Abstract
TREK channels belong to the superfamily of two-pore-domain K+ channels and are activated by membrane stretch, arachidonic acid, volatile anaesthetics and heat. TREK-1 is highly expressed in the atrium of the adult heart. In this study, we investigated the role of TREK-1 and TREK-2 channels in regulating the resting membrane potential (RMP) of isolated chicken embryonic cardiac myocytes. At room temperature, the average RMP of embryonic day (ED) 11 atrial myocytes was −22 ± 2 mV. Raising the temperature to 35°C hyperpolarized the membrane to −69 ± 2 mV and activated a large outwardly rectifying K+ current that was relatively insensitive to conventional K+ channel inhibitors (TEA, 4-AP and Ba2+) but completely inhibited by tetracaine (200 μm), an inhibitor of TREK channels. The heat-induced hyperpolarization was mimicked by 10 μm arachidonic acid, an agonist of TREK channels. There was little or no inwardly rectifying K+ current (IK1) in the ED11 atrial cells. In marked contrast, ED11 ventricular myocytes exhibited a normal RMP (−86.1 ± 3.4 mV) and substantial IK1, but no temperature- or tetracaine-sensitive K+ currents. Both RT-PCR and real-time PCR further demonstrated that TREK-1 and TREK-2 are highly and almost equally expressed in ED11 atrium but much less expressed in ED11 ventricle. In addition, immunofluorescence demonstrated TREK-1 protein in the membrane of atrial myocytes. These data indicate the presence and function of TREK-1 and TREK-2 in the embryonic atrium. Moreover, we demonstrate that TREK-like currents have an essential role in determining membrane potential in embryonic atrial myocytes, where IK1 is absent.
Background K+ channels play an essential role in setting the resting membrane potential (RMP) and modulating action potentials (AP) in cardiac myocytes. An increase in the RMP towards the potassium equilibrium potential (EK) during development, due to a gradual increase in inwardly rectifying K+ current (IK1), is one of the most prominent changes in the membrane properties of ventricular myocytes (Sperelakis & Shigenobu, 1972; Sperelakis & Pappano, 1983). In contrast, atrial cells (ED 6–18) had a stable RMP of about −65 mV when measured with microelectrodes at 30°C (Pappano, 1972, 1976). In Pappano's studies, the RMP was not significantly affected by conventional K+ channel inhibitors, 20 mm TEA and low concentrations of Ba2+ up to 1 mm, consistent with an absence of IK1. Furthermore, patch clamp analysis indicated the absence of background currents at potential between −50 and −30 mV in single chick embryonic atrial myocytes at room temperature (Clay et al. 1988). These reports led us to question the mechanism underlying the RMP of embryonic atrial myocytes in the absence of inwardly rectifying K+ currents. A potential candidate to set the RMP is TREK-1, a member of the two-pore-domain K+ channel superfamily (K2P) and K2P channels are thought to be involved in setting the RMP in mammalian excitable cells (Lesage & Lazdunski, 2000).
TREK-1 is one of only a few identified heat-sensitive channels (Benham et al. 2003). TREK-1 is in the closed state at room temperature and is open at body temperature (Maingret et al. 2000; Kang et al. 2005) and therefore, could contribute significantly to the background K+ conductance in cells where they are expressed. The activation of TREK-1 produces a large background K+ conductance with a single-channel conductance of 48 pS at 5.4 mm extracellular K+ and 101 pS in 145 mm symmetrical intracellular and extracellular K+ (Fink et al. 1996; Patel et al. 1998). Besides heat, TREK-1 is also activated by membrane stretch, arachidonic acid or other polyunsaturated fatty acids, volatile anaesthetics, intracellular acid pH and ATP (Patel et al. 1998, 1999; Tan et al. 2002). The TREK-1 channel is regulated by protein kinase C (PKC)- and protein kinase A (PKA)-dependent phosphorylation (Patel et al. 1998; Patel & Honore, 2001; Murbartian et al. 2005). PKC phosphorylation is excitatory while PKA-mediated phosphorylation of Ser-333 at the C-terminal inhibits TREK-1 channel activity (Murbartian et al. 2005). More interestingly, upon PKA phosphorylation of Ser-333, TREK-1 channels switch to a voltage-gated channel although only at positive potentials and with a lower open channel probability (Bockenhauer et al. 2001). These unique properties of TREK-1 suggest the importance in stabilizing the membrane potential, modulating the action potential duration and cardiac excitability in response to a complex milieu of regulatory stimuli.
Members of the TREK family have similar biophysical and pharmacological properties. TRAAK and TREK-2 are mainly expressed in mammalian brain (Fink et al. 1998; Goldstein et al. 2005) but in some cases has been found at a much lower level in mammalian heart (Gu et al. 2002; Ozaita & Vega-Saenz de Miera, 2002). On the other hand, functional TREK-1 channels are expressed in the rat heart (Aimond et al. 2000; Terrenoire et al. 2001; Liu & Saint, 2004; Kelly et al. 2006; Li et al. 2006), having a role in the repolarization of cardiac action potentials and could contribute to clinically important arrhythmias (Kelly et al. 2006). However, the existence and functional role of TREK channels in embryonic heart has not previously been demonstrated. The present report provides direct evidence that heat-activated TREK-like K+ channels significantly contribute to setting the RMP of chick embryonic atrial myocytes at ED11 (mid-gestation in chick) while IK1 plays the predominant role in ventricular myocytes at this stage of development.
Methods
All of the experiments were carried out in accordance with the guidelines laid down by the Institutional Animal Care and Use Committee of Duke University Medical Center.
Myocyte isolation
Fertilized Ross Hubert chicken eggs (Goldkist Hatchery, Siler City, NC, USA) were incubated at 37°C and 70% relative humidity until they reached embryonic day 11. ED11 embryos were decapitated, and hearts were quickly excised and transferred to sterile PBS solution. The ventricle and atria were separated before the enzymatic digestion. For the preparation of atrial myocytes, only auricles were used to prevent contamination from other heart tissues. The atria and ventricles were then incubated for 10 min in Ca2+-free Ringer solution containing 0.1 mg ml−1 Trypsin, 1 mg ml−1 collagenase and 0.2 mg ml−1 bovine serum albumin at room temperature. Three digestion steps (8 min each) were subsequently carried out at 37°C with gentle stirring. The dissociated myocytes were collected, centrifuged and resuspended in Modified M-199 medium (Gibco Corp.). The isolated myocytes were then incubated overnight in a plastic Petri dish to which the myocytes did not attach. The myocytes were used within 24 h of dissociation.
Electrophysiological recordings
Small aliquots of myocytes were aspirated from the Petri dish and transferred to a recording/perfusion chamber (250 μl maximum volume; Warner Instruments). The myocytes were perfused in normal Tyrode solution containing (in mm) NaCl 135, KCl 5.4, MgCl2 2, CaCl2 1.8, Dextrose 5.5, Hepes 10 and pH 7.4. The temperature of the perfusing solution was controlled by means of a Fisher thermal cycler (Fisher Scientific Corp.). Patch pipettes were pulled from 1.5 mm diameter borosilicate glass (Sutter Instrument Corp.) and had a resistance of 2–4 MΩ when filled with solution containing (in mm) 145 KCl, 5 MgATP, 0.5 EGTA, 2 MgCl2, 10 Hepes and pH 7.2. The liquid junction potential was nulled before the pipette contacted the myocyte. Whole-cell currents were filtered at 2 kHz and sampled at 5 kHz with an AxoPatch 200B voltage-clamp amplifier and a Digidata 1322A converter (Molecular Devices). Membrane potentials were recorded in current-clamp mode after establishing the whole-cell configuration.
The inwardly rectifying K+ and voltage-gated K+ currents were blocked by a cocktail of ‘conventional’ K+ channel inhibitors containing 10 mm tetraethyl-ammonium-Cl (TEA), 1 mm 4-aminopyridine (4-AP) and 200 μm BaCl2. Osmolarity was maintained by replacing NaCl in the extracellular Tyrode solution. ZD-7288 (5 μm) was also included with the conventional inhibitors to block hyperpolarization-activated pacemaker current If. All chemicals were purchased from Sigma except for ZD-7288 which was purchased from Tocris Cookson Inc., MO, USA.
Total RNA isolation and RT-PCR
The ventricles and atria of ED11 embryos were dissected and immediately homogenized in the presence of 1%β-mercaptoethanol (β-ME, Sigma). Total RNA was prepared by using the Qiagen RNeasy kit (Qiagen). Possible genomic DNA contamination was eliminated by on-column DNase I treatment (supplied with kit by Qiagen). Two-step RT-PCR and real-time PCR were used to study the expression of TREK-1 and TREK-2 in chicken ED11 embryonic heart. Generally, 1 μg DNase I-treated total RNA was used to synthesize the first strand cDNA by Bio-Rad iQ reverse transcriptase (Bio-Rad Corp.). The reverse transcription (RT) reaction was terminated by heating to 85°C for 5 min. Prior to PCR and real-time PCR experiments, the synthesized cDNA was diluted 10 times to minimize inhibitory effects. A 2.5 μl volume of the diluted cDNA was mixed with primers (0.25 μmoles l−1), PCR supermix and water to a final volume of 20 μl. Specific primers for TREK-1 and TREK-2 were designed from the cDNA sequences predicted from the chicken genome (accession number XM_001234629 and XM_428902) and spanned an intron: sense 5′-TTGCTCTTCCTGCGGTTATATT-3′ and antisense 5′-TAACCAGTCTCCAATCATGCTG-3′ for cTREK-1 and sense 5′-TTAAGGGTCCTGTCCAAGAAGA-3′ and antisense 5′-CTGTTGTTGATGCTTTCCTGAG-3′ for TREK-2. Glyceraldehyde-3-phosphate dehydrogenase (cGAPDH) was used as an internal control (sense 5′-TGGGAAGCTTACTGGAATGG-3′ and antisense 5′-ACCAGGAAACAAGCTTGACG-3′, accession number: NM_204305). RNA without RT was used as a PCR control (NRTC) to monitor the genomic DNA contamination and PCR without cDNA template was used as a negative control (NTC). PCR cycling conditions were as follows: a 2 min inactivation step at 94°C followed by a total of 40–45 cycles for melting, annealing and extension steps. SYBR Green-based real-time PCR was used to quantify the expression of TREK-1 and TREK-2 in chicken ED11 embryonic heart. A 10 μl volume of SYBR Green supermix (Bio-Rad Corp.) was mixed with primers, target template and water to a final volume of 20 μl. PCR amplification was detected at a 10 s additional step at 80°C just after the extension step to minimize possible primer dimerization and any non-specific amplification. Real-time experiments were performed on a Bio-Rad iQ5 96-well PCR thermal cycler with an optical module (Bio-Rad Corp.). Primer specificity was checked by melting curves after PCR reactions. The expression of TREK channels was normalized to the reference gene, GAPDH, by using standard curve methods (Muller et al. 2002).
Immunofluorescence
Isolated myocytes were plated on laminin-coated coverslips and were cultured overnight. The cells were rinsed with PBS and fixed in 4% paraformaldehyde for 20 min, permeabilized with 0.5% Triton X-100 in PBS for 10 min and then blocked with 10% goat serum for 1 h. The primary TREK-1 antibody was then incubated with fixed cells at 4°C overnight. TREK-1 polyclonal antibody prepared against a highly conserved amino acid sequence was purchased from Sigma and diluted 100 times before use.
Data analysis
All data are presented as mean ±s.e.m. For statistic analysis, Student's t test or one-way ANOVA was used to determine statistic significance. A P value < 0.05 was considered to be significant, and is indicated with an asterisk.
Results
Heat-induced stabilization of membrane potentials in ED11 atrial myocytes
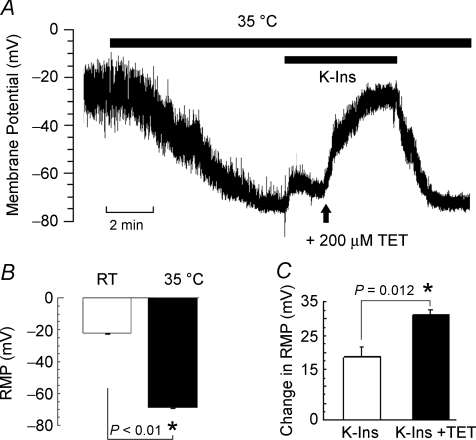
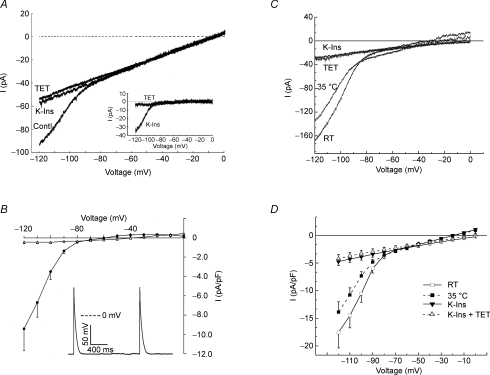
The temperature sensitivity of the RMP was determined by means of current clamp in single ED11 atrial cells (Fig. 1). Patch-clamp seals were obtained at room temperature (20 ± 2°C). At room temperature, most of these myocytes did not exhibit spontaneous activity and showed a low RMP with an average of −22.0 ± 1.9 mV (n = 9; Fig. 1B). Membrane potentials fluctuated between −49 and −20 mV (Fig. 1A). Raising the temperature to 35°C gradually hyperpolarized the membrane potential to −69.0 ± 1.7 mV (n = 9; Fig. 1B) and stabilized the membrane potential as shown in Fig. 1A. These results were consistent with earlier studies carried out at 30°C or room temperature (Pappano, 1972, 1976; Clay et al. 1988).
Figure 1. Effect of temperature, conventional K+ channel inhibitors (K-Ins) and tetracaine (TET) on resting membrane potential (RMP) in ED11 atrial myocytes.
Chicken ED11 atrial myocytes were perfused in normal Tyrode solution at room temperature (RT; 20 ± 2°C). Membrane potentials were recorded in current-clamp mode after formation of the whole-cell patch-clamp configuration. A, an example record of membrane potentials illustrating increased polarization when raising the bath temperature to 35°C and the effects of inhibitors. B, bar graph illustrating the mean increase in RMP when going from RT to 35°C (n = 9). Note that RMP at RT is an average of oscillating potentials. C, summarized change in RMP after addition of K-Ins and K-Ins plus TET (n = 5). Data were presented as mean ±s.e.m.
The current due to TREK channels is relatively insensitive to Ba2+, 4-AP and TEA at the concentrations that are usually employed to block K+ currents while 200 μm tetracaine inhibits TREK-1 (Patel et al. 1998). Therefore, to determine the currents underlying the heat-induced stabilization of membrane potentials, we employed a cocktail of conventional K+ channel inhibitors (K-Ins; in mm: TEA 10, 4-AP 1, Ba2+ 0.2) to block inwardly rectifying and voltage-gated K+ currents, and ZD-7288 (5 μm) to inhibit the hyperpolarizing activated current (If). The application of conventional inhibitors (K-Ins) depolarized the membrane by 18.5 ± 3.0 mV (n = 5) at 35°C (Fig. 1B). Subsequent addition of 200 μm tetracaine, a TREK-1 channel inhibitor, depolarized the membrane by an additional 30.9 ± 1.7 mV (n = 5; Fig. 1B). Since IK is activated at more positive membrane potentials, the second depolarization is more likely to be due to block of TREK-1. Washout of the conventional inhibitors and tetracaine restored the RMP to the level observed when the temperature was initially raised to 35°C (Fig. 1A). These data indicate that a stable, hyperpolarized RMP in these myocytes requires a temperature in the normal physiological range and the activation of a tetracaine-sensitive background current. These results are consistent with the presence of a current due to TREK channels, i.e. a current that is heat-activated, resistant to conventional K+ channel inhibitors and blocked by tetracaine.
Temperature-sensitive background K+ current
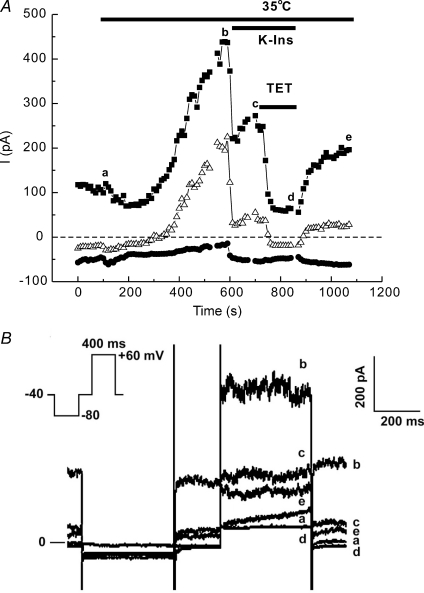
To demonstrate the existence of a temperature-sensitive background K+ current, atrial myocytes were warmed after being voltage-clamped to a holding potential of −40 mV and inhibitors were applied using the same protocol indicated for the data in Fig. 1 (Fig. 2). After establishing the whole-cell condition with the cell at room temperature, a voltage-step protocol was applied every 10 s to continuously monitor the background current at −80, −40 and +60 mV as the cell warmed (Fig. 2B inset). Raising the temperature to 35°C produced a gradual increase in the outward currents at all potentials positive to −80 mV, and only a small change in the background current near EK at −80 mV, as expected of a purely K+ background current. Application of conventional K+ channel inhibitors and ZD-7288 decreased the outward current at +60 mV by 53.4% and at −40 mV by about 80.6% (Fig. 2A, point b). Subsequent addition of tetracaine inhibited all of the remaining outward current at both potentials (Fig. 2A, point c). The current partially recovered during a brief washout period. These results demonstrate the presence of a temperature-sensitive K+ current that is activated at potentials too negative to activate IK and is noisy and time independent. A substantial portion of the measured current was not blocked by the cocktail, but was blocked by tetracaine, a drug known to block TREK channels. The cocktail itself probably blocked 25% or more of any TREK-like current present (Meadows et al. 2000; see Discussion), so it appears that the measured current may be mostly TREK currents. In fact, at −40 mV, where IK is not activated and there is little IK1, the cocktail blocked 80% of the current, suggesting that most of the heat-activated current at −40 mV was due to TREK channels.
Figure 2. Effect of temperature, conventional K+ channel inhibitors (K-Ins) and tetracaine (TET) on membrane current.
Heat slowly activates time-independent currents at positive potentials in ED11 atrial myocytes. A, the myocyte was held at −40 mV, hyperpolarized to −80 mV for 400 ms and subsequently depolarized to −40 mV for 200 ms and +60 mV for 400 ms. The protocol was repeated every 10 s. The magnitude of the current at the end of the 400 ms steps to −80 (•), −40 (▵) and +60 mV (▪) are plotted over time. Illustrated are the effects of temperature, K-Ins, K-Ins plus TET, and partial washout of inhibitors. B, representative recordings of currents during a single experiment using the voltage-step protocol shown in the inset. The data were taken at the time points indicated in A.
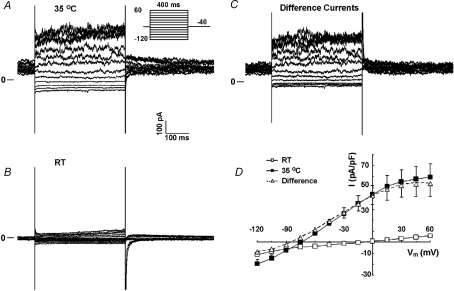
In other experiments, a complete current–voltage relationship (I–V) of ED11 atrial myocytes was determined by averaging the current over 50 ms near the end of 400 ms pulses between −120 mV and +60 mV from a holding potential of −40 mV, first at room temperature, then at 35°C (Fig. 3). The I–V at room temperature showed relatively little inward or outward current (Fig. 3B and D). Raising the temperature to 35°C increased time-independent background current substantially and particularly in the outward direction (Fig. 3A and D). Upon warming, the currents at +60 mV increased from 5.8 ± 0.6 to 53.8 ± 11.8 pA pF−1 (n = 13, P < 0.005), a ninefold increase. At positive potentials, the current also showed some time dependence along with deactivating tail currents, indicating the presence of delayed rectifier current (IK). However, the major component of the temperature-sensitive current was not time dependent (Fig. 3C). The current became quite noisy at positive potentials, consistent with the presence of large-conductance background channels. The I–V relationship of the temperature-sensitive current has a reversal potential near −80 mV, but also reaches a maximum at positive potentials (Fig. 3D, open triangles), indicating the existence of a large-conductance background K+ channel in the atrial myocytes.
Figure 3. Effect of temperature on K+ currents in ED11 atrial cells.
A, the current recorded at 35°C at various potentials. Inset indicates the voltage-step protocol. B, current at room temperature (RT). C, subtracted difference currents. Note the increase in instantaneous and time-dependent currents with increased temperature (discussed in text). D, mean current–voltage relationships showing outward rectification and a much larger current at 35°C with reversal near EK (n = 13).
The temperature-sensitive background K+ current is blocked by tetracaine
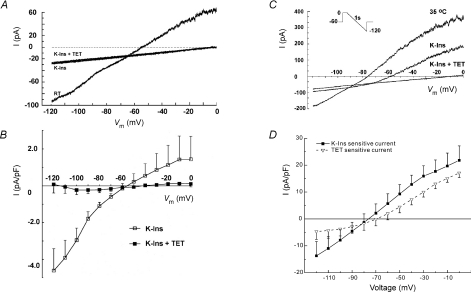
To characterize the temperature-sensitive background K+ currents, we employed a voltage ramp protocol shown in the inset of Fig. 4A in ED11 atrial myocytes. Myocytes were held at −60 mV and depolarized to 0 mV for 100 ms before initiating a 1 s hyperpolarizing ramp from 0 to −120 mV. Voltage-gated Na+ and Ca2+ channels were inactivated by this protocol. In the background range of potentials we could detect no tetracaine-sensitive currents in ED11 atrial myocytes at room temperature, though there was a small current that was completely blocked by the cocktail (Fig. 4A). The reversal potential of the blocked current was between −50 and −60 mV and that could reflect the presence of If, activated during the repolarizing ramp. The addition of tetracaine produced no further effect and indicated the absence of TREK-1 at room temperature. Results from six cells are summarized in Fig. 4B, showing that all of the background current at room temperature is blocked by the inhibitor cocktail and none is blocked by tetracaine applied after previous blockade by the cocktail.
Figure 4. Tetracaine-sensitive current is not detectable at room temperature (RT) but is present at body temperature in atrial myocytes.
A, representative recordings in an atrial myocyte of the current at RT, in the presence of conventional K+ channel inhibitors (K-Ins), and K-Ins plus 200 μm tetracaine (TET) using the voltage-ramp protocol shown in the inset. TET had no further effect on the whole-cell currents in the presence of K-Ins. B, mean I–V relationships of difference currents comparing conventional K+ inhibitor (K-Ins)-sensitive current and TET-sensitive current (n = 6). Note that TET-sensitive current is essentially absent. I–V curves are the average data plotted every 10 mV. C, representative current records from an atrial myocyte at body temperature. Illustrated are the currents at 35°C in the absence of inhibitors, in the presence of K-Ins, and K-Ins plus TET. D, mean difference currents illustrating the K-Ins- and TET-sensitive currents (n = 5).
In contrast, a large outwardly rectifying current was activated at 35°C, with a reversal potential near EK (Fig. 4C). Addition of conventional K+ channel inhibitor cocktail inhibited about 60% of the current (Fig. 4C and D, filled squares). Subsequent addition of tetracaine reduced the membrane current to a simple leak current (Fig. 4C). The tetracaine-sensitive current and cocktail-blocked current have a very similar I–V, particularly at potentials positive to the K+ equilibrium potential (Fig. 4D). This result is consistent with the idea that most or all of the heat-sensitive current is due to TREK channels.
Expression of TREK-1 in chick ED11 atrial myocytes
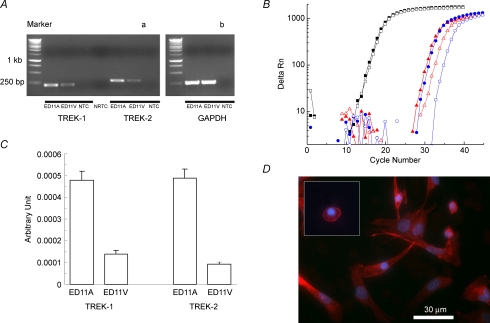
The TREK family is composed of TREK-1, TREK-2 and TRAAK. We next examined gene and protein expression of TREK-1 and TREK-2 during chicken heart development. As the first approach, the rat TREK family member (KCNK2, KCNK4 and KCNK10) coding sequences were used to blast the chicken genome. The results yielded the complete, predicted cDNA sequences for cTREK-1 and cTREK-2 (genebank nos, XM_001234629 and XM_426457). No TRAAK sequence was found in the chicken genome even with a reduced complexity search or more conserved region in TRAAK. Although we could not rule out the possibility of TRAAK expression in chicken embryonic heart, it is unlikely to be dominant. Using two-step RT-PCR, we found that both TREK-1 and TREK-2 were expressed in ED11 atria, but very little in ED11 ventricles (Fig. 5A). Relative quantification of TREK-1 and TREK-2 expression was done with SYBR Green-based real-time PCR. The real-time PCR data are summarized in Table 1 and Fig. 5C. The results show that TREK-1 and TREK-2 are almost equally expressed in chicken ED11 embryonic atria and suggest that both could contribute to TREK-like currents.
Figure 5. Gene and protein expression of TREK-1 in chicken ED11 embryonic heart.
A, RT-PCR showing TREK-1 and TREK-2 expression in ED11 embryonic heart. GAPDH was used as internal control (b) and PCR without cDNA template was used as a negative control for each set of primer pairs (NTC). Also, PCR with RNA having no reverse transcription was used to monitor the genomic DNA contamination (NRTC, a). The sizes of PCR products for GAPDH, TREK-1 and TREK-2 are 298, 221 and 293 bp, respectively. Forty cycles of PCR amplification were used in this experiment to ensure that there is no genomic DNA contamination and PCR amplification is optimal for further real-time PCR studies. B, the representative real-time PCR amplification of reference and target genes, GAPDH (squares), TREK-1 (triangles) and TREK-2 (circles). Threshold cycles were automatically calculated using Bio-Rad iQ5 software. The red filled symbols are for ED11 atria and open symbols for ED11 ventricles. C, the normalized expression of TREK-1 and TREK-2 compared to a reference gene GAPDH. D, antibody staining of TREK-1 in cultured ED11 atrial cells. Red indicates TREK-1 and blue is DAPI nuclear staining. The inset indicates staining of a round atrial myocyte similar to that used for patch-clamp studies (see Methods). TREK-1 is clearly expressed in the surface membrane of ED11 atrial cells.
Table 1.
Summary of real-time PCR in chicken ED11 embryonic heart
| Gene | Tissue | PCR efficiency | Threshold cycle | n |
|---|---|---|---|---|
| GAPDH | ED11A | 2.0606 | 16.36 ± 0.06 | 9 |
| ED11V | 16.72 ± 0.07 | 9 | ||
| TREK-1 | ED11A | 1.928 | 29.66 ± 0.12 | 9 |
| ED11A | 31.94 ± 0.18 | 8 | ||
| TREK-2 | ED11A | 1.8516 | 31.57 ± 0.12 | 14 |
| ED11V | 34.72 ± 0.18 | 12 |
However, mRNA expression levels do not necessarily indicate functional channel expression. Therefore, we used immunofluorescence to demonstrate the presence of TREK-1 channels in the cultured ED11 atrial cells (Fig. 5D). Importantly, surface membrane expression of TREK-1 channels was apparent both in the rounded myocytes used for patch clamp, and in well-attached myocytes that were spreading.
Lack of tetracaine-sensitive currents in ED11 ventricular myocytes
In contrast to the PCR results for atria, only faint, barely detectable bands were observed in ED11 ventricles. This allowed us to compare the electrophysiological and pharmacological properties of cells from embryos of the same age with and without the presence of TREK-1 channels. Ventricular myocytes from chicken embryos have been shown to have a stable RMP similar to adult heart even when measured at room temperature (Sperelakis & Shigenobu, 1972). There is no temperature-dependent difference in the RMP in ventricular cells (−90.8 ± 3.2 mV at room temperature, n = 2, and −83.0 ± 4.8 mV at 35°C, n = 3). We therefore repeated with ED11 ventricular cells some of the experiments we had previously carried out in atrial cells (Fig. 6).
Figure 6. ED11 ventricular myocytes have a large IK1 but no tetracaine-sensitive background K+ current both at room temperature and at 35°C.
A, representative recordings of currents at RT, in the presence of conventional K+ channel inhibitors (K-Ins) and K-Ins plus TET. The inset shows the K-Ins- and TET-sensitive currents (difference currents in the presence of K-Ins and K-Ins plus TET). The K-Ins-sensitive current showed strong inward rectification and had a reversal potential near EK indicating that this current was mostly IK1. B, mean I–V relationships for K-Ins (n = 5) and TET (n = 5)-sensitive currents. Action potentials recorded from single chick ED11 ventricular myocytes in current-clamp mode at room temperature are shown in the inset. C, representative current recordings at room temperature (RT), 35°C and in the presence of a cocktail of conventional K+ inhibitors (K-Ins) and a cocktail plus 200 μm tetracaine (TET). ED11 ventricular myocytes were held at −60 mV and depolarized to 0 mV for 100 ms, then a 1 s repolarizing ramp protocol was used to activate background currents. D, averaged I–V relationships (n = 9). Raising the temperature to 35°C reduced inwardly rectifying K+ currents, instead of activating a background K+ current.
Figure 6A shows representative recordings of currents during a 1 s voltage ramp from 0 to −120 mV at room temperature. There was a strong inwardly rectifying current, probably IK1, that was completely inhibited by conventional K+ channel inhibitors (Fig. 6A, blocked current shown in inset). As in the atrial cells, there was essentially no tetracaine-sensitive background K+ current in the presence of conventional K+ inhibitors in the ED11 ventricular cells when measured at room temperature (Fig. 6A and B).
However, at 35°C, the ventricular cells were not at all like atrial cells with respect to tetracaine-blockable currents. In atrial cells, raising the temperature activated about 40 pA pF−1 of current at 0 mV and 20 pA pF−1 at −40 mV (Fig. 4), about 60% of which was blockable by conventional inhibitors and the remainder by tetracaine (Fig. 4). In contrast, raising the temperature to 35°C in ventricular cells reduced inward currents (n = 9). There was a small current activated in ventricular cells at positive potentials that was completely blocked by tetracaine, but not by conventional K+ inhibitors (Fig. 4D). The most striking difference between the atrial and ventricular cells was the appearance of the background current in atrial cells when the bath temperature was raised and the complete absence of this phenomenon in ventricular cells (cf. Figs 3 and 6). The simplest explanation for this difference is that atrial cells have the strongly temperature-sensitive TREK channels while ventricular cells do not.
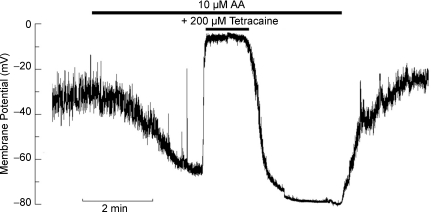
Effect of arachidonic acid on membrane potential of ED11 atrial myocytes
TREK channels are known to be activated by arachidonic acid (AA) (Patel et al. 1998). Thus, if the change in resting membrane potential observed in the atrial cells upon warming was indeed mostly due to the activation of TREK channels, AA should produce a similar change in the RMP with the cells at room temperature. Indeed, when ED11 atrial myocytes were exposed to 10 μm AA at room temperature, the RMP increased rapidly to nearly −70 mV and this change in potential was reversed by the application of 200 μm tetracaine (Fig. 7). The mean RMP in these experiments was −28.6 ± 2.6 mV in the absence of AA and −56.6 ± 8.2 mV in the presence of AA (n = 6, P = 0.003), while tetracaine reduced the RMP to −15.2 ± 3.4 mV (n = 6, P = 0.007). Taken together, these results are a strong indication that TREK channels are the major contributor to RMP in the embryonic chick atrium in contrast to the ventricle which relies on inwardly rectifying K+ channels.
Figure 7. Arachidonic acid hyperpolarized membrane potentials in chick ED11 atrial cells at room temperature.
Membrane potentials of chick ED11 atrial myocytes were recorded in current-clamp mode after formation of the whole-cell patch-clamp configuration. An example record of membrane potential illustrates the increased polarization after administering 10 μm arachidonic acid (AA) and the effects of inhibitors.
Discussion
Generally, the inwardly rectifying potassium channel (IK1) is predominant in determining the RMP in adult working myocardium. In this report, our electrophysiological and expression data together confirm the presence of functional TREK-1 and TREK-2 K+ channels as well as a significant role for these channels in setting the membrane potential in the embryonic atrium. This is the first report to demonstrate a functional role for TREK-1 and TREK-2 in maintaining and stabilizing the RMP in embryonic atrial myocytes. Since the embryonic chicken atrial cells lack IK1 (Fig. 3), our results show that the current we identify as being carried by TREK channels is sufficient and apparently necessary to maintain the RMP near EK.
TREK channels in the embryonic atrium
Our studies of the functional role of TREK-1 channels in chicken embryonic atrial cells is based on the pharmacological and biophysical properties of TREK-1 channels, which are relatively insensitive to conventional K+ channel inhibitors such as TEA, 4-AP and Ba2+ but are strongly inhibited by local anaesthetics including bupivacaine and tetracaine (Fink et al. 1996; Patel et al. 1998; Kindler et al. 1999). Although tetracaine-sensitive background K+ channels could include other K2P channels, the other K2P channels have different sensitivities to tetracaine and to the activators of TREK channels (Kindler et al. 1999). For example, the concentration of tetracaine used in this study is not enough to block TASK channels (IC50 668 μm) and TALK channels are activated by alkaline pH. Of all members of K2P K+ channels, only members of the TREK subfamily have heat sensitivity (Maingret et al. 2000; Kang et al. 2005). Therefore, the temperature-sensitive background K+ currents that are blocked by tetracaine are in fact TREK-like currents.
The TREK subfamily of K2P channels is composed of three members, TREK-1 TREK-2 and TRAAK. They have similar pharmacological and biophysical properties but TREK-2 and TRAAK have not been demonstrated to be functionally expressed in heart (Lesage et al. 2000; Gu et al. 2002; Putzke et al. 2007). On the other hand, TREK-1 and TREK-like current have been found in adult mammalian heart (Aimond et al. 2000; Terrenoire et al. 2001; Liu & Saint, 2004). Our gene expression analysis in developing mouse heart also shows that TREK-1 is the predominant K+ background channel (H. Zhang, unpublished observations). Relatively little TREK-2 and no TRAAK can be detected in developing mouse atrium. A very similar TREK expression pattern has been reported in embryonic rat heart (Liu & Saint, 2004). In the present study, we showed that both TREK-1 and TREK-2 are expressed in ED11 atria and can contribute to TREK-like currents. The expression of TREK-1 and TREK-2 in ventricle is relatively small. This is in good agreement with our electrophysiological results. Although TREK-1 can be electrophysiologically distinguished from TREK-2 by single-channel conductance and noisy opening kinetics (Kim, 2005), the contribution of them to RMP in chicken ED11 atrial myocytes cannot be separated due to the lack of specific inhibitors. We conclude that TREK channels are the predominant background K+ channels in embryonic chicken atrial cells.
The I–V relation of the heat-activated background K+ current
Our results at room temperature are consistent with single-channel studies showing that the activity of TREK-1 and TREK-2 is negligible (Maingret et al. 2000; Kang et al. 2005). At 42°C the I–V of cloned TREK-1 channels is outwardly rectifying, showing no saturation at positive potentials (Maingret et al. 2000). However, the activation of TREK-1 by AA or volatile anasthetics in the whole cell has been shown to give a current with an S-shaped I–V (Terrenoire et al. 2001), just as we find for the heat-activated current in atrial cells (Fig. 3). Although IK has a similar-shaped I–V in embryonic chicken heart cells, the voltage range of activation of IK is much more positive than what we found for the heat-sensitive current and IK is not strongly temperature sensitive (Clapham & Logothetis, 1988). Our electrophysiological data, taken together with the RT-PCR data, indicate that most, if not all, of the temperature-sensitive currents in embryonic chick atrial cells is due to TREK channels.
It is likely that the heat-activated current blocked by the cocktail is also largely, perhaps mostly, due to TREK channels. TREK channels are known to be blocked to some degree by Ba2+, TEA and 4-AP. In Xenopus oocytes, the expressed human TREK-1 was blocked about 22% by 100 μm Ba2+, 20% by 1 mm 4-AP and 9% by 1 mm TEA (Meadows et al. 2000). Therefore, we expect that TREK currents would be blocked by at least 25% by our cocktail (200 μm, 10 mm TEA and 1 mm 4-AP).
TREK channels and membrane potential
The addition of conventional K+ channel inhibitors produced only a slight decrease in membrane potential in comparison to a much larger depolarization with subsequent addition of tetracaine (Fig. 1). In contrast, tetracaine inhibited only about a third of the temperature-sensitive current (Fig. 4). Because tetracaine inhibits much of the background K+ currents, the reverse experiment of adding the tetracaine first followed by other K+ channel inhibitors would not have been informative. The apparent discrepancy can be explained by the following. At 35°C, membrane potential (Vm) (Fig. 1) is much closer to EK, reflecting, presumably, a relatively small Na+ conductance, gNa, about 0.08 of the conductance to K+ ions, gK (Sperelakis & Shigenobu, 1972). If [Na+]i is assumed to be ∼1 mm, this follows from the chord equation, wherein Vm at rest is equal to the sum of the products of the equilibrium potential for each ion, Ei, and the relative conductance for that ion, gi/gt, i.e. Vm= (Σgi/gt)Ei, where gt=Σgi. If about 1/2 of gK were blocked by the cocktail of conventional inhibitors (Fig. 1), gNa/gK would be 0.16 and Vm would be −56 mV, close to the measured value shown in Fig. 1. Blockade of another 1/4 of the total gK by tetracaine (i.e. 1/3 of the total block) would reduce Vm to −34 mV, again close to the measured value. Exact calculations are impossible because the blockers may affect conductance to ions other than potassium, and the origin of the conductance remaining in the presence of all blockers is unknown. Nevertheless, the approximate calculations show that the potential measurements give results which are completely consistent with the current measurements. It should be noted that a membrane potential change of 8–10 mV could have a substantial effect on the stability and excitability of cardiac membranes.
One of the important features of TREK channels is that they can be activated by arachidonic acid (AA). An AA-sensitive background K+ current with electrophysiological properties characteristic of TREK channels has been described in rat atrial myocytes (Kim & Clapham, 1989; Kim & Duff, 1990). We show in Fig. 7 that the membrane potential at room temperature is markedly increased by the presence of AA and that this increase is blocked by tetracaine. This result is completely consistent with an important role for TREK channels in setting the RMP of atrial cells in the ED11 embryo.
Lack of inwardly rectifying IK1 in embryonic atrial myocytes
We found very little IK1 in atrial myocytes, in contrast to ED11 ventricular myocytes where we show that IK1 is relatively large. The latter explains the more hyperpolarized potential in ventricle present at room temperature and at 35°C since IK1 is not known to be temperature sensitive. IK1 is lacking in myocytes of the primitive ventricle in early heart development (Josephson & Sperelakis, 1990). The RMP of these early ventricular myocytes is more depolarized and they are capable of rhythmic activity (Sperelakis & Pappano, 1983). As the heart matures, IK1 increases and the ventricular myocytes develop a stable, hyperpolarized RMP by ED11 in the chick. In contrast, TREK-1 is absent from ventricle but well-expressed and functional in the atria at ED11. This accounts for the temperature sensitivity and decreased stability of the RMP in atrial myocytes. ITREK-1 is outwardly rectifying and is larger at positive potentials. Thus, ITREK-1 probably serves as a repolarizing current in addition to stabilizing the RMP.
Conclusions
The heart is the first functional organ in embryonic development. Functional expression of TREK channels early in development suggests their physiological importance in embryonic heart. Unlike mammalian embryos, avian embryos do not have a constant temperature environment. Temperature-sensitive activation and inactivation of TREK channels could be important in controlling the heart rate, e.g. cooling would inactivate the channel and reduce the membrane potential, increasing excitability. Further, on a beat-to-beat basis, stretch activation of TREK channels is likely to be important for maintaining membrane stability during atrial filling (Niu & Sach, 2003). This idea is supported by the developmental regulation of expression of TREK-1 (see Fig. 1 in online Supplemental material). In the pre-septated chicken heart, TREK is expressed in both chambers of the heart, stabilizing the membrane potential to ensure proper activation of the contraction and prevent ectopic activity. Later, TREK-1 becomes restricted to atria, and stabilization of the ventricular membrane relies on IK1.
Acknowledgments
We appreciate Victoria Graham's assistance in the preparation of embryonic myocytes and immunohistochemistry. This work was supported by NIH grant HL71015 to T.L.C.
Supplementary material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2008.153395/DC1
References
- Aimond F, Rauzier JM, Bony C, Vassort G. Simultaneous activation of p38 MAPK and p42/44 MAPK by ATP simulates the K+ current ITREK in cardiomyocytes. J Biol Chem. 2000;275:39110–39116. doi: 10.1074/jbc.M008192200. [DOI] [PubMed] [Google Scholar]
- Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell Calcium. 2003;33:479–487. doi: 10.1016/s0143-4160(03)00063-0. [DOI] [PubMed] [Google Scholar]
- Bockenhauer D, Zilberberg N, Goldstein SA. KCNK2: Reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat Neurosci. 2001;4:486–491. doi: 10.1038/87434. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Logothetis DE. Delayed rectifier K+ current in embryonic chick heart ventricle. Am J Physiol Heart Circ Physiol. 1988;254:H192–H197. doi: 10.1152/ajpheart.1988.254.1.H192. [DOI] [PubMed] [Google Scholar]
- Clay JR, Hill CE, Roitman D, Shrier A. Repolarization current in embryonic chick atrial heart cells. J Physiol. 1988;403:525–537. doi: 10.1113/jphysiol.1988.sp017262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- Gu W, Schlichthorl G, Hirsch JR, Engels H, Karschin C, Karschin A, Derst C, Steinlein OK, Daut J. Expression pattern and functional characteristics of two novel splice variants of the two-pore-domain potassium channel TREK-2. J Physiol. 2002;539:657–668. doi: 10.1113/jphysiol.2001.013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson IR, Sperelakis N. Developmental increases in the inwardly-rectifying K+ current of embryonic chick ventricular myocytes. Biochim Biophys Acta. 1990;1052:123–127. doi: 10.1016/0167-4889(90)90066-m. [DOI] [PubMed] [Google Scholar]
- Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D, Mackenzie L, Hunter P, Smaill B, Saint DA. Gene expression of stretch-activated channels and mechanoelectric feedback in the heart. Clin Exp Pharmacol Physiol. 2006;33:642–648. doi: 10.1111/j.1440-1681.2006.04392.x. [DOI] [PubMed] [Google Scholar]
- Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharmaceut Design. 2005;11:2717–2736. doi: 10.2174/1381612054546824. [DOI] [PubMed] [Google Scholar]
- Kim D, Clapham DE. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989;244:1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Kim D, Duff RA. Regulation of K+ channels in cardiac myocytes by free fatty acids. Circ Res. 1990;67:1040–1046. doi: 10.1161/01.res.67.4.1040. [DOI] [PubMed] [Google Scholar]
- Kindler CH, Yost CS, Gray AT. Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem. Anesthesiology. 1999;90:1092–1102. doi: 10.1097/00000542-199904000-00024. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- Li XT, Dyachenko V, Zuzarte M, Putzke C, Preisig-Müller R, Isenberg G, Daut J. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc Res. 2006;69:86–97. doi: 10.1016/j.cardiores.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Liu W, Saint DA. Heterogeneous expression of tandem-pore K+ channel genes in adult and embryonic rat heart quantified by real-time polymerase chain reaction. Clin Exp Pharmacol Physiol. 2004;31:174–178. doi: 10.1111/j.1440-1681.2004.03964.x. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honore E. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows HJ, Benham CD, Cairns W, Gloger I, Jennings C, Medhurst AD, Murdock P, Chapman CG. Cloning, localization and functional expression of the human orthologue of the TREK-1 potassium channel. Pflugers Arch. 2000;439:714–722. doi: 10.1007/s004249900235. [DOI] [PubMed] [Google Scholar]
- Muller PY, Hanovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotech. 2002;32:1372–1379. [PubMed] [Google Scholar]
- Murbartian J, Lei Q, Sando JJ, Bayliss DA. Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. J Biol Chem. 2005;280:30175–30184. doi: 10.1074/jbc.M503862200. [DOI] [PubMed] [Google Scholar]
- Niu W, Sachs F. Dynamic properties of stretch-activated K+ channels in adult rat atrial myocytes. Prog Biophys Mol Biol. 2003;82:121–135. doi: 10.1016/s0079-6107(03)00010-5. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Vega-Saenz de Miera E. Cloning of two transcripts, HKT4.1a and HKT4.1b, from the human two-pore K+ channel gene KCNK4: Chromosomal localization, tissue distribution and functional expression. Mol Brain Res. 2002;102:18–27. doi: 10.1016/s0169-328x(02)00157-2. [DOI] [PubMed] [Google Scholar]
- Pappano AJ. Action potentials in chick atria. Increased susceptibility to blockade by tetrodotoxin during embryonic development. Circ Res. 1972;31:379–388. doi: 10.1161/01.res.31.3.379. [DOI] [PubMed] [Google Scholar]
- Pappano AJ. Action potentials in chick atria. Ontogenetic changes in the dependence of tetrodotoxin-resistant action potentials on calcium, strontium, barium. Circ Res. 1976;39:99–105. doi: 10.1161/01.res.39.1.99. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzke C, Wemhoner K, Sachse FB, Rinne S, Schlichthorl G, Li XT, Jae L, Eckhardt I, Wischmeyer E, Wulf H, Preisig-Muller R, Daut J, Decher N. The acid-sensitive potassium channel TASK-1 in rat cardiac muscle. Cardiovasc Res. 2007;75:59–68. doi: 10.1016/j.cardiores.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Sperelakis N, Pappano AJ. Physiology and pharmacology of developing heart cells. Pharmacol Ther. 1983;22:1–39. doi: 10.1016/0163-7258(83)90050-5. [DOI] [PubMed] [Google Scholar]
- Sperelakis N, Shigenobu K. Changes in membrane properties of chick embryonic hearts during development. J Gen Physiol. 1972;60:430–453. doi: 10.1085/jgp.60.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JH, Liu W, Saint DA. Trek-like potassium channels in rat cardiac ventricular myocytes are activated by intracellular ATP. J Membr Biol. 2002;185:201–207. doi: 10.1007/s00232-001-0123-0. [DOI] [PubMed] [Google Scholar]
- Terrenoire C, Lauritzen I, Lesage F, Romey G, Lazdunski M. A TREK-1-like potassium channel in atrial cells inhibited by b-adrenergic stimulation and activated by volatile anesthetics. Circ Res. 2001;89:336–342. doi: 10.1161/hh1601.094979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.