included among the in vitro properties of the flavonoids.
For the 20 compounds tested, there appear to be some structural requirements for FAAH inhibition, of which a hydroxy substituent at the position 7 of the benzopyran-4-one ring is probably the most important. In terms of drug design based on such compounds, the 7-hydroxy- and 3,7-dihydroxyflavones may be useful starting points for synthesis of compounds that are more potent FAAH inhibitors, while retaining other potentially useful biological properties, such as activation of PPARγ (Liang et al., 2001). Such compounds could, in theory, be useful for the treatment of inflammatory disorders and cancers, given that both components have potential in this regard (Bifulco et al., 2004; Holt et al., 2005; D'Argenio et al., 2006; Jayamanne et al., 2006; Moraes et al., 2006; Wang et al., 2006; Izzo et al., 2008).
With respect to the effects of the flavonoids on AEA uptake, metabolism and processing of metabolites, two observations are worth commenting on. Firstly, with respect to the effects on FAAH, higher concentrations are required for the adherent cells than for the cell-free homogenates. This pattern has been seen with other FAAH inhibitors (Bisogno et al., 1998) and presumably indicates that the cell membrane is a permeability barrier to the compounds. However, the compounds were rather efficaceous in preventing the FAAH-dependent accumulation of tritium in the membranes following incubation of RBL2H3 cells in suspension with AEA. The simplest explanation for this finding, given that the rank order of potencies were the same as for the cell-free homogenates, is that the cells in suspension, although viable (as checked using trypan blue), are more permeable to the flavonoids than when they are adherent.
Although this investigation has primarily been concerned with the structural requirements for the inhibition of FAAH by flavonoids, the nature of the compounds begs the question as to whether dietary flavonoid intake is sufficient to inhibit FAAH in vivo. At the outset, it should be pointed out that extrapolation of in vitro data, such as reported here, to the situation in man is difficult, to put it mildly, but ‘ballpark' estimates can be considered. In plants, flavones are often, but not exclusively, present as glycosides, but aglycones are produced after ingestion. The two most potent (with respect to FAAH inhibition) compounds were 7-hydroxyflavone and 3,7-dihydroxyflavone, but these compounds, although naturally occurring (in Dracaena cochinchinensis, Clerodendron phlomoidis and Platymiscium praecox Mart., found in China, India and Brazil, respectively; Braga De Oliveira et al., 1972; Roy and Pandey, 1994; Tu et al., 2003) cannot be described as commonly occurring compounds. The mean dietary intake of kaempferol by adults is ∼5 mg day−1, whereas that of quercetin is ∼16 mg day−1; intakes of apigenin, myricetin, fisetin and luteolin are much lower, although there is naturally a large inter-individual variation (Arai et al., 2000; Sampson et al., 2002; Johannot and Somerset, 2006). Following intake of a bowl of endive soup, containing 8.65 mg of kaempferol equivalent, a mean peak plasma kaempferol concentration of ∼0.1 μM was found for eight healthy subjects (DuPont et al., 2004). In another study, the plasma concentrations of kaempferol and quercetin following ingestion of concentrated black tea (providing 27 and 49 mg of these flavonoids, respectively) were found to be 15 and 29 μg L−1, corresponding to ∼0.05 and ∼0.1 μM, respectively (de Vries et al., 1998). These values for kaempferol are considerably lower than the concentrations needed for inhibition of FAAH activity in either cell-free homogenates or intact cells, and suggests that inhibition of FAAH following ingestion of dietary flavonoids is unlikely. Inhibition of FAAH may, however, occur following localized exposure to flavones. Tobacco leaves contain kaempferol glycoside (Pang et al., 2007), raising the possibility that a local inhibition of FAAH in the lungs occurs after cigarette consumption. Given that AEA, which is often produced at high levels following cellular damage (Kondo et al., 1998; Hansen et al., 2001), induces cough (Jia et al., 2002), a high local concentration of kaempferol would hardly be beneficial to smokers.
A separate question is whether inhibition of FAAH contributes to the pharmacological actions of flavonoids in experimental animals. As an example, kaempferol and quercetin (administered orally at a dose of 50 mg kg−1 as glycosides) produce antinociceptive effects in a model of visceral pain (acetic acid writhing) and anti-inflammatory effects in a carrageenan model (Toker et al., 2004). Such effects are also seen with URB597 (Holt et al., 2005; Naidu and Lichtman, 2007), so it is at least theoretically possible that inhibition of peripheral FAAH can contribute to such actions. However, the fact that kaempferol and quercetin glycosides were equally effective in the models at the dose given (Toker et al., 2004) would argue against a contribution mediated by FAAH. A definitive answer requires the measurement of local AEA levels in the tissues.
In conclusion, the present study has demonstrated that flavonoids inhibit FAAH at the concentrations used (with the notable exception of oestrogen receptors) to demonstrate other biological effects in vitro, with kaempferol being the most potent of the common naturally occurring compounds. Although it is unlikely that dietary ingestion of flavonoids will result in FAAH inhibition, the compounds may be useful templates for synthetic strategies for the discovery of novel anti-inflammatory and chemotherapeutic agents.
Acknowledgments
We thank Britt Jacobsson and Ingrid Persson for their expert technical assistance in the FAAH screening and adherent RBL2H3 AEA hydrolysis assays, respectively. The study was supported by grants from the Swedish Research Council (Grant no. 12158, medicine) and the Research Funds of the Medical Faculty (Umeå University). MB is a recipient of a grant from the EU Leonardo da Vinci programme.
Abbreviations
- AEA
anandamide (arachidonoylethanolamide)
- DMSO
dimethylsulphoxide
- EtOH
ethanol
- FAAH
fatty acid amide hydrolase
- OMDM-1
(9Z)-N-(1-((S)-4-hydroxybenzyl)-2-hydroxyethyl)-9-octadecenamide
- PPARγ
peroxisome proliferator activated receptor γ
- URB597
3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate
Conflict of interest
The authors state no conflict of interest.
References
- Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Rémésy C, Chap H, et al. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol. 1997;53:2087–2094. doi: 10.1016/s0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- Arai Y, Watanabe S, Kimmmira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- Bifulco M, Laezza C, Pisanti S, Gazzerro P. Cannabinoids and cancer: pros and cons of an antitumour strategy. Br J Pharmacol. 2006;148:123–135. doi: 10.1038/sj.bjp.0706632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifulco M, Laezza C, Valenti M, Ligresti A, Portella G, Di Marzo V. A new strategy to block tumor growth by inhibiting endocannabinoid inactivation. FASEB J. 2004;18:1606–1608. doi: 10.1096/fj.04-1754fje. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Melck D, De Petrocellis L, Bobrov MYu, Gretskaya NM, Bezuglov VV, et al. Arachidonoylserotonin and other novel inhibitors of fatty acid amide hydrolase. Biochem Biophys Res Commun. 1998;248:515–522. doi: 10.1006/bbrc.1998.8874. [DOI] [PubMed] [Google Scholar]
- Boldrup L, Wilson SJ, Barbier AJ, Fowler CJ. A simple stopped assay for fatty acid amide hydrolase avoiding the use of a chloroform extraction phase. J Biochem Biophys Methods. 2004;60:171–177. doi: 10.1016/j.jbbm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Braga De Oliveira A, Fonseca E, Silva LG, Gottlieb OR. Flavonoids and coumarins from Platymiscium praecox. Phytochemistry. 1972;11:3515–3519. [Google Scholar]
- Brožiè P, Šmuc T, Gobec S, Rižner TL. Phytoestrogens as inhibitors of the human progesterone metabolizing enzyme AKR1C1. Mol Cell Endocrinol. 2006;259:30–42. doi: 10.1016/j.mce.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem. 2005;280:5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- Chacko BK, Chandler RT, D'Alessandro TL, Mundhekar A, Khoo NKH, Botting N, et al. Anti-inflammatory effects of isoflavones are dependent on flow and human endothelial cell PPARγ. J Nutr. 2007;137:351–356. doi: 10.1093/jn/137.2.351. [DOI] [PubMed] [Google Scholar]
- Cortes JR, Perez-G M, Rivas MD, Zamorano J. Kaempferol inhibits IL-4-induced STAT6 activation by specificially targeting JAK3. J Immunol. 2007;179:3881–3887. doi: 10.4049/jimmunol.179.6.3881. [DOI] [PubMed] [Google Scholar]
- Dang Z-C, Audinot V, Papapoulos SE, Boutin JA, Löwik CWGM. Peroxisome proliferator-activated receptor γ (PPARγ) as a molecular target for the soy phytoestrogen genistein. J Biol Chem. 2003;278:962–967. doi: 10.1074/jbc.M209483200. [DOI] [PubMed] [Google Scholar]
- D'Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568–570. doi: 10.1096/fj.05-4943fje. [DOI] [PubMed] [Google Scholar]
- Day TA, Rakhshan F, Deutsch DG, Barker EL. Role of fatty acid amide hydrolase in the transport of the endogenous cannabinoid anandamide. Mol Pharmacol. 2001;59:1369–1375. doi: 10.1124/mol.59.6.1369. [DOI] [PubMed] [Google Scholar]
- de Vries JHM, Hollman PCH, Meyboom S, Buysman MNCP, Zock PL, van Staveren WA, et al. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol and biomarkers for dietary intake. Am J Clin Nutr. 1998;68:60–65. doi: 10.1093/ajcn/68.1.60. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- DuPont MS, Day AJ, Bennett RN, Mellon FA, Kroon PA. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur J Clin Nutr. 2004;58:947–954. doi: 10.1038/sj.ejcn.1601916. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Tiger G, Ligresti A, López-Rodríguez ML, Di Marzo V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis—a difficult issue to handle. Eur J Pharmacol. 2004;492:1–11. doi: 10.1016/j.ejphar.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Furusawa M, Tanaka T, Ito T, Nishikawa A, Yamazaki N, Nakaya K-i, et al. Antioxidant activity of hydroxyflavonoids. J Health Sci. 2005;51:376–378. [Google Scholar]
- Gates MA, Tworoger SS, Hecht JL, De Vivo I, Rosner B, Hankinson SE. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007;121:2225–2232. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Schmid PC, Bittigau P, Lastres-Becker I, Berrendero F, Manzaneres J, et al. Anandamide, but not 2-arachidonoylglycerol, accumulates during in vivo neurodegeneration. J Neurochem. 2001;78:1415–1427. doi: 10.1046/j.1471-4159.2001.00542.x. [DOI] [PubMed] [Google Scholar]
- Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Shi L, Tuniki VR, Falck JR, Campbell WB. Studies of anandamide accumulation inhibitors in cerebellar granule neurons: comparison to inhibition of fatty acid amide hydrolase. J Mol Neurosci. 2007;33:18–24. doi: 10.1007/s12031-007-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S, Comelli F, Costa B, Fowler CJ. Inhibitors of fatty acid amide hydrolase reduce carrageenan-induced hind paw inflammation in pentobarbital-treated mice: comparison with indomethacin and possible involvement of cannabinoid receptors. Br J Pharmacol. 2005;146:467–476. doi: 10.1038/sj.bjp.0706348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Aviello G, Petrosino S, Orlando P, Marsicano G, Lutz B, et al. Increased endocannabinoid levels reduce the development of precancerous lesions in the mouse colon. J Mol Med. 2008;86:89–98. doi: 10.1007/s00109-007-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, McLeod RL, Wang X, Parra LE, Egan RW, Hey JA. Anandamide induces cough in conscious guinea pigs through VR1 receptors. Br J Pharmacol. 2002;137:831–836. doi: 10.1038/sj.bjp.0704950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannot L, Somerset SM. Age-related variations in flavonoid intake and sources in the Australian population. Publ Health Nutr. 2006;9:1045–1054. doi: 10.1017/s1368980006009712. [DOI] [PubMed] [Google Scholar]
- Kaczocha M, Hermann A, Glaser ST, Bojesen IN, Deutsch DG. Anandamide uptake is consistent with rate-limited diffusion and is regulated by the degree of its hydrolysis by fatty acid amide hydrolase. J Biol Chem. 2006;281:9066–9075. doi: 10.1074/jbc.M509721200. [DOI] [PubMed] [Google Scholar]
- Keli SO, Hertog MGL, Feskens EJM, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156:637–642. [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- Ko W-C, Shih C-M, Lai Y-H, Chen J-H, Huang H-L. Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure–activity relationships. Biochem Pharmacol. 2004;68:2087–2094. doi: 10.1016/j.bcp.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Kondo S, Sugiura T, Kodaka T, Kudo N, Waku K, Tokumura A. Accumulation of various N-acylethanolamines including N-arachidonoylethanolamine (anandamide) in cadmium chloride-administered rat testis. Arch Biochem Biophys. 1998;354:303–310. doi: 10.1006/abbi.1998.0688. [DOI] [PubMed] [Google Scholar]
- Kuiper GJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, ven der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptorβ. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lenman A, Fowler CJ. Interaction of ligands for the peroxisome proliferator-activated receptor γ with the endocannabinoid system. Br J Pharmacol. 2007;151:1343–1351. doi: 10.1038/sj.bjp.0707352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y-C, Tsai S-H, Tsai D-C, Lin-Shiau S-Y, Lin J-K. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-γ by flavonoids in mouse macrophages. FEBS Lett. 2001;496:12–18. doi: 10.1016/s0014-5793(01)02393-6. [DOI] [PubMed] [Google Scholar]
- Maxwell CA, Hartwig UA, Joseph CM, Phillips DA. A chalcone and two related flavonoids released from alfalfa roots induce nod genes of Rizobium meliloti. Plant Physiol. 1989;91:842–847. doi: 10.1104/pp.91.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland MJ, Porter AC, Rakhshan FR, Rawat DS, Gibbs RA, Barker EL. A role for caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide. J Biol Chem. 2004;279:41991–41997. doi: 10.1074/jbc.M407250200. [DOI] [PubMed] [Google Scholar]
- Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther. 2006;110:371–385. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Lichtman AH.Synergistic antinociceptive effects of URB597 and diclofenac in a mouse visceral pain model 2007. 17th Annual Symposium on the Cannabinoids, Burlington, Vermont, International Cannabinoid Research Society, 2007, no. 172. Available online at
- Nöthlings U, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Flavonols and pancreatic cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;166:924–931. doi: 10.1093/aje/kwm172. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE. Cannnabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortar G, Ligresti A, De Petrocellis L, Morera E, Di Marzo V. Novel selective and metabolically stable inhibitors of anandamide cellular uptake. Biochem Pharmacol. 2003;65:1473–1481. doi: 10.1016/s0006-2952(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Pang T, Yuan Z, Dai Y, Wang C, Yang J, Peng L, et al. Identification and determination of glycosides in tobacco leaves by liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry. J Sep Sci. 2007;30:289–296. doi: 10.1002/jssc.200600236. [DOI] [PubMed] [Google Scholar]
- Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise. Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- Paylor B, Holt S, Fowler CJ.The potency of the fatty acid amide hydrolase inhibitor URB597 is dependent upon the assay pH Pharmacol Res 200654481–485.Corrigendum published in Pharmacol Res 55: 80 (2007) [DOI] [PubMed] [Google Scholar]
- Roy R, Pandey VB. A chalcone glycoside from Clerodendron phlomidis. Phytochemistry. 1994;37:1775–1776. doi: 10.1016/s0031-9422(00)89613-2. [DOI] [PubMed] [Google Scholar]
- Ruiz-Llorente L, Ortega-Gutiérrez S, Viso A, Sánchez MG, Sánchez AM, Fernández C, et al. Characterization of an anandamide degradation system in prostate epithelial PC-3 cells: synthesis of new transporter inhibitors as tools for this study. Br J Pharmacol. 2004;141:457–467. doi: 10.1038/sj.bjp.0705628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson L, Rimm E, Hollman PCH, de Vries JHM, Katan MB. Flavonol and flavone intakes in US health professionals. J Am Diet Assoc. 2002;102:1414–1420. doi: 10.1016/s0002-8223(02)90314-7. [DOI] [PubMed] [Google Scholar]
- Shukla S, MacLennan GT, Flask CA, Fu P, Mishra A, Resnick MI, et al. Blockade of ß-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007;67:6925–6935. doi: 10.1158/0008-5472.CAN-07-0717. [DOI] [PubMed] [Google Scholar]
- Thors L, Alajakku K, Fowler CJ. The ‘specific' tyrosine kinase inhibitor genistein inhibits the enzymic hydrolysis of anandamide: implications for anandamide uptake. Br J Pharmacol. 2007a;150:951–960. doi: 10.1038/sj.bjp.0707172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thors L, Eriksson J, Fowler CJ. Inhibition of the cellular uptake of anandamide by genistein and its analogue daidzein in cells with different levels of fatty acid amide hydrolase-driven uptake. Br J Pharmacol. 2007b;152:744–750. doi: 10.1038/sj.bjp.0707401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker G, Küpeli E, Memisoğlu M, Yesilada E. Flavonoids with antinociceptive and anti-inflammatory activities from the leaves of Tilia argentea (silver linden) J Ethnopharmacol. 2004;95:393–397. doi: 10.1016/j.jep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Tu P-F, Tao J, Hu Y-Q, Zhao M-B. Flavones from the wood Dracaena chonchinchinensis. J Nat Med. 2003;1:27–29. [Google Scholar]
- Wang T, Xu J, Yu X, Yang R, Han ZC. Peroxisome proliferator-activated receptor γ in malignant diseases. Crit Rev Oncol Hematol. 2006;58:1–14. doi: 10.1016/j.critrevonc.2005.08.011. [DOI] [PubMed] [Google Scholar]