Abstract
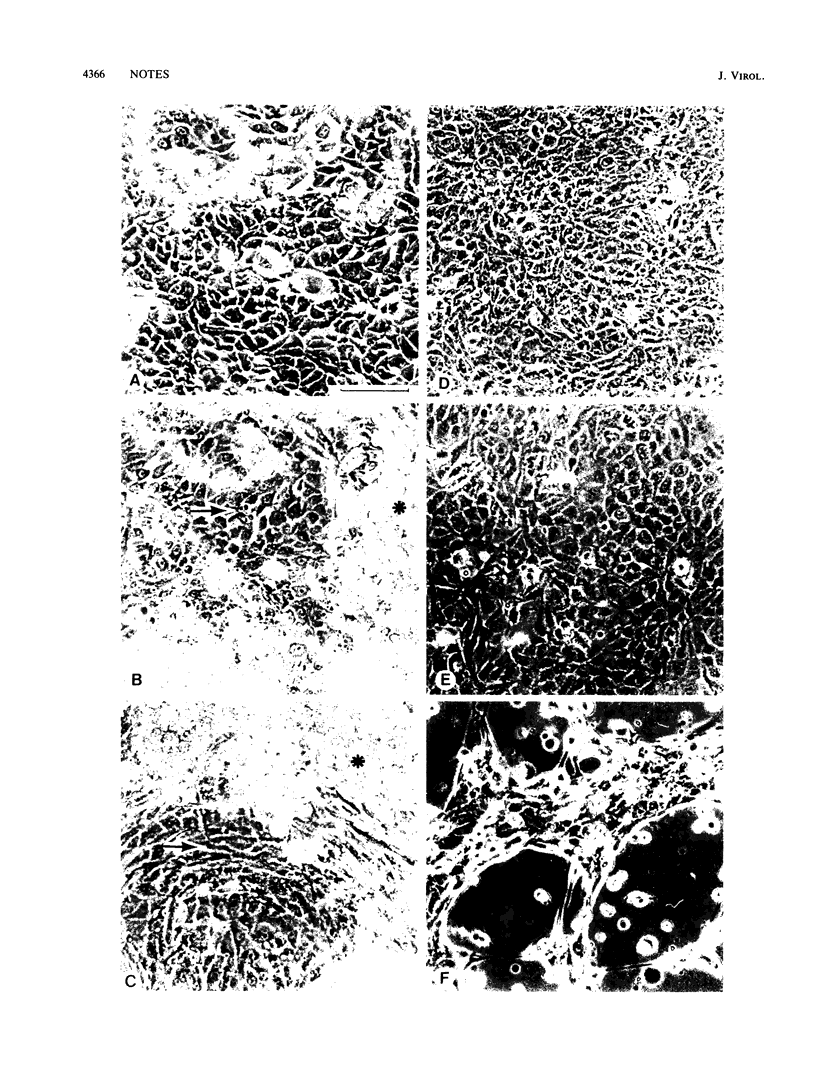
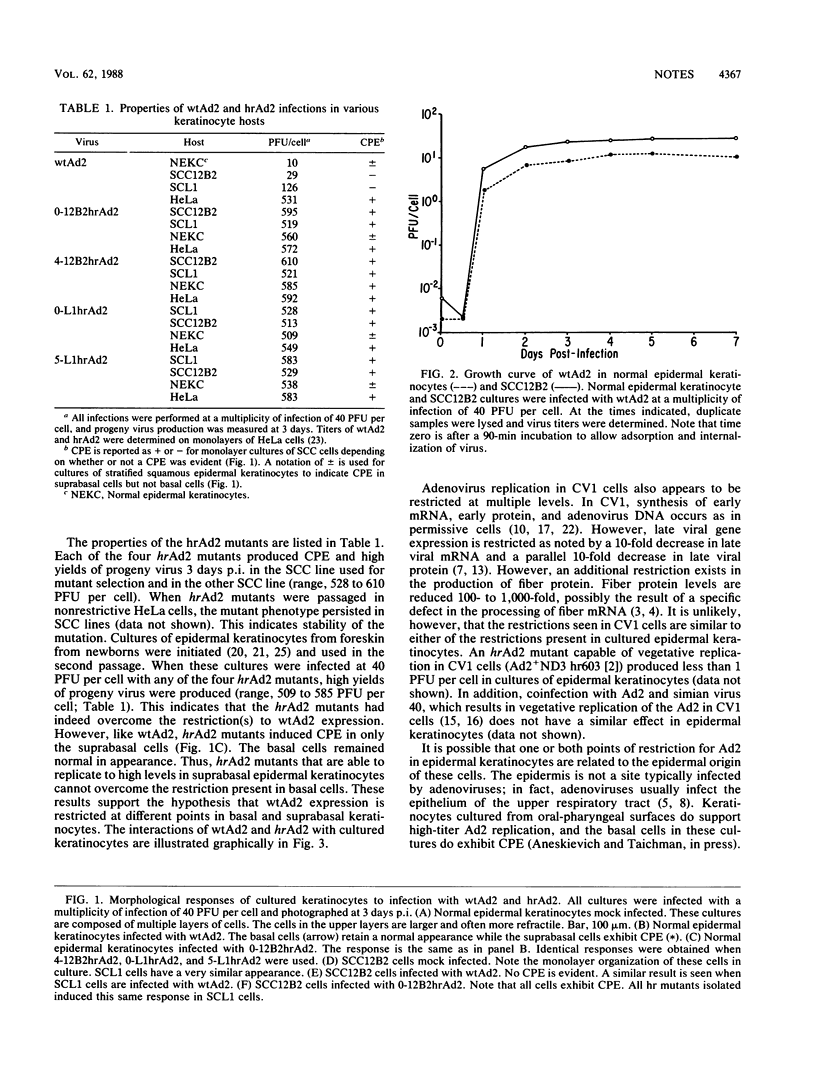
Cultures of epidermal keratinocytes contain two populations of cells, a basal undifferentiated population and a suprabasal terminally differentiated population. When exposed to wild-type adenovirus type 2 (wtAd2), the suprabasal cells are positive by immunofluorescence for capsid antigen and exhibit cytopathic effects (CPE) (R.F. LaPorta, and L.B. Taichman, Virology 110:137-146, 1981). The basal cells, although infected, are not positive for capsid antigen and do not display CPE. Despite CPE and capsid antigens in suprabasal cells, yields of virus from the entire culture are very low (10 PFU per cell). These observations suggest that Ad2 expression is restricted at different times in the viral life cycle in basal and suprabasal cells. To test this hypothesis, we isolated host range (hr) mutants of Ad2 on two lines of squamous cell carcinoma (SCC) keratinocytes which were shown to be restrictive for wtAd2 replication. The hrAd2 mutants produced high yields of progeny virus in epidermal cell cultures (500 to 600 PFU per cell). However, the pattern of CPE induction in these cultures was like that produced by wtAd2, i.e., basal cells were CPE negative and suprabasal cells were CPE positive. The high yield of hrAd2 progeny indicated that the restriction present in suprabasal cells was overcome. However, the failure of hrAd2 mutants to induce CPE in basal cells indicated that the hrAd2 mutants remain restricted in the basal population and supported our hypothesis that a second and distinct restriction exists in basal keratinocytes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers K. M., Greif F., Setzer R. W., Taichman L. B. Cell-cycle withdrawal in cultured keratinocytes. Differentiation. 1987;34(3):236–240. doi: 10.1111/j.1432-0436.1987.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Anderson C. W. Spontaneous mutants of the adenovirus-simian virus 40 hybrid, Ad2+ND3, that grow efficiently in monkey cells. Virology. 1981 May;111(1):263–269. doi: 10.1016/0042-6822(81)90670-x. [DOI] [PubMed] [Google Scholar]
- Anderson K. P., Klessig D. F. Altered mRNA splicing in monkey cells abortively infected with human adenovirus may be responsible for inefficient synthesis of the virion fiber polypeptide. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4023–4027. doi: 10.1073/pnas.81.13.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. P., Klessig D. F. Posttranscriptional block to synthesis of a human adenovirus capsid protein in abortively infected monkey cells. J Mol Appl Genet. 1983;2(1):31–43. [PubMed] [Google Scholar]
- Aneskievich B. J., Taichman L. B. Epithelium-specific response of cultured keratinocytes to infection with adenovirus type 2. J Invest Dermatol. 1988 Oct;91(4):309–314. doi: 10.1111/1523-1747.ep12475641. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Tilgen W., Dzarlieva R. T., Breitkreutz D., Haag D., Riehl R. K., Bohnert A., Fusenig N. E. Phenotypic and genotypic characteristics of a cell line from a squamous cell carcinoma of human skin. J Natl Cancer Inst. 1982 Mar;68(3):415–427. [PubMed] [Google Scholar]
- FRIED M. ISOLATION OF TEMPERATURE-SENSITIVE MUTANTS OF POLYOMA VIRUS. Virology. 1965 Apr;25:669–671. doi: 10.1016/0042-6822(65)90098-x. [DOI] [PubMed] [Google Scholar]
- Farber M. S., Baum S. G. Transcription of adenovirus RNA in permissive and nonpermissive infections. J Virol. 1978 Jul;27(1):136–148. doi: 10.1128/jvi.27.1.136-148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Nakajima K., Oda K., Shimojo H. Complementation of translational defect for growth of human adenovirus type 2 in Simian cells by a Simian virus 40-induced factor. J Mol Biol. 1973 Dec 5;81(2):207–223. doi: 10.1016/0022-2836(73)90190-3. [DOI] [PubMed] [Google Scholar]
- Holbrook K. A., Hennings H. Phenotypic expression of epidermal cells in vitro: a review. J Invest Dermatol. 1983 Jul;81(1 Suppl):11s–24s. doi: 10.1111/1523-1747.ep12540003. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Anderson C. W. Block to multiplication of adenovirus serotype 2 in monkey cells. J Virol. 1975 Dec;16(6):1650–1668. doi: 10.1128/jvi.16.6.1650-1668.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F. Isolation of a variant of human adenovirus serotype 2 that multiplies efficiently on monkey cells. J Virol. 1977 Mar;21(3):1243–1246. doi: 10.1128/jvi.21.3.1243-1246.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporta R. F., Taichman L. B. Adenovirus type-2 infection of human keratinocytes: viral expression dependent upon the state of cellular maturation. Virology. 1981 Apr 15;110(1):137–146. doi: 10.1016/0042-6822(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Levin M. J., Wiese W. H., Crumpacker C. S., Henry P. H. A nondefective (competent) adenovirus-SV40 hybrid isolated from the AD.2-SV40 hybrid population. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1128–1135. doi: 10.1073/pnas.63.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABSON A. S., O'CONOR G. T., BEREZESKY I. K., PAUL F. J. ENHANCEMENT OF ADENOVIRUS GROWTH IN AFRICAN GREEN MONKEY KIDNEY CELL CULTURES BY SV40. Proc Soc Exp Biol Med. 1964 May;116:187–190. doi: 10.3181/00379727-116-29197. [DOI] [PubMed] [Google Scholar]
- Reich P. R., Baum S. G., Rose J. A., Rowe W. P., Weissman S. M. Nucleic acid homology studies of adenovirus type 7-SV40 interactions. Proc Natl Acad Sci U S A. 1966 Feb;55(2):336–341. doi: 10.1073/pnas.55.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell. 1980 Nov;22(2 Pt 2):629–632. doi: 10.1016/0092-8674(80)90373-6. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981 May;41(5):1657–1663. [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Taichman L., Reilly S., Garant P. R. In-vitro cultivation of human oral keratinocytes. Arch Oral Biol. 1979;24(5):335–341. doi: 10.1016/0003-9969(79)90099-2. [DOI] [PubMed] [Google Scholar]
- Williams J. F. Enhancement of adenovirus plaque formation on HeLa cells by magnesium chloride. J Gen Virol. 1970 Dec;9(3):251–255. doi: 10.1099/0022-1317-9-3-251. [DOI] [PubMed] [Google Scholar]
- Williams J. F., Gharpure M., Ustacelebi S., McDonald S. Isolation of temperature-sensitive mutants of adenovirus type 5. J Gen Virol. 1971 May;11(2):95–101. doi: 10.1099/0022-1317-11-2-95. [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Parker L. M., Binder N. E., Beckett M. A., Sinard J. H., Griffiths C. T., Rheinwald J. G. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell. 1982 Dec;31(3 Pt 2):693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]



