Abstract
Neurotrophin-mediated signalling cascades can be initiated by activation of either the p75 neurotrophin receptor (p75NTR) or the more selective tyrosine kinase receptors. Previously, we demonstrated that nerve growth factor (NGF) increased the excitability of sensory neurons through activation of p75NTR to liberate sphingosine 1-phosphate. If neurotrophins can modulate the excitability of small diameter sensory neurons through activation of p75NTR, then brain-derived neurotrophic factor (BDNF) should produce the same sensitizing action as did NGF. In this report, we show that focally applied BDNF increases the number of action potentials (APs) evoked by a ramp of depolarizing current by reducing the rheobase without altering the firing threshold. This increased excitability results, in part, from the capacity of BDNF to enhance a tetrodotoxin-resistant sodium current (TTX-R INa) and to suppress a delayed rectifier-like potassium current (IK). The idea that BDNF acts via p75NTR is supported by the following observations. The sensitizing action of BDNF is prevented by pretreatment with a blocking antibody to p75NTR or an inhibitor of sphingosine kinase (dimethylsphingosine), but not by inhibitors of tyrosine kinase receptors (K252a or AG879). Furthermore, using single-cell RT-PCR, neurons that were sensitized by BDNF expressed the mRNA for p75NTR but not TrkB. These results demonstrate that neurotrophins can modulate the excitability of small diameter capsaicin-sensitive sensory neurons through the activation of p75NTR and its downstream sphingomyelin signalling cascade. Neurotrophins released upon activation of a variety of immuno-competent cells may be important mediators that give rise to the enhanced neuronal sensitivity associated with the inflammatory response.
Nerve growth factor (NGF) plays a key role in the initiation of the inflammatory response. This idea is supported by the observation that the levels of NGF are significantly elevated in the skin after blister formation or in pleural exudates after exposure to carrageenan (Weskamp & Otten, 1987). Furthermore, a number of immuno-competent cells upon activation release NGF (Ehrhard et al. 1993; Leon et al. 1994; Thacker et al. 2007). Associated with the inflammatory response is the activation of small diameter sensory neurons which, in turn, contributes to increased sensitivity, vasodilatation, and plasma extravasation. A number of inflammatory mediators including cytokines (Ferreira et al. 1988; Schweizer et al. 1988; Cunha et al. 1992), prostaglandins (Handwerker, 1976; Chahl & Iggo, 1977), and NGF (Lewin et al. 1993; Lewin & Mendell, 1993) heighten the sensitivity of nociceptors to noxious stimulation. When injected into the paw of a rat NGF produces hyperalgesia to both thermal and mechanical stimulation (Lewin et al. 1993). In addition, pretreatment with an antibody to NGF prevents the thermal hyperalgesia produced by injection of complete Freund's adjuvant into the paw of a rat (Lewin et al. 1994; Woolf et al. 1994). In an isolated skin–nerve type preparation, NGF increases the firing frequency of isolated saphenous nerve in response to thermal stimulation (Rueff & Mendell, 1996). The mechanisms giving rise to NGF-induced sensitization are not well understood. However, studies indicate that NGF acts directly on sensory neurons to modulate their excitability because NGF augments the capsaicin-evoked current (Shu & Mendell, 1999, 2001) as well as current-evoked AP firing (Zhang et al. 2002) in small diameter sensory neurons.
It is well established that NGF can activate the p75 neurotrophin receptor (p75NTR) and the tyrosine kinase receptor TrkA (Meakin & Shooter, 1992; Bothwell, 1995; Roux & Barker, 2002; Huang & Reichardt, 2003; Reichardt, 2006). However, the specific roles of each receptor and their downstream signalling cascades in the sensitizing actions of NGF remain poorly defined. We previously demonstrated that acute exposure to NGF enhances AP firing evoked by a ramp of depolarizing current in sensory neurons isolated from young adult rats. This effect of NGF appears to result from activation of the sphingomyelin signalling cascade via p75NTR to liberate ceramide, which is metabolized to sphingosine 1-phosphate (Zhang et al. 2002; Zhang & Nicol, 2004; Zhang et al. 2006). Unlike TrkA, p75NTR can be activated by all the neurotrophins (Rodriguez-Tébar et al. 1990, 1992; Squinto et al. 1991; Roux & Barker, 2002; Gentry et al. 2004), most notably brain-derived neurotrophic factor (BDNF). Therefore, to further define the role of p75NTR activation in the sensitization of small diameter capsaicin-sensitive sensory neurons, the capacity of acutely applied BDNF to augment neuronal excitability was examined. In this report, we show that BDNF, through the p75NTR signalling cascade, increases the number of APs evoked by a ramp of current through an enhancement of the TTX-R INa and a suppression of a delayed rectifier-like IK.
Methods
Isolation and maintenance of rat sensory neurons
The procedures for primary culture of rat sensory neurons have been previously described (Lindsay, 1988) with slight modification (Jiang et al. 2003). Briefly, male Sprague–Dawley rats (100–150 g) were killed by placing them in a chamber that was then filled with CO2. DRGs were removed and collected in a culture dish filled with sterilized Puck's solution. The ganglia were transferred to a conical tube filled with Puck's solution containing 10 U ml−1 of papain II, and incubated for 12 min at 37°C. The tube was centrifuged for 50 s at low speed (approximately 2000 g) and the pellet was resuspended in Puck's solution containing collagenase (1 mg ml−1, type 1A) and dispase II (2.5 mg ml−1). After a 14 min incubation at 37°C, the tube was centrifuged for 50 s before the enzyme-containing supernatant was removed. The pellet was resuspended in F-12 medium supplemented with 30 ng ml−1 7S nerve growth factor (Harlan Bioproducts, Indianapolis, IN, USA) and mechanically dissociated with fire-polished pipettes until all obvious chunks of tissues were gone. Isolated cells were plated onto plastic coverslips that had been previously coated with poly d-lysine and laminin. The cells were maintained in F-12 medium containing nerve growth factor at 37°C and 3% CO2 and used within 6–24 h for electrophysiological recordings and the RT-PCR experiments. All procedures have been approved by the Animal Use and Care Committee of the Indiana University School of Medicine.
Electrophysiology
Recordings were made using the whole-cell patch-clamp technique as previously described (Hamill et al. 1981; Zhang et al. 2002). Briefly, a coverslip with the sensory neurons was placed in a recording chamber where the neurons were bathed in normal Ringer solution of the following composition (in mm): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes and 10 glucose, pH adjusted to 7.4 with NaOH. Recording pipettes were pulled from borosilicate glass tubing and fire-polished. Whole-cell voltages or currents were recorded with an Axopatch 200 patch-clamp amplifier (Molecular Devices, Sunnyvale, CA, USA); the data were acquired and analysed using pCLAMP 6.04 or pCLAMP 9.0 (Molecular Devices). In the current clamp experiments, the neurons were held at their resting potentials and a depolarizing ramp (1000 ms in duration) was applied. The amplitude of the ramp was adjusted to produce between two and five action potentials (APs) under control conditions and then the same ramp was used throughout the recording period for each individual neuron. In voltage clamp experiments, both capacitance and series resistance compensation (typically 80%) were used. The mean series resistance before compensation was 1.7 ± 0.1 MΩ (n = 22). Linear leakage currents were subtracted by a P/4 protocol in the recordings of TTX-R INa but not in the IK recordings.
IK was isolated by superfusing the cells with 140 mm N-methyl-glucamine chloride (NMG)-containing Ringer solution (an equimolar substitution for NaCl); pH adjusted to 7.4 with KOH. Patch pipettes had resistances of 2–5 MΩ when filled with the following solution (in mm): 140 KCl, 5 MgCl2, 4 ATP, 0.3 GTP, 2.5 CaCl2, 5 EGTA (calculated free Ca2+ concentration of 100 nm, MaxChelator) and 10 Hepes, at pH 7.3 adjusted with KOH. This pipette solution was also used in the current clamp recordings. The TTX-R INa was isolated by superfusing the cells with the following Ringer solution (in mm): 30 NaCl, 65 NMG-Cl, 30 TEA, 0.1 CaCl2, 5 MgCl2, 10 Hepes, 10 glucose, 10 sucrose and 500 nm TTX; pH adjusted to 7.4 with TEAOH. The recording pipette was filled with (in mm): 110 CsFl, 25 CsCl, 10 NaCl, 5 MgCl2, 4 ATP, 0.3 GTP, 1 CaCl2, 10 EGTA, 10 glucose and 10 Hepes, at pH 7.3 (maintained with CsOH). Patch pipettes had resistances of 1–1.5 MΩ. The membrane voltage was held at −60 mV; activation of the currents was determined by voltage steps of 380 ms or 30 ms for IK or TTX-R INa, respectively, which were applied at 5 s intervals in +5 mV (TTX-R INa) or +10 mV (IK) increments to +60 mV. Kinase inhibitors were applied to the neurons by superfusion of the recording chamber prior to the focal application of BDNF. At the end of each recording, the neuron was exposed to 100 nm capsaicin. This neurotoxin was used to distinguish capsaicin-sensitive sensory neurons as these neurons are believed to transmit nociceptive information (Holzer, 1991). However, the correlation between capsaicin sensitivity and that a neuron is a nociceptor is not absolute. Some nociceptive neurons are insensitive to capsaicin and some capsaicin-sensitive neurons are not nociceptors (see Petruska et al. 2000). Therefore this agent was used to define a population of small-diameter sensory neurons that could serve a nociceptive function. The results reported in the following text were obtained from capsaicin-sensitive neurons only. All experiments were performed at room temperature (∼22°C).
Data analysis
Data are presented as the means ± s.e.m. The excitability parameters described in Table 1 were determined, in part, by differentiating the voltage trace (dV/dt) in the current-clamp recordings (sampling frequency of 500 Hz). The voltage and time at which the first AP was fired were taken as the point that exceeded the baseline value of dV/dt by > 20-fold. The baseline value of dV/dt was determined by averaging the points between the onset of the ramp and the next 100 ms (135–235 ms). The rheobase was measured as the amount of ramp current at the firing threshold. The resistance at threshold (RTh) was calculated as the difference between the firing threshold and the resting membrane potential divided by the rheobase current. The voltage dependence for activation of TTX-R INa and IK was fitted with the Boltzmann equation where:
where G is the conductance (G = I/(Vm − Erev)), Gmax is the maximal conductance obtained from the Boltzmann fit under control conditions, V0.5 is the voltage for half-maximal activation, Vm is the membrane potential, and k is a slope factor. The Boltzmann parameters were determined for each individual neuron and were used to calculate the means ± s.e.m. Statistical differences between the control recordings and those obtained under various treatment conditions were determined by using either Student's paired t test, ANOVA, or repeated-measures (RM) ANOVA. When a significant difference was obtained with an ANOVA, post hoc analysis was performed using Tukey's test. Values of P < 0.05 were judged to be statistically significant.
Table 1.
The effects of BDNF on parameters of excitability
| RMP (mV) | FT (mV) | Rheobase (pA) | RTh (MΩ) | Normalized RTh | |
|---|---|---|---|---|---|
| Control | −61.0 ± 1.6 | −12.3 ± 3.2 | 853 ± 305 | 214 ± 52 | 1.0 |
| BDNF | |||||
| 2 min | −57.2 ± 1.8* | −13.5 ± 3.1 | 555 ± 249* | 288 ± 64* | 1.61 ± 0.21* |
| 6 min | −56.0 ± 2.1* | −13.1 ± 2.3 | 383 ± 134* | 312 ± 63* | 1.89 ± 0.24* |
| 10 min | −56.7 ± 2.1* | −13.1 ± 3.1 | 414 ± 152* | 295 ± 56* | 1.85 ± 0.24* |
RMP: resting membrane potential. FT: firing threshold. RTh: resistance at the firing threshold n = 15. P < 0.05, RM ANOVA
Single cell RT-PCR
The presence of gene transcripts for p75NTR and TrkB was detected after electrophysiological recording using techniques described by Song et al. (1998) with modification. Briefly, APs before and after treatment with BDNF were recorded from a small diameter sensory neuron; the cell was aspirated into another sterilized micropipette containing DEPC water. The contents of the micropipette were forced into a 0.2 ml microtube that contained 5 μl DEPC water, 1 μl 10× DNase I buffer and 1 μl DNase I, and allowed to incubate at room temperature for 15 min. EDTA (1 μl, 25 mm) was then added and this solution incubated at 65°C for 10 min (DNase I amplification kit, Invitrogen, Carlsbad, CA, USA). The single-cell RNA was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions. The cDNA was stored at −20°C prior to the PCR detection. Two 5 μl aliquots from the cDNA isolated from each neuron were then used to detect the presence of p75NTR and full-length TrkB in that cell. Amplification of the p75NTR was performed using the forward primer (bases 27): GGTCTATTCTGATGGAGTCAAGCTAAG (2152–2178) and the reverse primer (bases 22): CCAAGAATGAGCGCACTAACAG (2240–2219) using the Platinum PCR Supermix (Invitrogen). Amplification of the full-length TrkB was performed using the forward primer (bases 21): TTCGGTATCACCAACAGCCAG (2210–2230) and the reverse primer (bases 21): CTCGGTGGGCGGGTTACCCTC (2602–2582) using the Platinum PCR Supermix (Invitrogen). The TrkB primer sequences were obtained from Lee et al. (1999). These PCR reactions ran for 45 cycles (94°C for 1 min, 56°C for p75NTR and TrkB for 1 min, 72°C for 2 min). The PCR products were sequenced using an ABI Prism 3100 genetic analyser at facilities in the Department of Biochemistry and Molecular Biology, Indiana University School of Medicine.
Chemicals
BDNF was obtained from R&D Systems (Minneapolis, MN, USA). The p75NTR blocking antibody was kindly provided by Dr Louis Reichardt (University of California, San Francisco). K252a and AG879 were purchased from EMD Chemicals Inc. (San Diego, CA, USA). Dimethylsphingosine (DMS) was obtained from Avanti Polar Lipids (Alabaster, AL, USA). Tissue culture supplies were purchased from Invitrogen (Carlsbad, CA, USA). All other chemicals were obtained from Sigma Chemical Co. (St Louis, MO, USA). DMS and capsaicin were dissolved in 1-methyl-2-pyrrolidinone (HPLC grade). K252a came from the supplier in DMSO and was diluted into Ringer solution just before use. We have demonstrated previously that the vehicle, 1-methyl-2-pyrrolidinone, has no effect on AP firing or the activation of either TTX-R INa or IK (Zhang et al. 2002).
Results
BDNF enhances the excitability of sensory neurons
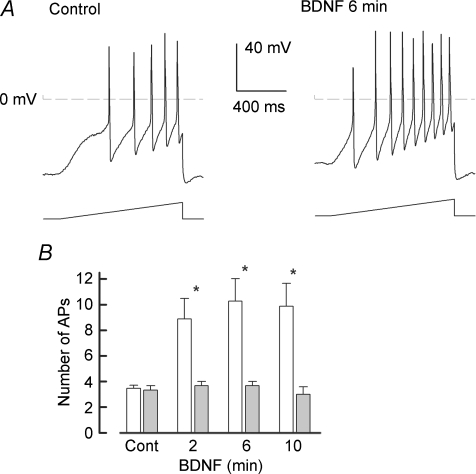
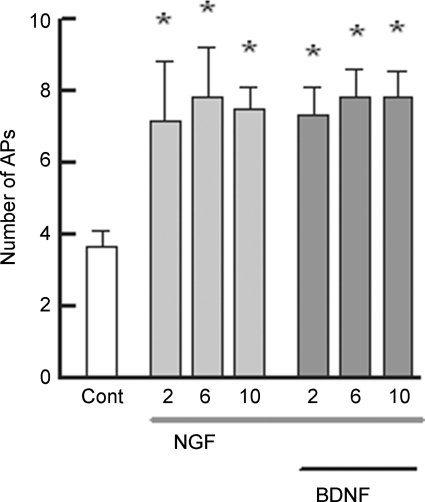
We previously demonstrated that NGF enhanced the excitability of rat sensory neurons via activation of the p75NTR. Because the p75NTR can be activated by all members of the neurotrophin family, BDNF should produce a similar increase in the excitability as did NGF. To determine whether BDNF modulates the excitability of small-diameter capsaicin-sensitive sensory neurons, BDNF was applied focally through a microperfusion pencil applicator (∼10 p.s.i., 5 s duration, Smart Squirt, AutoMate Scientific Inc., Berkeley, CA, USA) placed near the neuronal cell body. The effect of BDNF on the firing of APs evoked by a ramp of depolarizing current was tested 2, 6, 10 and 20 min after the focal application. Indeed, BDNF augmented AP firing in 12 of 15 small diameter capsaicin-sensitive sensory neurons. A representative recording (Fig. 1A, left panel) illustrates that the ramp of current evoked five APs under control conditions, whereas 6 min after exposure to BDNF (50 ng ml−1), the same ramp of current now elicited nine APs (right panel). The capacity of BDNF to enhance AP firing is summarized in Fig. 1B. Focally applied BDNF significantly increased the number of APs elicited by the ramp from an average control value of 3.5 ± 0.2 to 9.9 ± 1.8 APs (n = 15, RM ANOVA) after a 10 min exposure to BDNF. There was no significant difference in the number of APs at the different times of exposure to BDNF. After a 20 min exposure to BDNF the number of evoked APs (8.9 ± 2.0 APs, n = 10, data not shown) was not different from that at 10 min. In 3 of the 15 neurons, BDNF appeared to have only a small effect on AP firing wherein after a 10 min exposure the number of APs evoked by the ramp was increased by one or two APs compared to the control values.
Figure 1. BDNF augments the excitability of capsaicin-sensitive small diameter sensory neurons.
A, in a representative neuron, 6 min after the focal application of 50 ng ml−1 BDNF the number of evoked APs increases from a control value of 5 to 9 APs. In this particular neuron, the resting membrane potential was depolarized by 10 mV from a control value of −64 mV. B, summary of the capacity of BDNF (open bars) to sensitize sensory neurons (n = 15). The focal application of Ringer solution alone (grey bars) had no effect on the number of evoked APs (control 3.3 ± 0.3 APs versus 3.0 ± 0.6 APs after 10 min, n = 3, P = 0.67 RM ANOVA). Asterisks indicate a significant difference from control (P < 0.05, RM ANOVA).
It is possible that these neurons did not express the p75NTR (see below). In a separate series of experiments, focal application of Ringer solution alone had no effect on AP production over time (see Fig. 1B). In addition to augmenting the number of APs, BDNF produced a significant depolarization of the resting membrane by ∼5 mV, a twofold reduction in the rheobase, and about a twofold increase in the resistance measured at threshold (RTh). However, the firing threshold was not altered by BDNF. These actions of BDNF are summarized in Table 1. The changes in the AP properties produced by BDNF are similar to those we previously reported for NGF (Zhang et al. 2002). None of the APs properties shown in Table 1 were altered significantly by the focal application of Ringer solution alone (data not shown). For example, the resting membrane potential after a 10 min exposure to focally applied Ringer solution (−57.3 ± 3.4 mV, n = 3) was unchanged from a control value of −57.2 ± 3.9 mV (RM ANOVA). To examine whether the increase in APs was reversible, neurons were superfused with normal Ringer solution for 10 min after a 20 min exposure to BDNF; however, the number of APs elicited by the ramp remained elevated at 14.5 ± 2.7 (n = 4). Therefore, these results demonstrate that exposure to BDNF can lead to a rapid and sustained enhancement of the excitability of adult sensory neurons by significantly altering the resting membrane potential and the rheobase without changing the apparent firing threshold.
BDNF-induced sensitization is prevented by the blocking antibody to p75NTR but not by inhibition of tyrosine kinase receptors
To determine whether the sensitization produced by BDNF was mediated by activation of the p75NTR, sensory neurons were pretreated with a blocking antibody to p75NTR (courtesy of Dr Louis Reichardt, see Weskamp & Reichardt, 1991) for 1 h at a concentration of 50 μg ml−1. As shown in Fig. 2, after pretreatment with the blocking antibody, BDNF failed to increase the number of APs evoked by the depolarizing ramp (control 3.8 ± 0.2 APs versus 3.7 ± 0.3 APs after 10 min treatment with BDNF, n = 9, RM ANOVA). Also, pretreatment with the p75NTR blocking antibody prevented the other changes in AP properties that are represented in Table 1 (data not shown). In a parallel series of experiments on sensory neurons obtained from the same tissue harvest but not pretreated with the blocking antibody, BDNF significantly increased the number of APs from a control value of 4.0 ± 0.6 to 7.0 ± 0.8 APs (6 min exposure, n = 4, RM ANOVA). A similar suppression of sensitization was observed previously wherein the blocking antibody to p75NTR prevented the enhancement of excitability by NGF (Zhang & Nicol, 2004). These results indicate that activation of the p75NTR plays a critical role in enhancing the excitability of these small diameter sensory neurons.
Figure 2. Pretreatment with a blocking antibody to p75NTR prevented the increase in AP firing by BDNF.
Summary of results from nine sensory neurons that had been pretreated with a blocking antibody to p75NTR for 1 h. Focal application of 50 ng ml−1 BDNF (open bars) failed to increase AP firing. In a parallel series of experiments, sensory neurons that were not treated with blocking antibody were sensitized by BDNF (grey bars). Asterisks indicate a significant difference from control (P < 0.05, RM ANOVA).
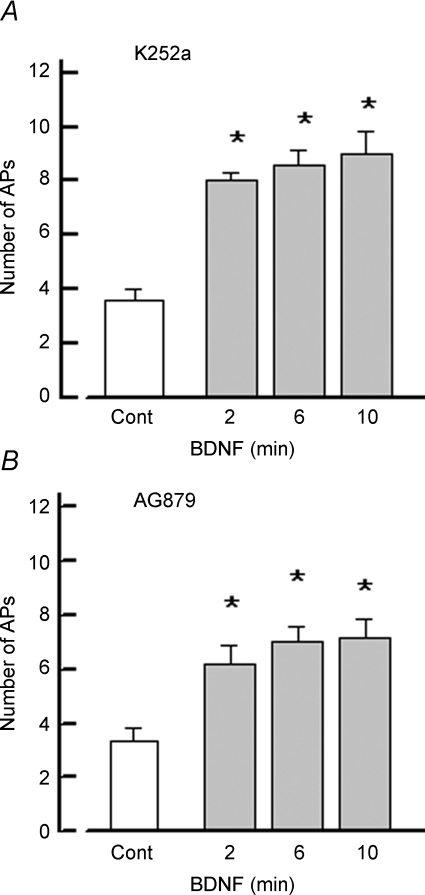
In addition to p75NTR, it is possible that BDNF sensitizes these sensory neurons through activation of the tyrosine kinase receptor, TrkB. To examine this possibility, sensory neurons were pretreated for 20 min with an antagonist of tyrosine kinase receptors, K252a (Tapley et al. 1992; Knüsel & Hefti, 1992; but see Kase et al. 1987). As shown in Fig. 3A, treatment with 400 nm K252a did not suppress the sensitizing effect of BDNF on sensory neurons. In untreated parallel neurons, BDNF produced a significant increase in the number of evoked APs (control 3.6 ± 0.4 versus 10 min BDNF treatment 9.0 ± 0.8 APs, n = 5, RM ANOVA). K252a was dissolved in DMSO and was diluted with Ringer solution just before use. Recordings from other neurons demonstrated that the vehicle alone (0.04% DMSO Ringer solution) did not alter the excitability (control 4.6 ± 0.2 APs versus 4.4 ± 0.04 APs after 20 min exposure, n = 5, data not shown). Similar to the neurons described in Table 1, in this series of K252a experiments, untreated neurons exposed to BDNF exhibited a significant increase in AP firing that was associated with a 30 ± 9% decrease in the value of the rheobase measured after 10 min whereas the firing threshold was unchanged (control −15.4 ± 3.5 mV versus −16.9 ± 3.2 mV, n = 5, P = 0.09 RM ANOVA).
Figure 3. Treatment with antagonists of tyrosine kinase receptors does not block the increase in AP firing produced by BDNF.
A, summary of the results obtained from five sensory neurons that were pretreated with 400 nm K252a before exposure to 50 ng ml−1 BDNF. B, pretreatment with 30 μm AG879 also had no effect on the sensitization produced by 50 ng ml−1 BDNF (n = 6). Asterisks indicate a significant difference from control (P < 0.05, RM ANOVA).
To further examine the idea that the augmentation of excitability of sensory neurons produced by BDNF did not occur through activation of Trk receptors, a different structurally unrelated inhibitor of Trk receptors was used. Another series of experiments examined the effects of AG879, which is a member of the tyrphostin family of Trk inhibitors (Yaish et al. 1988; Lyall et al. 1989; Ohmichi et al. 1993), on the sensitizing capacity of BDNF. Sensory neurons were pretreated with 30 μm AG879 for 30 min and then BDNF was applied focally. As shown in Fig. 3B, treatment with AG879 had no effect on the ability of BDNF to sensitize sensory neurons (control 3.3 ± 0.5 APs versus 10 min BDNF treatment 7.2 ± 0.7 APs, n = 6, RM ANOVA). In the presence of AG879, BDNF, after only a 2 min exposure, produced a significant depolarization of the resting membrane potential and ∼50% decrease in the rheobase without altering the firing threshold (data not shown). These results were similar to those observed with BDNF in the untreated sensory neurons (see Table 1). Thus, BDNF rapidly augments the firing capacity of small diameter sensory neurons through activation of the p75NTR rather than TrkB.
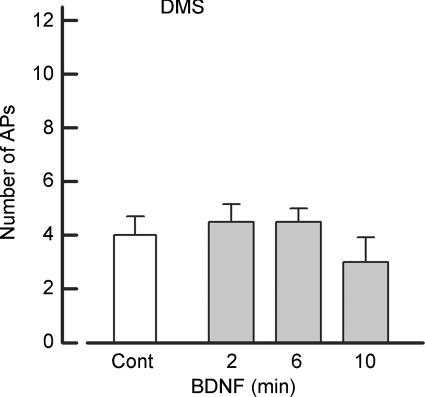
Dimethylsphingosine, an inhibitor of sphingosine kinase, blocks the sensitization produced by BDNF
Our previous studies indicated that NGF increased the excitability of sensory neurons through activation of p75NTR (Zhang & Nicol, 2004) and the NGF-induced increase in excitability was dependent on the conversion of ceramide to sphingosine 1-phosphate (S1P) (Zhang et al. 2006). Our results presented above suggest that BDNF may sensitize sensory neurons though the p75NTR with downstream activation of the S1P signalling pathway. To examine this idea, sensory neurons were pretreated for 20 min with 20 μm N,N-dimethylsphingosine (DMS), a specific competitive inhibitor of sphingosine kinase (Olivera & Spiegel, 1993; Yatomi et al. 1996; Edsall et al. 1998), which is the kinase that converts sphingosine to S1P. As shown in Fig. 4, pretreatment with DMS blocked the ability of BDNF to augment AP firing (control 4.0 ± 0.7 APs versus 10 min BDNF exposure 3.0 ± 0.9, n = 4, P = 0.07 RM ANOVA). In contrast to the Trk inhibitors, DMS blocked the BDNF-induced depolarization of the resting membrane potential (control −67.2 ± 3.1 mV versus 10 min BDNF exposure −68.8 ± 4.4, n = 4, P = 0.50 RM ANOVA) and the decrease in the rheobase (control 898 ± 275 pA versus 10 min BDNF exposure 935 ± 239, n = 4, P = 0.22 RM ANOVA). Taken together, these results indicate that BDNF enhances the excitability of small diameter sensory neurons through the activation of the p75NTR–S1P cascade rather than the TrkB pathway.
Figure 4. Pretreatment with an inhibitor of sphingosine kinase, N,N-dimethylsphingosine, blocks the ability of BDNF to increase AP firing.
Summary of the results obtained for four sensory neurons that were exposed to 50 ng ml−1 BDNF after pretreatment with 20 μm N,N-dimethylsphingosine (DMS).
Neurons sensitized by BDNF express the mRNA for p75NTR but not TrkB
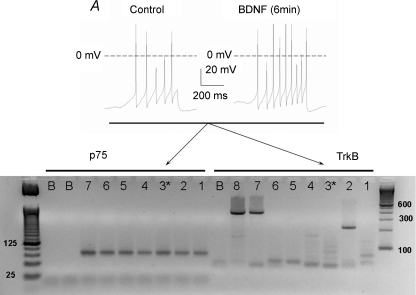
In using pharmacological inhibitors to elucidate signalling cascades, there are always questions regarding the specificity of these agents and the consequent interpretation of their results. To further establish that BDNF augments AP firing through activation of the p75NTR and not TrkB, we used single-cell RT-PCR to determine the expression of p75NTR and TrkB in neurons that were sensitized by BDNF. These results are shown in Fig. 5. The top panel shows that after a 6 min exposure to BDNF, the number of APs evoked from a representative small diameter sensory neuron was increased by ∼twofold. This neuron was then aspirated into another pipette from which single-cell RT-PCR was performed. The bottom panel represents the PCR products obtained from this neuron (cell 3, lanes labelled 3). The gel shows that neuron 3 expressed the mRNA for p75NTR but not TrkB. In addition, the gel demonstrates that neurons 1–6, which were all small diameter (< 25 μm) and sensitized by BDNF (summarized in Fig. 3B), expressed the mRNA for p75NTR but not TrkB. It is well established that TrkB is infrequently expressed in small diameter sensory neurons, but expressed more highly in medium to large diameter sensory neurons (see Discussion). To test this idea, single-cell RT-PCR was performed using a large diameter neuron (> 50 μm). Indeed, neuron 7 (lanes labelled 7) expressed the mRNA for TrkB and also p75NTR. As another positive control, mRNA was isolated from five additional large diameter (> 50 μm) sensory neurons wherein the RT-PCR indicated that these neurons expressed the mRNA for TrkB (lane 8). The sizes of the PCR products for p75NTR (89 bp) and TrkB (392 bp) were as predicted and sequencing revealed the products to match those expected for the targeted sites (data not shown). It is unclear what the product detected in lane 2 for TrkB represents as the size is only about half that of the expected TrkB product. It is possible that this band resulted from a non-specific interaction with the random hexamer primers in the PCR that were used to enhance the sensitivity. It seems unlikely that this band is a specific reaction product since no other bands at this size were detected in any of the TrkB reactions for the other neurons. Also, there is no band at this size detected in the reaction for p75NTR using cDNA obtained from this same neuron. The lanes labelled B (blank) represent reactions carried out in the absence of cDNA template and indicate that there is no amplification of non-specific targets. Therefore, these results indicate that small diameter sensory neurons that were sensitized by BDNF expressed the mRNA for the p75NTR but not TrkB and that TrkB was detected in large diameter sensory neurons.
Figure 5. Sensory neurons that were sensitized by BDNF express the mRNA for p75NTR but not TrkB.
A, a recording obtained from a representative neuron wherein the focal application of 50 ng ml−1 BDNF increased the number of evoked APs. B, an ethidium bromide gel representing the RT-PCR results obtained for six individual capsaicin-sensitive small diameter sensory neurons. The left side of the gel labelled p75 shows the results obtained for the detection of p75NTR in six individual sensory neurons that were sensitized by BDNF (lanes 1–6). Each neuron expressed the mRNA for p75NTR (product size 89 bp). Lane 7 shows that a single large diameter (> 50 μm) sensory neuron expressed the mRNA for p75NTR (no recordings were obtained from this neuron). The base pair ladder is shown on the left. The right side of the gel illustrates the results obtained for the detection of TrkB in those same six sensory neurons that were sensitized by BDNF (lanes 1–6). None of these neurons expressed the mRNA for TrkB (392 bp). Lane 7 shows that the same single large diameter (> 50 μm) sensory neuron that expressed the mRNA for p75NTR also expressed the mRNA for TrkB. Lane 8 shows that the cDNA isolated from five large diameter sensory neurons expressed the mRNA for TrkB at the appropriate size. Lane 3* represents the RT-PCR results obtained for the neuron shown in panel A. Lanes marked B represent RT-PCR results wherein the reaction contained no cDNA (blanks).
If these neurotrophins, NGF and BDNF, are sensitizing sensory neurons through activation of p75NTR, then this suggests that prior sensitization by one neurotrophin should occlude the actions of the other. Indeed, treatment with NGF produced about a twofold increase in the number of APs compared to the control condition (see Fig. 6). BDNF was then added in the presence of NGF. Under these conditions, BDNF did not cause an additional increase in the number of APs. These results suggest that NGF and BDNF activate the same receptor to enhance the excitability of small diameter sensory neurons. These results in combination with those obtained for K252a and AG879 as well as the single-cell RT-PCR demonstrate that activation of p75NTR, but not TrkB, mediates the sensitization produced by BDNF.
Figure 6. BDNF does not further enhance the increase in excitability produced by NGF.
Recordings were obtained from six small-diameter sensory neurons under control conditions wherein the ramp elicited 3.7 ± 0.4 APs. These neurons were then exposed to 100 ng ml−1 NGF and the AP firing was determined at 2, 6 and 10 min. NGF produced about a twofold increase in the number of APs. After the 10 min exposure to NGF, the neurons were then exposed to 50 ng ml−1 BDNF in the presence of NGF. Recordings of APs evoked by the ramp of current were obtained after 2, 6 and 10 min exposure to NGF and BDNF. Asterisks indicate a significant difference from control (P < 0.05, RM ANOVA).
BDNF enhances TTX-R INa
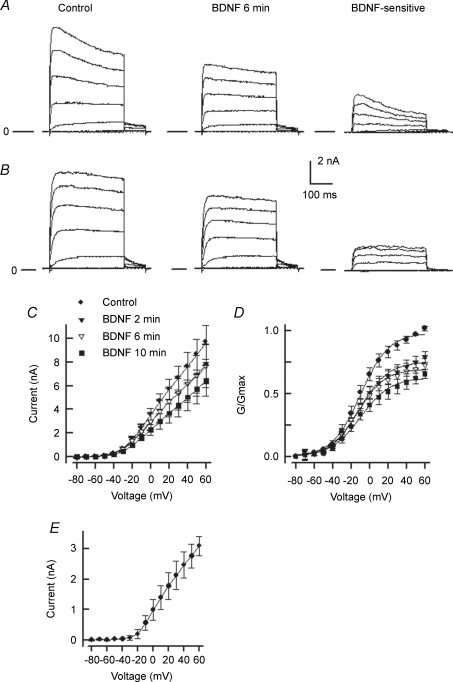
Previous studies have shown that the enhanced excitability of small diameter sensory neurons produced by the pro-inflammatory prostaglandin, PGE2, results, in part, from an augmentation of the amplitude of TTX-R INa (Gold et al. 1996a; England et al. 1996). Also, we have previously shown that NGF increased the amplitude of TTX-R INa in a time-dependent manner (Zhang et al. 2002). Therefore, we examined whether BDNF modulated TTX-R INa and could account for the enhanced AP firing produced by BDNF. As shown in Fig. 7A, exposure to 50 ng ml−1 BDNF enhanced the amplitude of TTX-R INa wherein after 6 min the peak amplitude was increased from a control value of −1.80 nA to −2.35 nA. The results obtained from eight sensory neurons are summarized in Fig. 7B (left panel). BDNF rapidly and significantly increased the peak amplitude of TTX-R INa from the control value of −1.95 ± 0.13 to −2.24 ± 0.11 nA (n = 8, RM ANOVA) after only a 2 min exposure. The average peak currents obtained for the 2 and 6 min exposures to BDNF were not significantly different. Although one neuron was lost after 6 min of recording, the average value for the peak amplitude of TTX-R INa after 6 min (n = 8) was not different from that obtained after 10 min (−2.32 ± 0.15 nA, n = 7, ANOVA, data not shown). The currents obtained after exposure to BDNF were normalized to their respective peak currents obtained under control conditions. As shown in Fig. 7B (right panel) BDNF significantly augmented TTX-R INa by about 1.17-fold for both time points.
Figure 7. BDNF enhances the TTX-R INa in capsaicin-sensitive small diameter sensory neurons.
A, left, a representative recording of TTX-R INa obtained for voltage steps between −60 and +35 mV (in 5 mV increments) under control conditions. Right, after a 6 min exposure to 50 ng ml−1 BDNF the amplitude of TTX-R INa was increased. B, left, summary of the effects of focally applied BDNF (50 ng ml−1) on the current–voltage relation obtained from eight sensory neurons. The average current values after 2 and 6 min exposure to BDNF were significantly different from the control values for the voltages between −35 and +60 mV (RM ANOVA). Right, summary of the I/Imax–voltage relation in which the values of the current after BDNF treatment were normalized to their respective peak amplitude values for the control condition. The values of I/Imax after 2 and 6 min of BDNF were significantly different from their control values for voltages between −35 and +60 mV. C, left, the G/Gmax–voltage relation for the control condition and after 6 min exposure to BNDF. Right, expansion of this relation for voltages between −30 and 0 mV where the asterisks represent a significant difference between the control values and after the 6 min BDNF treatment (RM ANOVA). In addition, the values of G/Gmax were significantly different between the control and after 2 min exposure to BDNF for the voltages between −30 and −15 mV (data not shown).
We noted that over the time of exposure to BDNF there was a small rightward shift in the reversal potential for TTX-R INa. Under control conditions, ENa was measured to be 26.7 ± 1.0 mV (n = 8) and was not significantly different from the calculated ENa of 27.7 mV. However, after the 6 min exposure to BDNF, ENa had shifted to 30.4 ± 1.0 mV and although only ∼3 mV, was significantly different from ENa measured for the control (RM ANOVA). The origins of this are unclear. It is possible that BDNF somehow modifies the screening of the membrane surface charge which then alters the permeability of the channel. Elevations in extracellular Ca2+ or a lowering of intracellular Ca2+ concentrations can produce a small rightward shift in ENa which has been attributed to alterations in screening of surface charge. However, this shift was also accompanied by a rightward shift in the activation curve with a large decrease in the peak INa amplitude (Ohmori & Yoshii, 1977; Kostyuk & Krishtal, 1977). This is contrary to the leftward shift in the current–voltage relation observed after treatment with BDNF. It is also possible that BDNF somehow alters the activity of the Na+/K+-ATPase so that intracellular levels of Na+ are reduced. Calculations using the Nernst equation indicate that a lowering of intracellular Na+ by 1 mm (from 10 to 9 mm) would account for the 3 mV shift in ENa.
The current values were converted to conductance and then normalized to the fitted value of Gmax obtained for each respective neuron; the G/Gmax versus voltage relation is summarized in Fig. 7C (left panel). The G/Gmax versus voltage relation was fitted with the Boltzmann equation wherein the fitting parameters are summarized in Table 2. After a 6 min exposure to BDNF, V0.5 was significantly shifted by about 7 mV to more hyperpolarized voltages whereas the value of k was unchanged. The right panel of Fig. 7C expands the Boltzmann fit of the G/Gmax versus voltage relation and shows that that the values of G/Gmax were significantly larger after BDNF exposure between the voltages of −25 and −10 mV. Therefore, these results show that BDNF rapidly augments TTX-R INa by shifting the voltage dependence for activation to more hyperpolarized voltages and it is over this voltage range where such changes can have an impact on the AP firing.
Table 2.
Boltzmann parameters for TTX-R INa and IK
| V0.5 (mV) | k (mV) | n | |
|---|---|---|---|
| TTX-R INa | |||
| Control | −1.7 ± 1.4 | 11.9 ± 0.6 | 8 |
| BDNF 2 min | −5.2 ± 1.6 | 12.2 ± 0.7 | 8 |
| BDNF 6 min | −8.9 ± 1.5* | 11.3 ± 0.9 | 8 |
| BDNF 10 min | −8.8 ± 3.2 | 11.3 ± 0.8 | 7 |
| IK | |||
| Control | −8.4 ± 4.1 | 15.2 ± 1.1 | 7 |
| BDNF 2 min | −9.0 ± 3.9 | 16.2 ± 1.6 | 7 |
| BDNF 6 min | −10.3 ± 4.9 | 15.5 ± 1.7 | 7 |
| BDNF 10 min | −3.5 ± 6.7 | 18.2 ± 3.0 | 7 |
P < 0.05 RM ANOVA comparing Control, BDNF 2 min, and BDNF 6 min.
BDNF suppresses IK
It is well established that a reduction in potassium currents enhances the excitability of sensory neurons (Weinreich & Wonderlin, 1987; Gold et al. 1996b; Nicol et al. 1997; Cordoba-Rodriguez et al. 1999; Zhang et al. 2002). Therefore, we explored the idea that the sensitizing actions of BDNF observed in the current-clamp experiments may result from the inhibition of an outward potassium current (IK) in capsaicin-sensitive sensory neurons. As illustrated in Fig. 8, externally applied BDNF produced a time-dependent suppression of IK in adult rat sensory neurons (7 of 8 cells). Figure 8A shows a representative recording obtained before (left) and after a 6 min exposure to 50 ng ml−1 BDNF (middle) wherein BDNF reduced the peak IK by ∼28% measured at +60 mV. The IK that was sensitive to BDNF (right panel) was obtained by subtraction of the traces shown in the middle panel from the left panel. The BDNF-sensitive IK exhibited rapid activation with a slow relaxation suggesting that NGF acts on a delayed rectifier-like IK. Figure 8B illustrates the suppression of IK (∼19%) in another representative neuron that exhibited outward currents that had relatively little time-dependant relaxation. Again, the BDNF-sensitive current exhibits the properties of a delayed rectifier type of IK. Figure 8C summarizes the effects of BDNF on the current–voltage relation obtained from seven neurons. Under control conditions, the average peak value of IK was 9.69 ± 1.42 nA as measured at +60 mV whereas after a 10 min exposure to 50 ng ml−1 BDNF the average value was significantly reduced to 6.45 ± 1.31 nA. The extent of inhibition measured after a 20 min exposure (6.28 ± 1.14 nA) was not different from that obtained after 10 min (t test, P > 0.05) suggesting that, for this concentration, maximal inhibition was achieved after 10 min. These neurons then were washed with NMG-Ringer solution for 10 min after the 20 min treatment with BDNF. IK was not significantly altered (4.96 ± 0.51 nA) from the average value at 20 min suggesting that over this time frame, the suppression of IK was not readily reversible. The conductance–voltage relation is summarized in Fig. 8D. Conductance values determined from currents before and after the application of BDNF were fitted by the Boltzmann relation and were normalized to their respective values for the fits of Gmax obtained for the control condition. The conductance–voltage relation demonstrates that treatment with BDNF significantly reduced G/Gmax by approximately 21, 27 and 34% after 2, 6 and 10 min, respectively, without altering either V0.5 or k (see Table 2). The current–voltage relation for the BDNF-sensitive IK obtained from these seven neurons is summarized in Fig. 8E. The BDNF-sensitive IK begins to activate around −20 mV, which places the suppression of this IK at the appropriate voltage range to influence the value of the rheobase and therefore modulate the capacity of these neurons to fire APs.
Figure 8. BDNF suppresses IK in capsaicin-sensitive small diameter sensory neurons.
In panels A and B, the traces on the left illustrate the recordings of IK for voltage steps between −80 and +60 mV (in 20 mV increments) for two neurons exhibiting different recovery kinetics (A: fast; B: slow). The middle traces represent the IK remaining after 6 min exposure to 50 ng ml−1 BDNF. The traces on the right illustrate the IK that was sensitive to BDNF and were obtained by the subtracting the middle traces from the control traces on the left. The lines labelled with zero mark the zero current level for each panel. C, summary of the current–voltage relation showing the time-dependent suppression of IK obtained from seven sensory neurons. The current values after 2 and 6 min exposures to BDNF were significantly different from the control values for the voltages between +10 and +60 mV, for the 10 min exposure current values were different between −10 and +60 mV (RM ANOVA). D, the G/Gmax relation for the seven neurons shown in C. The values of G/Gmax after 2 and 6 min exposures to BDNF were significantly different from the control values for the voltages between 0 and +60 mV; for the 10 min exposure the values of G/Gmax were different between −50 and +60 mV (RM ANOVA). E, the current–voltage relation for the IK that was sensitive to BDNF for these seven sensory neurons.
Discussion
Our results demonstrate that acutely applied BDNF enhances the excitability of small diameter capsaicin-sensitive sensory neurons. Previously, we showed that activation of p75NTR by NGF increased the capacity of sensory neurons to fire APs in response to a ramp of depolarizing current through the intracellular metabolism of ceramide to sphingosine 1-phosphate (Zhang et al. 2002, 2006). If NGF sensitizes sensory neurons through activation of p75NTR, then BDNF should produce similar effects like NGF. Indeed, our current findings support this idea wherein a blocking antibody to p75NTR prevents the sensitization produced by BDNF, the increase in AP firing produced by BDNF is blocked by inhibition of sphingosine kinase and not inhibitors of tyrosine kinase receptors, those neurons sensitized by BDNF expressed the mRNA for only p75NTR but not TrkB, and in the presence of NGF, BDNF did not produce an additional increase in AP firing.
Analogous to NGF, BDNF can be synthesized and released from a variety of immuno-competent cells. Human platelets express BDNF at levels that are 50–100 times that found in extracts of porcine brain (Yamamoto & Gurney, 1990). These platelets release BDNF, which may have an important role in supporting the outgrowth and survival of sensory neurons as well as potentially regulating vascular permeability (Yamamoto & Gurney, 1990). Later studies showed that other types of immuno-competent cells such as T cells (CD4+ and CD8+), B cells, macrophages, monocytes and dendritic cells release BDNF upon activation by such agents as LPS (Batchelor et al. 1999; Braun et al. 1999; Kerschensteiner et al. 1999; Noga et al. 2008). Interestingly, a local wound to the striatum promotes the release of BDNF from microglia (Batchelor et al. 1999). More recently, intrathecal injection of BDNF produced a decrease in the paw withdrawal threshold, indicating that BDNF was involved in the initiation of tactile allodynia (Coull et al. 2005). This response to BDNF was comparable to that caused by the intrathecal injection of microglia that had been previously activated by ATP. In support of the important role of neurotrophin release from immuno-competent cells, Coull et al. (2005) found that when the microglia were pretreated with siRNA targeted to BDNF and then activated by ATP, they failed to sensitize the withdrawal response. These results suggest that local injury releases ATP to the extracellular environment, where it can activate both neurons and microglia (see reviews of ATP and pain, Ding et al. 2000; Hamilton & McMahon, 2000; North, 2004; Inoue, 2006). The microglia then release BDNF which enhances the sensitivity of dorsal horn neurons to peripheral stimulation (Coull et al. 2005). Our results are consistent with this idea wherein BDNF through activation of p75NTR augments the capacity of sensory neurons to fire APs.
Using single-cell RT-PCR, we observed that those small diameter sensory neurons that were sensitized by BDNF expressed the mRNA for p75NTR and not TrkB. Based on previous studies, it is not surprising that we detected p75NTR and failed to detect TrkB in small diameter neurons. The mRNA for p75NTR colocalizes with TrkA mRNA in small diameter sensory neurons of the lumbar DRG (Verge et al. 1992; Wright & Snider, 1995). However, the mRNA for p75NTR was observed in only approximately 8% of those sensory neurons expressing TrkB mRNA (Wright & Snider, 1995). In addition, the expression of TrkB in the lumbar DRG is essentially limited to medium/large-sized sensory neurons with the values ranging from lower levels of 5–12% (Wright & Snider, 1995; Kashiba et al. 1995; Kobayashi et al. 2005) to higher levels of 27–33% (McMahon et al. 1994; Wetmore & Olson, 1995; Karchewski et al. 1999). In addition, our current clamp recording were obtained from only capsaicin-sensitive neurons wherein the coexpression of TrkB and TRPV1 is limited to 0.4% of the lumbar 4/5 DRG (Kobayashi et al. 2005). Based on these observations, it seems unlikely that BDNF was acting through TrkB to augment the excitability of capsaicin-sensitive small diameter sensory neurons.
Previous studies have shown that other neurotrophins can augment the sensitivity of sensory neurons in a manner similar to NGF. The application of BDNF or neurotrophin-5, which is also a ligand at p75NTR and TrkB, lowered the thermal threshold for AP firing in the isolated saphenous nerve–skin preparation (Rueff & Mendell, 1996; Shu et al. 1999). Neurotrophin-4/5 also enhanced the amplitude of the capsaicin-evoked current in sensory neurons isolated from the DRG (Shu & Mendell, 1999). The injection of neurotrophin-4/5 or BDNF, but not neurotrophin-3, into the hind paw of a rat significantly decreased the withdrawal latency to a thermal stimulus (Shu et al. 1999). It is not clear why neurotrophin-3 did not produce thermal hyperalgesia; however, previous studies suggest that neurotrophin-3 may have antinociceptive actions. Treatment with neurotrophin-3 suppressed, rather than enhanced, the release of substance P evoked by electrical stimulation in the isolated spinal cord (Malcangio et al. 1997). Although neurotrophin-3 does not alter neuronal sensitivity under normal conditions, exposure to this neurotrophin reverses the mechanical hyperalgesia produced by injection of complete Freunds adjuvant into the paw (Watanabe et al. 2000) or acid into the muscle (Gandhi et al. 2004) and the thermal hyperalgesia produced by chronic constriction injury of the sciatic nerve (Wilson-Gerwing et al. 2005). Because neurotrophin-3 reversed thermal hyperalgesia, it seems unlikely that these antinociceptive effects are mediated by TrkC pathways (Wilson-Gerwing et al. 2005). These results all suggest that neurotrophin-3 can effectively reverse the heightened sensitivity produced by either inflammation or injury; however, the mechanisms of action remain to be determined.
The work by Mendell's laboratory suggests that BDNF or neurotrophin-5 alters the sensitivity of sensory neurons by modulating the activity of ion channels that regulate AP firing (Rueff & Mendell, 1996; Shu et al. 1999). Our results demonstrating that BDNF increases AP firing through an augmentation of the amplitude of TTX-R INa and suppression of a delayed rectifier-like IK are consistent with this idea. Previous studies have demonstrated that BDNF, through activation of either TrkB or p75NTR, can modulate the activity of ion channels. In PC12 cells, which express p75NTR but not TrkB, exposure to BDNF produced an increase in the peak amplitude of the L-type calcium current (Jia et al. 1999). Application of BDNF to isolated neurons of the CNS evoked an inward current that gave rise to a rapid depolarization (Kafitz et al. 1999). The BDNF-induced inward current was blocked by pretreatment with K-252a but not by TTX, suggesting that BDNF was acting through TrkB. Also, BDNF activates a slow non-selective cationic current thought to be conducted by TRPC3 in which the actions of BDNF were blocked by K-252a (Li et al. 1999). Later studies indicate that BDNF activation of this TRPC3 current plays a critical role in the guidance of nerve growth cones (Li et al. 2005; Amaral & Pozzo-Miller, 2007).
In terms of regulating membrane excitability, BDNF can suppress the current conducted by potassium channels, such as those carried by heteromultimers of the Kir3 family (Rogalski et al. 2000) and Kv1.3 (Tucker & Fadool, 2002). In addition, BDNF increases the excitability of neurons in brain slices isolated from the medial nucleus of the trapezoid body through the suppression of both low- and high-threshold IK (Youssoufian & Walmsley, 2007). In contrast to the BDNF-induced enhancement of TTX-R INa in sensory neurons, BDNF via the activation of Fyn kinase inhibits the peak current conducted by Nav1.2 (Ahn et al. 2007). Since both TrkB and p75NTR were coexpressed with Nav1.2 and Fyn kinase, it is difficult to know which pathway resulted in the activation of Fyn. Taken together, these results demonstrate that BDNF, through the modulation of different ion channels, can increase the excitability in a variety of neurons.
In summary, our results show that BDNF through activation of p75NTR can enhance the excitability of small diameter capsaicin-sensitive sensory neurons through the modulation of both TTX-R INa and a delayed rectifier-like IK. Inhibition of sphingosine kinase prevents the sensitization produced by BDNF suggesting that activation of p75NTR leads to the liberation of S1P, which somehow modulates the excitability of the sensory neuron. Thus, neurotrophins released by activated immune cells could augment the excitability of nociceptive sensory neurons through activation of p75NTR-mediated signalling pathways to produce the heightened sensitivity associated with the inflammatory response.
Acknowledgments
This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR015481-01 from the National Center for Research Resources, NIH. This work was supported by NIH NINDS NS46084.
References
- Ahn M, Beacham D, Westenbroek RE, Scheuer T, Catterall WA. Regulation of Nav1.2 channels by brain-derived neurotrophic factor, TrkB, and associated Fyn kinase. J Neurosci. 2007;27:11533–11542. doi: 10.1523/JNEUROSCI.5005-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral MD, Pozzo-Miller L. TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J Neurosci. 2007;27:5179–5189. doi: 10.1523/JNEUROSCI.5499-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Mannsfeldt A, Neuhaus-Steinmetz U, Fischer A, Schnoy N, Lewin GR, Renz H. Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am J Respir Cell Mol Biol. 1999;21:537–546. doi: 10.1165/ajrcmb.21.4.3670. [DOI] [PubMed] [Google Scholar]
- Chahl LA, Iggo A. The effects of bradykinin and prostaglandin E1 on rat cutaneous afferent nerve activity. Br J Pharmacol. 1977;59:343–347. doi: 10.1111/j.1476-5381.1977.tb07498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba-Rodriguez R, Moore KA, Kao JP, Weinreich D. Calcium regulation of a slow post-spike hyperpolarization in vagal afferent neurons. Proc Natl Acad Sci U S A. 1999;96:7650–7657. doi: 10.1073/pnas.96.14.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Ferriera SH. The pivotal role of tumour necrosis factor α in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Cesare P, Drew L, Nikitaki D, Wood JN. ATP, P2X receptors and pain pathways. J Auton Nerv Syst. 2000;81:289–294. doi: 10.1016/s0165-1838(00)00131-4. [DOI] [PubMed] [Google Scholar]
- Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. 1998;37:12892–12898. doi: 10.1021/bi980744d. [DOI] [PubMed] [Google Scholar]
- Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Natl Acad Sci U S A. 1993;90:10984–10988. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via a cyclic AMP–protein kinase A cascade. J Physiol. 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1b as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- Gandhi R, Ryals JM, Wright DE. Neurotrophin-3 reverses chronic mechanical hyperalgesia induced by intramuscular acid injection. J Neurosci. 2004;24:9405–9413. doi: 10.1523/JNEUROSCI.0899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry JJ, Barker PA, Carter BD. The p75 neurotrophin receptor: multiple interactors and numerous functions. Prog Brain Res. 2004;146:25–39. doi: 10.1016/S0079-6123(03)46002-0. [DOI] [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci U S A. 1996a;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Role of a Ca2+-dependent slow afterhyperpolarization in prostaglandin E2-induced sensitization of cultured rat sensory neurons. Neurosci Lett. 1996b;205:161–164. doi: 10.1016/0304-3940(96)12401-0. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton SG, McMahon SB. ATP as a peripheral mediator of pain. J Auton Nerv Syst. 2000;81:187–194. doi: 10.1016/s0165-1838(00)00137-5. [DOI] [PubMed] [Google Scholar]
- Handwerker HO. Influences of algogenic substances and prostaglandins on the discharges of unmyelinated cutaneous nerve fibres identified as nociceptors. Adv Pain Res Ther. 1976;1:41–45. [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109:210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Jia M, Li M, Liu XW, Jiang H, Nelson PG, Guroff G. Voltage-sensitive calcium currents are acutely increased by nerve growth factor in PC12 cells. J Neurophysiol. 1999;82:2847–2852. doi: 10.1152/jn.1999.82.6.2847. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhang YH, Clark JD, Tempel BL, Nicol GD. Prostaglandin E2 inhibits the potassium current in sensory neurons from hyperalgesic Kv1.1 knockout mice. Neuroscience. 2003;119:65–72. doi: 10.1016/s0306-4522(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Karchewski LA, Kim FA, Johnston J, McKnight RM, Verge VM. Anatomical evidence supporting the potential for modulation by multiple neurotrophins in the majority of adult lumbar sensory neurons. J Comp Neurol. 1999;413:327–341. doi: 10.1002/(sici)1096-9861(19991018)413:2<327::aid-cne11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent kinases. Biochem Biophys Res Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Noguchi K, Ueda Y, Senba E. Coexpression of trk family members and low-affinity neurotrophin receptors in rat dorsal root ganglion neurons. Brain Res Mol Brain Res. 1995;30:158–164. doi: 10.1016/0169-328x(94)00249-e. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knüsel B, Hefti F. K-252 compounds: modulators of neurotrophin signal transduction. J Neurochem. 1992;59:1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with aδ/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Krishtal OA. Effects of calcium and calcium-chelating agents on the inward and outward current in the membrane of mollusc neurones. J Physiol. 1977;270:569–580. doi: 10.1113/jphysiol.1977.sp011969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SL, Kim JK, Kim DS, Cho HJ. Expression of mRNAs encoding full-length and truncated TrkB receptors in rat dorsal root ganglia and spinal cord following peripheral inflammation. Neuroreport. 1999;10:2847–2851. doi: 10.1097/00001756-199909090-00027. [DOI] [PubMed] [Google Scholar]
- Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci. 1993;9:353–359. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci. 1994;6:1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, Yuan XB. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- Li H-S, Xu XZ, Montell C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron. 1999;24:261–273. doi: 10.1016/s0896-6273(00)80838-7. [DOI] [PubMed] [Google Scholar]
- Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci. 1988;8:2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall RM, Zilberstein A, Gazit A, Gilon C, Levitzki A, Schlessinger J. Tyrphostins inhibit epidermal growth factor (EGF)-receptor tyrosine kinase activity in living cells and EGF-stimulated cell proliferation. J Biol Chem. 1989;264:14503–14509. [PubMed] [Google Scholar]
- Malcangio M, Garrett NE, Cruwys S, Tomlinson DR. Nerve growth factor- and neurotrophin-3-induced changes in nociceptive threshold and the release of substance P from the rat isolated spinal cord. J Neurosci. 1997;17:8459–8467. doi: 10.1523/JNEUROSCI.17-21-08459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Meakin SO, Shooter EM. The nerve growth factor family of receptors. Trends Neurosci. 1992;15:323–331. doi: 10.1016/0166-2236(92)90047-c. [DOI] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR, Evans AR. Prostaglandins suppress an outward potassium current in embryonic rat sensory neurons. J Neurophysiol. 1997;77:167–176. doi: 10.1152/jn.1997.77.1.167. [DOI] [PubMed] [Google Scholar]
- Noga O, Peiser M, Altenähr M, Schmeck B, Wanner R, Dinh QT, Hanf G, Suttorp N. Selective induction of nerve growth factor and brain-derived neurotrophic factor by LPS and allergen in dendritic cells. Clin Exp Allergy. 2008;38:473–479. doi: 10.1111/j.1365-2222.2007.02907.x. [DOI] [PubMed] [Google Scholar]
- North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol. 2004;554:301–308. doi: 10.1113/jphysiol.2003.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmichi M, Pang L, Ribon V, Gazit A, Levitzki A, Saltiel AR. The tyrosine kinase inhibitor tyrphostin blocks the cellular actions of nerve growth factor. Biochemistry. 1993;32:4650–4658. doi: 10.1021/bi00068a024. [DOI] [PubMed] [Google Scholar]
- Ohmori H, Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977;267:429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol. 2000;84:2365–2379. doi: 10.1152/jn.2000.84.5.2365. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Tébar A, Dechant G, Barde YA. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990;4:487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Tébar A, Dechant G, Götz R, Barde YA. Binding of neurotrophin-3 to its neuronal receptors and interactions with nerve growth factor and brain-derived neurotrophic factor. EMBO J. 1992;11:917–922. doi: 10.1002/j.1460-2075.1992.tb05130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski SL, Appleyard SM, Pattillo A, Terman GW, Chavkin C. TrkB activation by brain-derived neurotrophic factor inhibits the G protein-gated inward rectifier Kir3 by tyrosine phosphorylation of the channel. J Biol Chem. 2000;275:25082–25088. doi: 10.1074/jbc.M000183200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Rueff A, Mendell LM. Nerve growth factor and NT-5 induce increased thermal sensitivity of cutaneous nociceptors in vitro. J Neurophysiol. 1996;76:3593–3596. doi: 10.1152/jn.1996.76.5.3593. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Feige U, Fontana A, Müller K, Dinarello CA. Interleukin-1 enhances pain reflexes. Mediation through increased prostaglandin E2 levels. Agents Actions. 1988;25:246–251. doi: 10.1007/BF01965025. [DOI] [PubMed] [Google Scholar]
- Shu XQ, Llinas A, Mendell LM. Effects of trkB and trkC neurotrophin receptor agonists on thermal nociception: a behavioral and electrophysiological study. Pain. 1999;80:463–470. doi: 10.1016/S0304-3959(99)00042-1. [DOI] [PubMed] [Google Scholar]
- Shu X-Q, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- Shu X-Q, Mendell LM. Acute sensitization by NGF of the response of small-diameter sensory neurons to capsaicin. J Neurophysiol. 2001;86:2931–2938. doi: 10.1152/jn.2001.86.6.2931. [DOI] [PubMed] [Google Scholar]
- Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. J Neurosci. 1998;18:3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squinto SP, Stitt TN, Aldrich TH, Davis S, Bianco SM, Radziejewski C, Glass DJ, Masiakowski P, Furth ME, Valenzuela DM, Distefano PS, Yancopoulos GD. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991;65:885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105:838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- Tucker K, Fadool DA. Neurotrophin modulation of voltage-gated potassium channels in rat through TrkB receptors is time and sensory experience dependent. J Physiol. 2002;542:413–429. doi: 10.1113/jphysiol.2002.017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge VM, Merlio JP, Grondin J, Ernfors P, Persson H, Riopelle RJ, Hökfelt T, Richardson PM. Colocalization of NGF binding sites, trk mRNA, and low-affinity NGF receptor mRNA in primary sensory neurons: responses to injury and infusion of NGF. J Neurosci. 1992;12:4011–4022. doi: 10.1523/JNEUROSCI.12-10-04011.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Endo Y, Kimoto K, Katoh-Semba R, Arakawa Y. Inhibition of adjuvant-induced inflammatory hyperalgesia in rats by local injection of neurotrophin-3. Neurosci Lett. 2000;282:61–64. doi: 10.1016/s0304-3940(00)00842-9. [DOI] [PubMed] [Google Scholar]
- Weinreich D, Wonderlin WF. Inhibition of calcium-dependent spike after-hyperpolarization increases excitability of rabbit visceral sensory neurones. J Physiol. 1987;394:415–427. doi: 10.1113/jphysiol.1987.sp016878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp G, Otten U. An enzyme-linked immunoassay for nerve growth factor (NGF): a tool for studying regulatory mechanisms involved in NGF production in brain and in peripheral tissues. J Neurochem. 1987;48:1779–1786. doi: 10.1111/j.1471-4159.1987.tb05736.x. [DOI] [PubMed] [Google Scholar]
- Weskamp G, Reichardt LF. Evidence that biological activity of NGF is mediated through a novel class of high affinity receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Wetmore C, Olson L. Neuronal and nonneuronal expression of neurotrophins and their receptors in sensory and sympathetic ganglia suggest new intercellular trophic interactions. J Comp Neurol. 1995;353:143–159. doi: 10.1002/cne.903530113. [DOI] [PubMed] [Google Scholar]
- Wilson-Gerwing TD, Dmyterko MV, Zochodne DW, Johnston JM, Verge VM. Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons. J Neurosci. 2005;25:758–767. doi: 10.1523/JNEUROSCI.3909-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Safieh-Garabedian B, Ma Q-P, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Wright DE, Snider WD. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol. 1995;351:329–338. doi: 10.1002/cne.903510302. [DOI] [PubMed] [Google Scholar]
- Yaish P, Gazit A, Gilon C, Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988;242:933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Gurney ME. Human platelets contain brain-derived neurotrophic factor. J Neurosci. 1990;10:3469–3478. doi: 10.1523/JNEUROSCI.10-11-03469.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Megidish T, Toyokuni T, Hakomori S, Igarashi Y. N,N-Dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry. 1996;35:626–633. doi: 10.1021/bi9515533. [DOI] [PubMed] [Google Scholar]
- Youssoufian M, Walmsley B. Brain-derived neurotrophic factor modulates cell excitability in the mouse medial nucleus of the trapezoid body. Eur J Neurosci. 2007;25:1647–1652. doi: 10.1111/j.1460-9568.2007.05428.x. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Nicol GD. NGF-mediated sensitization of the excitability of rat sensory neurons is prevented by a blocking antibody to the p75 neurotrophin receptor. Neurosci Lett. 2004;366:187–192. doi: 10.1016/j.neulet.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na+ current and delayed rectifier K+ current in rat sensory neurons. J Physiol. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD. Intracellular sphingosine 1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J Physiol. 2006;575:101–113. doi: 10.1113/jphysiol.2006.111575. [DOI] [PMC free article] [PubMed] [Google Scholar]