Abstract
High single-channel conductance K+ channels, which respond jointly to membrane depolarization and micromolar concentrations of intracellular Ca2+ ions, arise from extensive cell-specific alternative splicing of pore-forming α-subunit mRNAs. Here, we report the discovery of an endogenous BKCa channel α-subunit intron-containing mRNA in the cytoplasm of hippocampal neurons. This partially processed mRNA, which comprises ≈10% of the total BKCa channel α-subunit mRNAs, is distributed in a gradient throughout the somatodendritic space. We selectively reduced endogenous cytoplasmic levels of this intron-containing transcript by RNA interference without altering levels of the mature splice forms of the BKCa channel mRNAs. In doing so, we could demonstrate that changes in a unique BKCa channel α-subunit intron-containing splice variant mRNA can greatly impact the distribution of the BKCa channel protein to dendritic spines and intrinsic firing properties of hippocampal neurons. These data suggest a new regulatory mechanism for modulating the membrane properties and ion channel gradients of hippocampal neurons.
Keywords: dendrite, epilepsy, intron-retention, KCNMA1, local splicing
In mammalian neurons, the firing of an action potential requires the coordinated gating of at least a dozen different classes of voltage-gated ion channels. The integration of these currents manifests itself as characteristic input–output properties intrinsic to each neuron. One such firing property of some neurons, such as those in the hippocampus (1, 2) or cerebellum (3), is the ability to initiate repetitive burst firing in response to depolarizing current. Voltage-clamp analysis suggests that the small net inward current that drives the depolarizing momentum is a result of a subtle balance of the sum of inward and outward postspike currents (4). Although short-term and long-term feedback mechanisms exist to preserve burst firing (5), it is known that relatively small changes in the size of an individual current may have a dramatic impact on firing activity (6).
One transient current activated during the falling phase of the action potential is the BKCa channel. In the central nervous system, BKCa channels are localized to the cell soma as well as the pre- and postsynaptic terminals of neurons, where they regulate fundamental neuronal functions such as burst firing, neurotransmitter release, shaping action potential waveforms, and frequency tuning (7). Native channels are assembled as tetramers of the pore-forming α-subunits encoded by a single gene, KCNMA1 (8), which is subject to vast tissue-specific (9) and cell-specific alternative splicing (10). The resulting functional heterogeneity in BKCa channel currents is due in part to these splicing events generating BKCa channels with altered Ca2+ sensitivity and gating kinetics (7) as well as altered channel trafficking (11). Channel differences also arise from modulation via a family of tissue-specific auxiliary β-subunits (10). Currently, the functional diversity among BKCa channels in neurons is not fully resolved, but the expression and subcellular distribution patterns of splice variants are expected to be one functionally significant factor in determining their characteristic input–output properties. Indeed, there is some evidence for members of the K+ channel family, for example BKCa (12) or A-type (13), establishing functional gradients within the dendritic processes of mature neurons, where they transform the shape of local synaptic potentials or the size of somatic action potentials (14).
Here we report a novel form of posttranscriptional gene expression (cytoplasmic splicing of a RNA with a retained intron) that plays a role in generating local populations of BKCa channel mRNAs and proteins in hippocampal neurons. These results show that the regulation of a cytoplasmic BKCa channel intron-containing mRNAs partially underlie the characteristic input–output properties of hippocampal neurons.
Results
BKCa Channel Intron-Containing mRNAs Are Present in Hippocampal Dendrites.
To determine the repertoire of mRNA splice variants in the postsynaptic compartment, we prepared a cDNA template derived from antisense RNA amplification of polyA mRNA isolated from rat hippocampal dendrites, which were harvested from 14- to 21-day-old cultures in vitro (15). Dendrites were carefully harvested individually to ensure that no cell bodies, and thus nuclei, of any neuron or nonneuronal cell were harvested. From this starting material, we detected two separate PCR products using primers specific to intron 16 of KCNMA1 [supporting information (SI) Fig. 5a]. Intron 16 (i16) is ≈6,000 nt long and lies upstream of a previously described “hot spot” for alternative exon usage (16). Exons surrounding i16 encode the 3′-end of the first of two highly conserved Ca2+-binding RCK (regulators of conductance K+ channel) domains and the nonconserved linker region spanning the first and second RCK module (17) (see Fig. 1a). The nucleotide sequence of the PCR products (330 and 390 nt) proved to be identical to the rat genomic BKCa channel genomic DNA sequence deposited in GenBank. We also observed the presence and absence of introns in other mRNAs in amplified polyA mRNA dendrite samples subjected to microarray analysis (SI Fig. 5b). The presence of select introns in multiple mRNAs, as evidenced by hybridization, shows that intron retention is a generalized phenomenon in hippocampal neurons and not simply restricted to the BKCa channel gene. The absence of hybridization for multiple select introns further demonstrates that there was no genomic contamination of the dendrite samples. For this study, we will restrict our focus to the analysis of the BKCa channel mRNA.
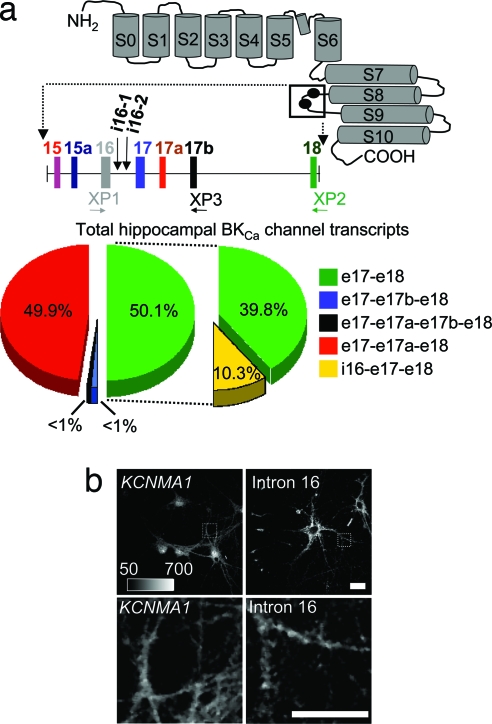
Fig. 1.
BKCa channel intron-containing mRNAs are present in the cytoplasm of hippocampal neurons. (a) Putative membrane topology of the BKCa channel α-subunit and the location of two sites of alternative splicing (black circles) are shown. Exons are denoted with vertical solid bars and introns with horizontal lines. Exon lengths are 69, 12, 87, 77, 9, 174, and 94 nt for e15, e15a, e16, e17, e17a, e17b, and e18, respectively. Intron lengths are 2,340, 6,222, 6,654, 4,528, 6,421, and 30,920 nt for i15, i15a, i16-containing, i17, i17a, and i17b, respectively. KCNMA1 splice variant analysis was performed in hippocampal tissue. These data show the abundance of four KCNMA1 splice variants and the exonic linkage analysis of each splice variant to i16-containing region. For the alternative splicing analysis, the “all transcript” products were analyzed with XP1, XP2, and XP3 on two separate hippocampus samples in replicate (n = 16). For i16 transcripts, two samples of both hippocampus and hippocampal dendrites were analyzed in replicate (n = 2) with XP2 and XP3. (b) ISH was used to verify the subcellular localization of KCNMA1 mRNA (e22–e25) and i16-containing BKCa channel mRNA (n = 3). Anti-digoxigenin Fab fragments conjugated to Qdot-565 were used to enhance the detection and visualization. (Upper) Photomicrographs showing ISH of cultured hippocampal neurons with KCNMA1 e22–e25 probe and i16-containing BKCa channel mRNA probe. (Lower) Photomicrographs showing enlarged marked regions from Upper.
i16-Containing Transcripts Are Associated with a Single BKCa Channel Splice Variant.
The levels and endogenous populations of i16-containing transcripts were resolved by MALDI-TOF MS base extension (18). This PCR method uses an identical probe to analyze multiple transcripts (see SI Experimental Procedures). Therefore, it was chosen over real-time PCR, which requires different exon-spanning probes to identify alternative spliced transcripts. Using this approach, we first determined the levels of i16-containing transcripts. Hippocampal tissue cDNA was amplified with PCR primers in exon 16 (e16) forward and e18 reverse to capture the “all BKCa channel transcripts” population. A single extension primer (XP1) was used to assay the PCR products. The i16-containing transcript represented 10.3% ± 2.3% of the total BKCa channel transcript population (Fig. 1a). The levels of i16-containing transcripts reported here are in agreement with a previous study showing other KCNMA1 splice variants typically represent a small subset (≈10%) of the total KCNMA1 transcript population (19).
We next determined the endogenous KCNMA1 exonic combination between i16 to e18 in both hippocampal tissue and isolated dendrites. This region consists of four downstream exons: two constitutive exons (e17 and e18) and two alternatively spliced exons (e17a and e17b). Here we amplified cDNA with two sets of PCR primers: (i) e16 forward and e18 reverse to capture “all BKCa channel transcripts” or (ii) i16–1 forward and e18 reverse to capture “only i16-containg transcripts” (Fig. 1a). The PCR products were simultaneously assayed with the two different extension primers (XP2 and XP3) to capture all four exonic combinations (Fig. 1a). In the “all BKCa channel transcripts” population, every exonic combination was detected, but two highly abundant variants, e17–e18 and e17–e17a–e18, made up the bulk of the BKCa channel transcript population (Fig. 1a). A remarkably different pattern of splicing was detected in the “only i16-containing transcript” population. Unexpectedly, only one splice variant was detected; all of the i16-containing transcripts skip e17a and e17b and splice e17 directly to e18 (Fig. 1a). These data show that i16-containing mRNAs are restricted to a subset of the KCNMA1 transcript population containing correctly spliced downstream exons, e17 and e18. Furthermore, these results highlight two key properties of i16-contiaining transcripts. First, they are present at biologically significant levels in the hippocampus, and, more surprisingly, the overall complexity of the i16-contiaining transcripts transcript population is less diverse then would be possible if all combinations of the downstream exons were associated with the retained intron.
The Subcellular Localization of BKCa Channel and the i16-Containing Splice Variant mRNAs in Hippocampal Neurons.
In situ hybridization (ISH) is one method for assessing the dendritic localization of mRNA transcripts in neurons, but the methodology as standardly practiced is not particularly robust. For our studies, we developed a novel, highly sensitive Quantum Dot (Qdot)-based ISH protocol with i16-containing BKCa channel mRNA-specific probes to visualize its endogenous subcellular localization throughout the somatodendritic compartment (Fig. 1b and SI Fig. 6). This procedure allows detection of low abundance signals because of a prephotobleaching step eliminates any endogenous background cellular autofluorescence (SI Fig. 6). For the i16-containing BKCa channel mRNA, a series of puncta are detectable in the cell soma that extends into the proximal and distal dendrite. Signal intensity is strongest in the first 50-μm proximal segment of the dendrite and diminishes as a function of distance from the cell soma (Fig. 1b and SI Fig. 7). A comparison with the ISH signal of the KCNMA1 e22–e25 exon probe of the mature BKCa channel mRNA shows a similar pattern of distribution (Fig. 1b and SI Fig. 7). This pattern is coincident with where dendritic BKCa channel activity is most predominate in the most proximal portions of the dendrite while decaying with distance from the cell body (12). The importance of these data are twofold. First, they provide independent corroboration of the somatodendritic presence of the i16-containing BKCa channel mRNA. Second, they represent, to our knowledge, the first report of an endogenous intron-containing mRNA that is exported from the nucleus and transported to the somatodendritic cytoplasm. Furthermore, these phenomena are not restricted to cultured hippocampal neurons. Similar levels of i16-containing BKCa channel mRNA were detected by our quantitative PCR approach in fetal and adult brain tissues (data not shown). We followed this observation by using ISH to show the presence of i16-containing BKCa channel mRNA in the somatodendritic compartment of neurons in the hippocampus and striatum of adult rat brains (SI Fig. 5).
Reducing BKCa Channel i16-Containing Splice Variant mRNA Levels Alters the Distribution of BKCa Channel Protein in Dendrites.
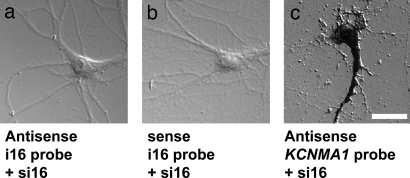
Given the subcellular distribution and localization of i16-containing BKCa channel mRNA, we next determined whether i16-containing BKCa channel mRNAs contribute to the abundance and localization patterns of BKCa channel protein. Two nonoverlapping siRNAs specific for the i16 sequence (si16) were synthesized and transfected into primary hippocampal neurons. The siRNA-treated hippocampal neurons maintained normal cellular morphology (Fig. 2). Using ISH, we next show i16-specific siRNA treatment depleted the pools of i16-containing BKCa channel mRNA in the cytoplasm of hippocampal neurons (Fig. 2a). Control sense i16–1 probes do not show any significant signal in untreated cultures (Fig. 2b). As a control for off-target effects, each of the two nonoverlapping i16–1 siRNAs were transfected individually, yielding the same phenotype (data not shown). The mature BKCa channels α-subunit mRNAs expressing backbone exons (e22–e25) were unchanged in abundance and subcellular distribution in si16-treated neurons (Fig. 2c) as expected because siRNAs modulate their target mRNA effects through cytoplasmic reduction of the target RNA. For these photomicrographs, we chose to use used ISH with alkaline phosphatase and NBT/BCIP (Nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate) detection with differential interference contrast optics to visually highlight the cellular morphology of the siRNA-treated neurons
Fig. 2.
i16-specific siRNA treatment reduces i16-containing BKCa channel mRNA levels but not the levels of BKCa channel mRNA containing exons only. Cultured hippocampal neurons were transfected with a pool of i16-specific siRNAs. ISH was used to analyze the changes in KCNMA1 (e22–e25) and i16-containing BKCa channel mRNA levels in si16-treated hippocampal neurons. (a) Differential interference contrast (DIC) photomicrograph showing the ISH staining with antisense i16-containing probe. (b) DIC photomicrograph showing the ISH staining with sense i16-containing probe. (c) DIC photomicrograph showing the ISH staining with KCNMA1 e22–e25 probe. (Scale bars: 25 μm.)
BKCa Channel i16-Containing mRNAs Contribute Significantly to the Populations of BKCa Channel in Dendritic Spines.
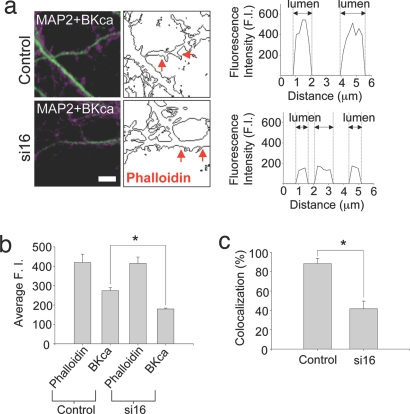
Having established an effective siRNA treatment protocol to selectively reduce i16-containing BKCa channel mRNA levels, we next assessed the subcellular distribution of BKCa channel protein in the dendritic spines of i16-specific siRNA-treated and untreated neurons by using triple label fluorescence. MAP2 (microtubule-associated protein 2) staining was used to identify dendritic processes in hippocampal neurons (Fig. 3a Left). Phallotoxins bind with high affinity to the filamentous actin (F-actin) and are frequently used to identify and quantify the levels of the cytoskeletal protein in dendritic spines. To quantify the distribution of BKCa channels in dendritic spines, we determined how frequently BKCa channel puncta were colocalized with F-actin in spine structures by using volume measurement analysis. We then compared the fluorescence intensity signal observed from BKCa channels and phalloidin in dendritic spines of untreated and si16-treated neurons (Fig. 3). In Fig. 3a, we have taken one plane in a z-stack to show the representative signal obtained with MAP2 and BKCa channel antibodies. Outlined next to the photomicrograph is the boundary of Alexa 488-phalloidin staining within the same optical section (Fig. 3a Center). For the initial analysis, a line scan across dendritic spines (as represented between the two arrows) highlights the presence of BKCa channel protein within the lumen of the spine. In si16-treated neurons, a smaller but consistently detectable BKCa channel fluorescence intensity was noted in comparison to controls (Fig. 3a Right). These data were the first suggestive evidence of a differential distribution of BKCa channels in the lumen of dendritic spines in untreated versus si16-treated neurons.
Fig. 3.
i16-specific siRNAs modify the differential distribution of BKCa channel protein in dendritic spines. (a Left) The merged confocal images of BKCa channel (magenta) and MAP2 (green) are shown. (Center) Outlines of AlexaFluor 488 phalloidin immunostaining are shown. Control and si16-treated cultures show a differential distribution of BKCa channel immunostaining. The line scan profile (Right) represents BKCa channel fluorescence intensity between where the red arrowheads point (Center). The higher BKCa channel staining was apparent in the lumen of control dendritic spines. The gray dotted lines in Right demarcate the edge of phalloidin staining which corresponds to the morphology of spines. (Scale bars: 5 μm.) (b) To quantify the differential distribution of BKCa channel in dendritic spines, we randomly selected dendritic spine heads based on phalloidin staining and measured the intensity of phalloidin and BKCa channel fluorescence intensities. There was no significant difference in phalloidin signal (control, 419.09 ± 43.01; si16, 415.20 ± 32.46). However, significant differences in BKCa channel staining were apparent (control, 274.47 ± 14.97; si16, 179.44 ± 4.74; n = 60; P < 0.001). (c) The correlation of dendritic spine and BKCa channel colocalization with si16 treatment. The incidence of colocalization observed for BKCa channels and phalloidin in dendritic spines of si16-treated neurons was dramatically reduced compared with control samples: 88.33% (41.67%, si16 treated culture; n = 60; P < 0.001). Data are mean ± SEM.
Dendritic spines are dynamic structures with variable three-dimensional topography. It is possible that the line scan analysis may be biased if the pixels we are analyzing correspond to some spines that are only partially in the optical section while others are being bisected directly in the middle of the spine lumen. To address this possibility (Fig. 3b), a region of interest was selected in multiple, randomly selected spine heads in the same optical section. We first analyzed phalloidin fluorescence. Any difference in phalloidin signal would strongly imply that we were not comparing similar areas in a region of interest within the spine head. Importantly, there was no difference in the intensity of phalloidin staining between untreated and si16-treated neurons (Fig. 3b, control; 419.09 ± 43.01, n = 60 and si16; 415.20 ± 32.46, n = 60). There was, in contrast, a quantifiable difference in BKCa channel protein distribution (Fig. 3b, control; 274.47 ± 14.97 and si16; 179.44 ± 4.74, n = 60, P < 0.001). To further refine this difference, we transformed the BKCa channel fluorescence intensity differences relative to phalloidin signal in Fig. 3b to reflect a measure of their incidence of colocalization (Fig. 3c). The observed BK channel fluorescence ranged from ≈50% to 75% of the overall phalloidin signal in the spine heads of untreated neurons. Therefore, to be more rigorous we selected a lower value (50%) as our minimum parameter to establish the incidence of BKCa channel and phalloidin colocalization. The spine heads showing ≥50% BKCa channel fluorescence intensity relative to phalloidin intensity were annotated as normal. By using these parameters of colocalization within a region of interest in a spine head, untreated cells are about two times more likely to have BKCa channel protein in their spine heads above our 50% threshold as compared with si16-treated cells (Fig. 3c). In untreated neurons, the BKCa channel puncta predominately showed normal colocalization with phalloidin (≈88%; Fig. 3c). We see a dramatic change in si16-treated neurons in the colocalization pattern of BKCa channel puncta in dendritic spines. Here, the colocalization incidence was significantly reduced (≈42%; Fig. 3c). Collectively, these experiments highlight the local contribution i16-containing mRNAs make to the BKCa channel protein distribution in hippocampal dendritic spines.
In parallel control experiments, we examined the differential distribution of another member of the K+ channel family, Kv2.1. As a prevalent component of the somatodendritic delayed-rectifier potassium currents in mammalian neurons, Kv2.1 plays a prominent role in regulating Ca2+ influx and suppressing neuronal excitability (20). We assessed the colocalization of Kv2.1 channels with the phalloidin signature of individual dendritic spines. In contrast to the changes observed in BKCa channel differential distribution, the localization of Kv2.1 was unaltered between control and si16-treated cultures (n = 15; control, 85.0 ± 7.2; si16, 83.3 ± 8.0; Student's t test, P = 0.88; image shown in SI Fig. 8). As an additional control, we assessed the spine colocalization of the NR1 subunit of the NMDA receptor (21) with and without i16 siRNA treatment. Again, no discernible difference was found (n = 13; control, 80.8 ± 6.4, si16-treated, 75.0 ± 9.4; Student's t test, P = 0.66). These controls show that the siRNA-induced difference in BKCa channel spine localization is selective. Given this striking change in the pattern of BKCa channel protein distribution in dendritic spines, we next screened for associated functional consequences.
BKCa Channel Intron-Containing mRNAs Contribute to the Excitability of Hippocampal Neurons.
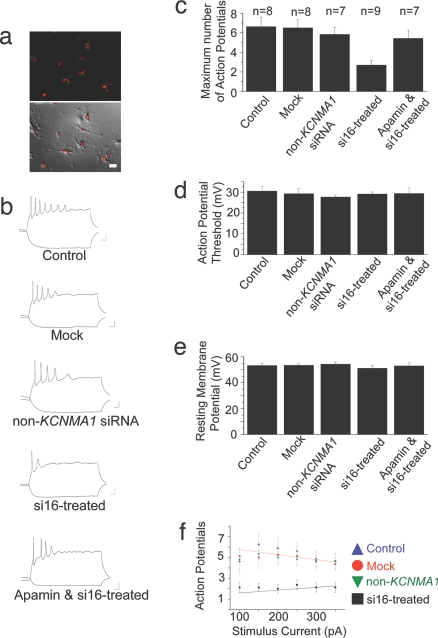
Hippocampal cells typically fire a burst of action potentials characterized by spike accommodation in which subsequent action potentials broaden often leading to spike failure (22). In hippocampal neurons, BKCa channels play a role in both action potential repolarization (12) as well as spike broadening during repetitive firing (23). Complex spike bursts of this sort are thought to underlie some adaptive processes during the acquisition of learning and memory (24). Abnormally large BKCa channel currents are the primary cause for changes in the patterns of complex spikes in some forms of epilepsy and dyskinesia (25). Having established an effective siRNA treatment protocol to selectively reduce i16-containing BKCa channel splice variant mRNA and protein levels, we next recorded from hippocampal neurons and analyzed their firing patterns and action potential profiles. Mock-treated, non-KCNMA1 siRNA-treated (Ambion negative controls), or si16-treated neurons were not detectably different from the control sample in either the shape of the evoked actions potentials, action potential generation threshold, resting membrane potential, or input resistance (Fig. 4). However, the maximum number of evoked action potentials was significantly reduced in the si16-treated neurons compared with control, mock-treated, and non-KCNMA1 siRNA-treated neurons (Fig. 4c). These findings show that the i16-containing mRNA is integral to the regulation of functional BKCa channel expression mediated membrane excitability.
Fig. 4.
Firing properties of hippocampal neurons are altered by i16-specific siRNAs. Hippocampal neurons were transfected with pools of siRNAs directed against separate KCNMA1 i16 sequences or a non-KCNMA1 target sequence (Ambion negative control nos. 1 and 7) and 20 nM siRNA-Glo Rhodamine marker. The neurons were current-clamped at −80 mV, and brief current injections (500 ms) were applied to evoke a train of action potential. The input impedance of treated neurons was not significantly different compared with control neurons (control, 0.484 ± 0.09, n = 8; mock-treated, 0.479 ± 0.064, n = 8; non-KCNMA1 siRNA-treated, 0.487 ± 0.080, n = 7; si16-treated, 0.590 ± 0.070, n = 9; apamin and si16-treated, 0.422 ± 0.07, n = 7). (a Upper) A fluorescence image showing the siRNA-Glo Rhodamine signal in the transfected neurons. (Lower) Photomicrograph is a merged image of fluorescence signal over DIC image. (b) Representative action potential traces from −150 and +150 pA current injection. (c) Histogram summary of the maximum number of action potentials generated from one current injection for each condition. (d) Histogram summary of the action potential threshold for each condition. (e) Histogram summary of the resting membrane for each condition. (f) Summary plot of the maximum number of action potentials generated at each current injection from si16-treated and mock neurons. Data are means ± SEM.
Spike Accommodation in Hippocampal Neurons Requires the Presence of BKCa Channel Intron-Containing mRNAs.
To further characterize the functional significance of the i16-containing BKCa channel transcript, we compared the input–output function of mock-treated and i16-specific siRNA-treated neurons. With small current injections, the control, mock-treated or non-KCNMA1 siRNA-treated neurons showed brisk spiking activity. However, as the size of the current injections increased, spike accommodation became more apparent and reduced the number of spikes fired (Fig. 4f). In contrast, the si16-treated cells exhibited marked spike accommodation even for small current injections, essentially leaving the neurons unable to encode different stimulus levels (Fig. 4f). These results show that a reduction in BKCa channel α-subunit intron-containing mRNA levels alters membrane excitability of hippocampal neurons. These data are consistent with previous studies demonstrating that changes in BKCa channel activity alter the membrane properties (2).
SK Channel Inhibition Increases the Excitability of i16-Specific siRNA-Treated Neurons.
Reduction of BKCa channel intron-containing mRNA levels mimics the proposed role for the β4 subunit in the regulation of the KCNMA1 α-subunit, which has been reported to play the functional role of inhibiting BKCa channels from contributing to membrane repolarization (26). BKCa channels are negative feedback regulators of calcium influx in hippocampal and many other neurons; therefore, the down-regulation of BKCa channel activity by si16-treated or β4 subunit regulation should result in sustained calcium influx. This indeed could reduce the firing properties of si16-treated neurons by enhancing the activation of calcium-meditated after-hyperpolarization currents via SK channels. One apamin-sensitive isoform, SK2, is localized throughout the postsynaptic compartment in both the shaft and spines of dendrites (27). Thus, to test the notion that the reduction of i16-containing splice variant levels is reducing functional BKCa channel activity and increasing SK channel activity, we analyzed the firing patterns of si16-treated neurons in the presence of SK channel blocker apamin. Here, we show the maximum number of evoked action potentials is significantly increased in si16-treated neurons in the presence of apamin (Fig. 4c). Collectively, these results are consistent with previous studies, which suggest that BKCa channel activity is an intrinsic determinant of membrane properties. Moreover, they offer the first evidence for a functional role of a cytoplasmically localized, endogenous intron-containing mRNA in altering the membrane excitability of hippocampal neurons.
Discussion
Under normal cellular conditions, unspliced or incompletely spliced intron-containing mRNAs are routinely sequestered within the nucleus (28) and when transported to the cytoplasm they are subject to cellular nonsense-mediated degradation (29). Although intron retention is not an entirely unique phenomenon to neurons, having been described for cyclooxygenase 3 (30) and EAAT2 variants (31), these events are rarely observed in higher eukaryotes (32). In fact, database entries annotating intron-containing mRNAs often represent them as artifacts with little likelihood of influencing cellular physiology. Yet, several reports have begun to pinpoint a mechanism for promoting the nuclear export of incompletely spliced intron-containing mRNAs (33, 34).
At least two posttranscriptional regulatory mechanisms are used to generate BKCa channel heterogeneity: (i) extensive alternative splicing of BKCa mRNA and (ii) differential use of auxiliary β-subunits. We have previously speculated that by coupling the extranuclear splicing capability of the somatodendritic compartment with local protein synthesis (15) or simply locally translating (35, 36) intron-containing mRNAs as readthrough products, there may be another novel layer of posttranscriptional regulation supplementing the functional complexity of individual synapses (15). As recent reports suggest, splicing activity residing outside the nucleus exists as both stimulus-dependent and constitutive mechanisms for regulating gene expression (33, 37). With particular reference to the i16-containing BKCa channel mRNA, the position of the intron is notable. For maximal activation, BKCa channels require a source of intracellular Ca2+. Often, these spikes of Ca2+ are generated by selectively coupling the opening of Cav channels subtypes in close proximity to BKCa channels (38) and perhaps as part of large macromolecular signaling complexes (39). Intron 16 immediately precedes an exon that codes for the nonconserved region linking the Ca2+-sensing RCK1 and RCK2 domains. Within the proline-rich sequence encoded in this exon, a noncanonical Src homology domain 3 (SH3) is expressed which allows BKCa channels to coordinate their spatial distribution within the actin cytoskeleton through the actin adapator protein cortactin (40). In the i16-containing mRNA knockdown experiments, we observed a radically different distribution where the BKCa channel protein was far less likely to colocalize with filamentous actin in dendritic spines. Changes in BKCa channels firing properties are synchronized with alterations in actin cytoskeletal dynamics (41) in some forms of stroke and epilepsy (40). One obvious question is to what extent neurons use an i16-containing mRNA to generate a functional BKCa channel that can properly coordinate with the actin cytoskeleton. The anti-BKCa channel antibody used here recognizes an epitope encoded within final intracellular C-terminal residues (e.g., exons 24 and 25). In the context of our BKCa channel immunofluorescence data, the most likely scenario is the cytoplasmic pre-mRNA splicing of the endogenous i16-containing BKCa transcript yielding a protein with C-terminal sequence intact. Such a cytoplasmic pre-mRNA splicing regulatory checkpoint by spliceosome-competent ribonucleoprotein complexes has recently been described in anucleate platelets (33). It is unclear if such a cellular process in the nervous system may create any novel, alternatively spliced mRNAs encoding a protein which generates a gain-of-function characteristic that alters the excitability of hippocampal neurons.
Functionally, most K+ channels tend to hyperpolarize the cell and moderate the effects of excitatory input (22). In contrast, BKCa channels cause rapid spike repolarization and a post-burst fast after hyperpolarization to facilitate repetitive firing in hippocampal neurons (2). The facilitory effect of BKCa channels is most conspicuous in the form of gain-of-function phenotypes that greatly enhance neuronal excitability to great detriment. A single point mutation in the first RCK domain underlies an enhanced rate of repolarization in the human syndrome of coexistent generalized epilepsy and paroxysmal dyskinesia (25). A similar gain-of-function phenotype that sharpens action potentials, supports higher firing rates with the loss of SK channel recruitment, and shows distinctive temporal lobe seizures was revealed when the auxiliary BKCa channel β4 subunit was deleted in β4-null mice (26). In the absence of BKCa channel activity whether genetically (42) or pharmacologically induced (2), the characteristic bursting properties of neurons are attenuated with increasing current injection as spike accommodation becomes more apparent. BKCa channels are potential drug targets for several clinical disorders such as autism (43) and epilepsy (25). Therefore, the functional significance of cytoplasmic intron-containing BKCa channel mRNAs suggests that intron-retained channel mRNAs and their locally translated proteins may be a novel class of therapeutic targets for disorders linked to this channel.
Although small changes in an individual ion channel conductance or localization can greatly impact both action potential generation and the intrinsic firing properties of a neuron, this role has never been assigned to an endogenous cytoplasmic intron-containing mRNA. Furthermore, these data highlight the influence BKCa channel α-subunit intron-containing mRNAs and mRNA splice variant expression will have on heterogeneity of BKCa currents at the subcellular level in hippocampal neurons. The interrelationship of these distinct subclasses may account, in part, for flexibility in the creation of functional phenotypes for the encoded protein.
Experimental Procedures
Hippocampal Cultures.
Primary cultures were plated as previously described (15).
Dendrite RT-PCR Analysis.
The pools of dendrites were harvested, subjected to two rounds of antisense RNA amplification procedure as previously described (15), and used as a template for PCR.
MALDI-TOF MS and Quantitative KCNMA1 Splice Variant Detection.
MALDI-TOF MS analysis of PCR amplicons of dendrite and hippocampus KCNMA1 cDNA was performed by using PCR primers selecting for either all transcripts or intron-containing transcripts using previously described methods (18). See SI Experimental Procedures.
ISH Using Cultured Neurons.
Antisense digoxigenin-labeled KCNMA1 RNA probes were generated by in vitro transcription. Anti-digoxigenin Fab fragments conjugated to Qdot 565 were used for detection (Invitrogen). The samples were subjected to photobleaching, and the Qdot signal was detected by using the Olympus Fluoview 1000 confocal scan head attached on inverted microscope. See SI Experimental Procedures.
ISH Using Adult Rat Brain Sections.
Fresh frozen adult rat brains were sectioned at 15-μm thickness. Anti-digoxigenin Fab Fragments conjugated to alkaline phosphatase (Roche) were used for detection with NBT/BCIP (Nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate; Roche) for visualization under brightfield optics.
Immunocytochemistry.
Primary neurons were fixed 10–14 days after plating and as described in SI Experimental Procedures. For each cell, five randomly placed line scans were taken from three separate regions of interest for each dendritic segment and analyzed with Metamorph image processing software.
siRNA Treatments.
Cultured primary rat hippocampal neurons were transfected in DharmaFect 3 (Dharmacon) 7–9 days after plating (see SI Experimental Procedures). The cultures were maintained at 37°C with 5% CO2 for 72 hr.
Whole-Cell Recordings.
Bathing solution consisted of 140 mM NaCl, 3 mM KCl, 1 mM CaCl2, 1 mM Mg Cl2, and 10 mM Hepes, adjusted to a pH of 7.4 with NaOH. The internal solution consisted of 120 mM potassium gluconate, 20 mM KCl, 10 mM Hepes, 0.1 mM EGTA, 2 mM MgCl2, 2 mM ATP, and 0.25 mM GTP, adjusted to a pH of 7.4 with KOH.
Supplementary Material
ACKNOWLEDGMENTS.
We thank J. Saunders for use of his electrophysiology equipment, M. Maronski and D. Scarsell for help with cell culture, L. Barrett and J. C. Oberholtzer for helpful discussions, C. Garner for the polyclonal MAP2 antibody, and V. Lee for the monoclonal MAP2 antibody. This work was funded in part by Grants MH58371, AG9900, CH 41699, and T32 DC 005363-01A1, and Health Research Fund funds from the Commonwealth of Pennsylvania.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711796105/DC1.
References
- 1.Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurons. J Physiol. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol. 2007;580:859–882. doi: 10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callaway JC, Ross WN. Spatial distribution of synaptically activated sodium concentration changes in cerebellar Purkinje neurons. J Neurophysiol. 1997;77(1):145–152. doi: 10.1152/jn.1997.77.1.145. [DOI] [PubMed] [Google Scholar]
- 4.Swensen AM, Bean BP. Ionic mechanisms of burst firing in dissociated Purkinje neurons. J Neurosci. 2003;23:9650–9663. doi: 10.1523/JNEUROSCI.23-29-09650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swensen AM, Bean BP. Robustness of burst firing in dissociated purkinje neurons with acute or long-term reductions in sodium conductance. J Neurosci. 2005;25:3509–3520. doi: 10.1523/JNEUROSCI.3929-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdakov D, Ashcroft FM. Cholecystokinin tunes firing of an electrically distinct subset of arcuate nucleus neurons by activating A-type potassium channels. J Neurosci. 2002;22:6380–6387. doi: 10.1523/JNEUROSCI.22-15-06380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salkoff L, et al. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7(12):921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- 9.Tseng-Crank J, et al. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 10.Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–834. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- 11.Zarei MM, et al. An endoplasmic reticulum trafficking signal prevents surface expression of a voltage- and Ca2+-activated K+ channel splice variant. Proc Natl Acad Sci USA. 2004;101:10072–10077. doi: 10.1073/pnas.0302919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poolos NP, Johnston D. Calcium-activated potassium conductances contribute to action potential repolarization at the soma but not the dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:5205–5212. doi: 10.1523/JNEUROSCI.19-13-05205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA, PKC. J Neurosci. 1998;18:3521–3528. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston D, Hoffman DA, Poolos NP. Potassium channels and dendritic function in hippocampal pyramidal neurons. Epilepsia. 2000;41:1072–1073. doi: 10.1111/j.1528-1157.2000.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 15.Glanzer J, et al. RNA splicing capability of live neuronal dendrites. Proc Natl Acad Sci USA. 2005;102:16859–16864. doi: 10.1073/pnas.0503783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, et al. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) α-subunits generated from a single site of splicing. J Biol Chem. 2005;280:33599–33609. doi: 10.1074/jbc.M505383200. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 18.McCullough RM, Cantor CR, Ding C. High-throughput alternative splicing quantification by primer extension and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Nucleic Acids Res. 2005;33:e99. doi: 10.1093/nar/gni098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDonald SH, et al. Increased large conductance calcium-activated potassium (BK) channel expression accompanied by STREX variant downregulation in the developing mouse CNS. BMC Dev Biol. 2006;6:37. doi: 10.1186/1471-213X-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misonou H, et al. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- 21.Mu Y, et al. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron. 2003;40:581–594. doi: 10.1016/s0896-6273(03)00676-7. [DOI] [PubMed] [Google Scholar]
- 22.Hille B. Ion Channels of Excitable Membranes. 3rd Ed. Sunderland, MA: Sinauer; 2001. p. xviii. [Google Scholar]
- 23.Shao LR, et al. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol. 1999;521:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas MJ, et al. Postsynaptic complex spike bursting enables the induction of LTP by theta frequency synaptic stimulation. J Neurosci. 1998;18:7118–7126. doi: 10.1523/JNEUROSCI.18-18-07118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du W, et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37:733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- 26.Brenner R, et al. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- 27.Cai X, et al. Unique roles of SK, Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Rutz B, Seraphin B. A dual role for BBP/ScSF1 in nuclear pre-mRNA retention and splicing. EMBO J. 2000;19:1873–1886. doi: 10.1093/emboj/19.8.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillman RT, Green RE, Brenner SE. An unappreciated role for RNA surveillance. Genome Biol. 2004;5:R8. doi: 10.1186/gb-2004-5-2-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaftel SS, et al. COX-3: A splice variant of cyclooxygenase-1 in mouse neural tissue and cells. Brain Res Mol Brain Res. 2003;119:213–215. doi: 10.1016/j.molbrainres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Lin CL, et al. Aberrant RNA processing in a neurodegenerative disease: The cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron. 1998;20:589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- 32.Galante PA, et al. Detection and evaluation of intron retention events in the human transcriptome. RNA. 2004;10:757–765. doi: 10.1261/rna.5123504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denis MM, et al. Escaping the nuclear confines: Signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, et al. An intron with a constitutive transport element is retained in a Tap messenger RNA. Nature. 2006;443:234–237. doi: 10.1038/nature05107. [DOI] [PubMed] [Google Scholar]
- 35.Job C, Eberwine J. Identification of sites for exponential translation in living dendrites. Proc Natl Acad Sci USA. 2001;98:13037–13042. doi: 10.1073/pnas.231485698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- 37.Konig H, et al. Splicing segregation: The minor spliceosome acts outside the nucleus and controls cell proliferation. Cell. 2007;131:718–729. doi: 10.1016/j.cell.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 38.Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- 39.Berkefeld H, et al. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- 40.Tian L, et al. A noncanonical SH3 domain binding motif links BK channels to the actin cytoskeleton via the SH3 adapter cortactin. FASEB J. 2006;20:2588–2590. doi: 10.1096/fj.06-6152fje. [DOI] [PubMed] [Google Scholar]
- 41.Brainard AM, et al. Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K+ current. Am J Physiol Cell Physiol. 2005;289:C49–C57. doi: 10.1152/ajpcell.00399.2004. [DOI] [PubMed] [Google Scholar]
- 42.Sausbier M, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci USA. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laumonnier F, et al. Association of a functional deficit of the BKCa channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. Am J Psychiatry. 2006;163:1622–1629. doi: 10.1176/ajp.2006.163.9.1622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.