Abstract
Although the fetal pineal gland does not secrete melatonin, the fetus is exposed to melatonin of maternal origin. In the non-human primate fetus, melatonin acts as a trophic hormone for the adrenal gland, stimulating growth while restraining cortisol production. This latter physiological activity led us to hypothesize that melatonin may influence some fetal functions critical for neonatal adaptation to extrauterine life. To test this hypothesis we explored (i) the presence of G-protein-coupled melatonin binding sites and (ii) the direct modulatory effects of melatonin on noradrenaline (norepinephrine)-induced middle cerebral artery (MCA) contraction, brown adipose tissue (BAT) lypolysis and ACTH-induced adrenal cortisol production in fetal sheep. We found that melatonin directly inhibits the response to noradrenaline in the MCA and BAT, and also inhibits the response to ACTH in the adrenal gland. Melatonin inhibition was reversed by the melatonin antagonist luzindole only in the fetal adrenal. MCA, BAT and adrenal tissue displayed specific high-affinity melatonin binding sites coupled to G-protein (Kd values: MCA 64 ± 1 pm, BAT 98.44 ± 2.12 pm and adrenal 4.123 ± 3.22 pm). Melatonin binding was displaced by luzindole only in the adrenal gland, supporting the idea that action in the MCA and BAT is mediated by different melatonin receptors. These direct inhibitory responses to melatonin support a role for melatonin in fetal physiology, which we propose prevents major contraction of cerebral vessels, restrains cortisol release and restricts BAT lypolysis during fetal life.
The pineal hormone melatonin is one of the few maternal hormones that crosses the placenta unaltered. The pineal production of melatonin starts postnataly in humans, sheep and rats (Deguchi, 1975; Nowak et al. 1990; Kennaway et al. 1992); nevertheless, passage of maternal melatonin to the fetal circulation exposes the fetus to a daily melatonin rhythm of low concentrations during the day and high concentrations at night (Yellon & Longo, 1988; McMillen & Nowak, 1989; Tamura et al. 2008). Recently, potential therapeutic uses of melatonin in perinatal medicine has been proposed to simulate a fetal-like environment in pre-term babies (Jan et al. 2007), and to prevent cerebral damage induced by fetal hypoxia based on experiments in sheep and rats (Okatani et al. 2000; Miller et al. 2005; Welin et al. 2007). However, the normal physiological role of melatonin and its full implication on fetal physiology and perinatal medicine is unknown.
Most of the studies on the effect of maternal melatonin in the fetus have been orientated towards chronobiotic actions of this hormone, providing circadian and seasonal time cues (Parraguez et al. 1996; Torres-Farfan et al. 2006a; Bellavía et al. 2006). In contrast, experimental evidence supporting other potential physiological functions of melatonin in fetal organ systems is limited. Some of the few studies available, using fetal capuchin monkeys, demonstrate that maternal melatonin has a trophic role on the fetal adrenal gland, stimulating growth while restraining cortisol production, thus limiting cortisol exposure to the fetus and early newborn (Torres-Farfan et al. 2004, 2006b) We speculate that maternal melatonin may influence additional fetal organ systems. These considerations led us to hypothesize that maternal melatonin plays a key role in the regulation of fetal organs that will have vital importance in the successful adaptation of the neonate to extra-uterine life.
To test this hypothesis, we chose to study direct effects of melatonin in two fetal sheep tissues critical for successful adaptation in postnatal life, brown adipose tissue (BAT), important for thermogenic function after delivery, and the adrenal gland, vital for fetal organ maturation elicited by cortisol at the end of fetal life. Additionally, we chose to assess the action of melatonin in sheep fetal cerebral arteries, fundamental vessels to preserve the integrity of the fetal central nervous system (reviewed by Pearce, 2006). Brown adipose tissue in sheep and humans is accrued, but not used during gestation. The action of noradrenaline, a main stimulus for newborn BAT lipolysis, is inhibited in utero by placental or maternal factors (Power et al. 2004). Lastly, the fetal adrenal gland produces increases in cortisol at term responsible for maturation of fetal organs, such as lungs in humans and other species (Liggins, 1976; Challis & Brooks, 1989) and induction of parturition in sheep (Liggins et al. 1973). In mammals, melatonin acts through two G-protein-coupled melatonin membrane receptor subtypes, MT1 and MT2, which are distributed in a number of adult and fetal tissues (Williams et al. 1991, 1997; Rivkees & Reppert, 1991; Helliwell & Williams, 1994; Drew et al. 1998; Thomas et al. 2002; Dubocovich & Markowska, 2005; Jimenez-Jorge et al. 2005). Most melatonin actions involve modulation of stimulatory responses (Dubocovich & Markowska, 2005). An important consideration in studying fetal sheep cerebral arteries, fetal BAT and fetal adrenal was the fact that we could follow direct effects of melatonin in response to known stimuli. In the present study, we investigated (i) the presence of G-protein-coupled melatonin binding sites in fetal sheep cerebral arteries, fetal BAT and fetal adrenal gland and (ii) direct modulatory effects of melatonin on noradrenaline-induced cerebral artery contraction and BAT lipolysis as well as on fetal adrenal cortisol production in response to ACTH.
Methods
Animals
Eight pregnant sheep were maintained in an open pen under a photoperiod of 12 h: 12 h (light: dark); food (dry alfalfa) and water were available ad libitum. At about 90% gestation (term 145 days), the mothers and fetuses were killled between 14.00 h and 16.00 h with an overdose of sodium thiopental (100 mg (kg of weight)−1), given in the maternal jugular vein). Fetuses (5 male and 3 female; age 132.3 ± 0.62 days of gestation, n = 8) were obtained by hysterotomy. The first branch of the middle cerebral arteries, the perirenal brown adipose tissue (BAT), and the adrenal glands were dissected in sterile conditions. Tissues from four fetuses were cut into pieces. A piece of BAT, adrenal and a piece of the middle cerebral artery were fixed by immersion in 4% paraformaldehyde–PBS for 1 h, cryoprotected with 30% sucrose and stored at –80°C for autoradiographic analysis. The remaining middle cerebral arteries, BAT and adrenal tissue from four fetuses were used fresh in ex vivo studies. Additional adrenal glands and BAT pieces from the other four 90% gestational fetuses were dissected to be used for membrane preparation. Other fetal tissues were dissected and incorporated into a Tissue Bank kept at −80°C. Procedures were in accordance with NIH Animal Care and Use Guidelines and were approved by the the Animal Experimentation Ethical Committee of the Facultad de Medicina, Universidad de Chile.
Materials
DMEM–F12, cocktail of protease inhibitors, melatonin, luzindole (MT1 and MT2 receptor antagonist), glycerol, guanosine 5′-[γ-thio]triphosphate (GTPγ-S; non-hydrolysable GTP analogue) and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St Louis, MO, USA). Noradrenaline (norepinephrine; Levofed, 1 mg ml−1) was purchased from Hospira Inc. (Mcpherson, KS, USA).
1–24 ACTH (Cortrosyn) was purchased from Organon Laboratories (Oss, Holland). Superfrost slides were purchased from Thomas (Swedesboro, NJ, USA). 2-[125I]iodomelatonin (specific activity, 2200 Ci mmol−1) was purchased from NEN Life Science Products (Boston, MA, USA). Borosilicate microfibre membrane filters (pore size: 1 μm, GC50) was purchased from Advantec MFS Inc. (Pleasanton, CA, USA). 125I-Hyperfilm was purchased from Amersham Pharmacia Biotech (Buckinghamshire, UK).
Experiments
1 Response to melatonin ex vivo
(a) Cerebral arteries
Ring segments of the middle cerebral arteries (2 mm long) were isolated and mounted in an isometric force transducer (model DSC 6; Kistler Morce, Seattle, WA, USA) and a displacement device in a myograph (dual-wire myograph; Danish Myo Technologies, Aarhus, Denmark) using two stainless steel wires (diameter: 40 μm). Mounting of the arteries was performed under a dissecting microscope. During mounting and experimentation, the myograph organ bath was filled with 10 ml Krebs–Henseleit buffer maintained at 39°C and aerated with 95% O2–5% CO2. Each ring artery was stretched to its individual optimal lumen diameter (250 ± 12.5 μm) as previously described (Ruijtenbeek et al. 2002; Villamor et al. 2002; Herrera et al. 2007). Duplicate arterial rings were exposed first for 2 min at different concentrations of KCl (4.75–125 mm), washing the segment with Krebs–Henseleit buffer before the next concentration was introduced. The maximal contraction was obtained at 50 mm of KCl (Fig. 1A). After, a cumulative concentration–response curve for noradrenaline (100 pm–100 mm) was performed. Maximal tension was obtained with 1 mm noradrenaline, and this dose was used to test the effects of 10 pm melatonin and 10 pm melatonin plus 1 μm luzindole (MT1 and MT2 melatonin receptor antagonist). The mean contraction values of arterial ring duplicates for each condition were calculated and expressed as percentage of maximal noradrenaline contraction.
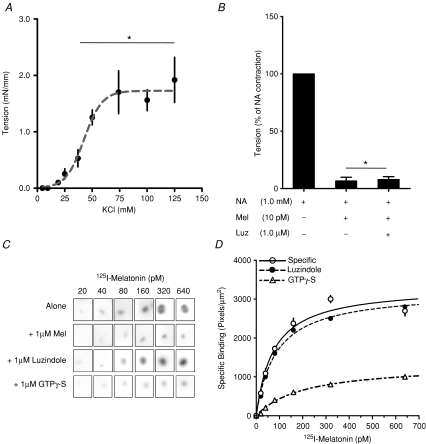
Figure 1. Detection of a functional melatonin receptor in sheep fetal cerebral arteries at 90% of gestation.
A, contractile response of cerebral arteries to potassium chloride (KCl). Values are means ±s.e.m. of tension. *Different to basal tension (P < 0.05; ANOVA and Newman–Keuls). B, effect of melatonin (Mel) on maximal contraction induced by 1 mm noradrenaline (NA) in presence or absence of luzindole (Luz). Values are percentage of maximal NA contraction. *Different to NA contraction (P < 0.05; ANOVA and Newman–Keuls). C, specific 2-[125I]iodomelatonin saturation binding by whole-mount autoradiography of cerebral artery rings sections exposed to increasing concentrations of 2-[125I]iodomelatonin. Each incubation condition (2-[125I]iodomelatonin alone, plus 1 μm cold melatonin; plus 1 μm luzindole and 1 μm GTPγ-S), is indicated in the figure. D, saturation isotherms of specific 2-[125I]iodomelatonin binding in cerebral arteries in presence of luzindole or GTPγ-S.
(b) Fetal BAT
BAT from individual fetuses was cut into small explants (about 25 mg), which were mixed and suspended in culture medium (D-MEM-F12). Triplicate explants were pre-incubated in culture medium for 6 h at 37°C and aerated with O2, then explants were incubated in triplicate for 6 h in 2 ml medium alone (basal) or containing 0.01, 0.1, 1 or 10 μm of noradrenaline. A second set of explants were incubated with 1 or 10 μm of noradrenaline in the presence or absence of 1 or 10 nm melatonin and in the presence or absence of 1 μm luzindole. After incubation, the medium was collected, explants were weighed and lipolysis was determined, measuring the glycerol present in the supernatant fraction by the glycerol oxidase method using a working reagent prepared by Valtek Diagnostics (Santiago, Chile) containing glycerolkinase, glycerolphosphate oxidase, peroxidase and ATP. The samples (10 μl) were incubated for 10 min at 37°C with 500 μl of working reagent and the absorbance was read at 520 nm in a Thermospectronic Genesis 10 UV (Waltham, MA, USA). The range of the standard curve was 65–1040 ng tube−1 of glycerol standard. Coefficients of variation for inter- and intra-assays were less than 5%. Production of glycerol was calculated as μg (mg of tissue)−1 and expressed as percentage production by basal explants.
(c) Fetal adrenal
Fetal adrenal gland explants were prepared as described before (Torres-Farfan et al. 2003) with slight modifications. In brief, fetal adrenal glands from individual fetuses were cut into small explants which were mixed, suspended and pre-incubated for 6 h in culture medium (D-MEM–F12) aerated with 95% O2–5% CO2. Explants were then incubated in triplicate for 12 h in 0.5 ml medium alone (basal) or containing 0.1, 1, 10 or 100 nm of 1–24 ACTH. In a second set of experiments, 10 nm of ACTH were incubated in the presence or absence of 1 or 10 nm melatonin and in the presence or absence of 1 μm luzindole. After incubation, the medium was collected, the explants were weighed and cortisol in culture medium was measured by radioimmunoassay (RIA) as described previously (Torres-Farfan et al. 2006a). The range of the standard curve was 12.5–800 pg tube−1. Culture medium was diluted in 0.1% gelatin PBS (pH 7.0) buffer to fit into the standard curve. The inter- and intra-assay coefficients were less than 10%. Production of cortisol was calculated as ng (mg of tissue)−1 and expressed as percentage production by basal explants.
2 Identification of specific binding sites for melatonin
We searched for specific melatonin binding sites in fetal cerebral arteries, BAT and fetal adrenals by whole-mount quantitative autoradiography following the procedure of Capsoni et al. (1994), with slight modifications. In addition, we examined melatonin binding in fetal sheep BAT and adrenal preparations (see below).
Middle cerebral arteries, adrenal and BAT frozen sections (20 μm) were thaw-mounted on superfrost slides. Sections were stored at −70°C until processed. Tissue sections were pre-incubated with Tris-Ca buffer for 15 min at 37°C and then with 600 μl of 20–640 pm 2-[125I]iodomelatonin for 90 min at 37°C. Non-specific binding was determined in adjacent sections incubated in the presence of 1 μm melatonin. To investigate whether the binding sites were coupled to G-protein, we incubated simultaneously a third group of sections with 1 μm GTPγ-S. We also tested the effect of 1 μm luzindole upon the binding of 2-[125I]iodomelatonin. After incubation, the sections were washed 5 times with Tris-Ca buffer and dried at room temperature. The sections were left in contact with 125I-Hyperfilm in an X-ray cassette overnight at −70°C. After exposure, the films were developed using a Kodak image station XP-100 system. Each autoradiographic image was scanned using a digital scanner (AGFA Snapscan E10, Mortsel Belgium), then the optical density (pixels mm−2) of a square of fixed size comprising most of the section was measured with the software Scion Image (ScionCorporation, http://www.scioncorp.com). The maximum number of 2-[125I]iodomelatonin binding sites (Bmax) and dissociation constant (Kd) were determined by Scatchard analysis using GraphPad Prism 4.00 (GraphPad Software, Inc. San Diego, CA, USA).
Membranes from BAT and adrenal glands were prepared by homogenizing the tissues in 20 vol of ice-cold Tris buffer (0.1 m, pH 7.4), containing a 0.1 m cocktail of protease inhibitors. The homogenates were centrifuged at 8000 g for 30 min at 4°C, the pellets were discarded and the supernatants were centrifuged for 90 min at 40 000 g at 4°C. The membrane pellet was resuspended by sonication in Tris-Ca buffer (25 mm Tris-HCl, 25 mm CaCl2, 0.2% BSA, pH 7.5). Protein concentration was measured by spectrophotometry at 280 nm using 1 mg ml−1 albumin solution as standard. Triplicate aliquots of membrane preparations (250 μg of protein) were incubated at 37°C for 90 min with 25–400 pm 2-[125I]iodomelatonin, in the presence or absence of 1 μm melatonin, in a final volume of 200 μl. The reaction was stopped by adding ice-cold Tris-Ca buffer (2 ml) and the membranes were separated by immediately filtering through borosilicate microfibre membrane filters. The amount of 2-[125I]iodomelatonin retained in the filter was measured in a gammacounter. Specific binding was calculated by substracting the non-specific binding from the total binding. We tested the effect of luzindole (MT1 and MT2 receptor antagonist) and of GTPγ-S upon 2-[125I]iodomelatonin binding. The Bmax and Kd were determined by Scatchard analysis using GraphPad Prism 4.00.
Data analysis
Data are expressed as mean ±s.e.m. Differences between means were calculated by ANOVA and Newman–Keuls test. Data in percentage was transformed to arcsine prior to analysis. Statistical analyses were performed using GraphPad Prism 4.00. Differences were considered significant when P < 0.05.
Results
Detection of a functional melatonin receptor in sheep fetal tissues
The three fetal tissues studied – middle cerebral arteries, BAT and fetal adrenal – presented a functional melatonin receptor, coupled to G-protein. However, differences in the ability to recognize luzindole suggest that melatonin acts on a similar receptor in cerebral arteries and BAT, but different from the one detected in the fetal adrenal.
(a) Cerebral arteries
Fetal sheep middle cerebral arteries contracted in response to increasing doses of potassium and noradrenaline, as reported by others (Pearce et al. 1999; Bishai et al. 2002). For noradrenaline, the maximal contraction was obtained at the 1 mm dose, and was similar to the maximal contraction induced with KCl (P < 0.05 ANOVA and Newman–Keuls; shown in Fig. 1A). The addition of melatonin inhibited the contraction induced by 1 mm noradrenaline, relaxing the vessel to the tension present in the basal condition (P < 0.05 ANOVA and Newman–Keuls). Addition of the MT1 and MT2 antagonist luzindole did not reverse the effect of melatonin (Fig. 1B). Neither melatonin nor luzindole changed basal contraction and the arteries recovered contractile capacity to KCL after washing out melatonin (not shown).
Cerebral arteries displayed a single specific binding site for melatonin with a Kd of 64.1 ± 9.8 pm, which was displaced by GTPγ-S, indicating a membrane-bound site coupled to G-protein. However, the 2-[125I]iodomelatonin binding was not displaced by luzindole (Fig. 1C and D).
(b) Brown adipose tissue (BAT)
Fetal BAT explants responded to increasing doses of noradrenaline by augmenting glycerol release (Fig. 2A), as shown by others in dispersed sheep perirenal fat adipocytes (Fain et al. 1984). The response to noradrenaline was maximal at 10 μm noradrenaline (P < 0.05 ANOVA and Newman–Keuls). Addition of 1 nm or 10 nm of melatonin decreased the glycerol release stimulated by 1.0 μm (data no shown) and 10.0 μm noradrenaline (P < 0.05 ANOVA and Newman-Keuls, Fig. 2B). The inhibitory effect of melatonin on noradrenaline-stimulated glycerol release was not reversed by luzindole. Neither melatonin (not shown) nor luzindole changed the basal glycerol production (Fig. 2B).
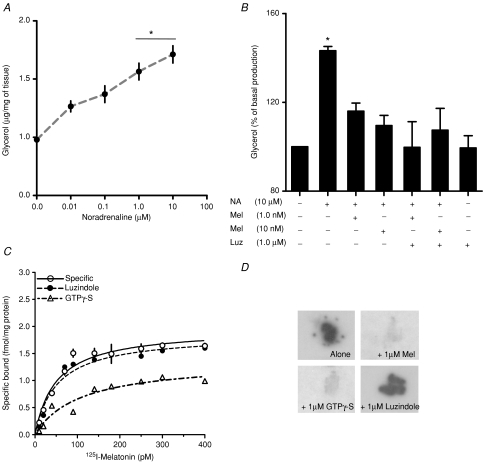
Figure 2. Detection of a functional melatonin receptor in sheep fetal brown adipose tissue at 90% of gestation.
A, glycerol response of BAT explants in culture to increasing concentrations of noradrenaline (NA). Values are means ±s.e.m. (μg (mg of tissue)−1). *Different to basal production (P < 0.05; ANOVA and Newman–Keuls). B, effect of melatonin on maximal response to noradrenaline in presence or absence of luzindole (Luz). Values are percentage of basal production. *Different to basal production (P < 0.05; ANOVA and Newman–Keuls). C, saturation isotherms of specific 2-[125I]iodomelatonin binding in BAT membrane preparation in presence of luzindole or GTPγ-S. D, specific 50 pm 2-[125I]iodomelatonin binding in serial BAT sections by whole-mount autoradiography. Each incubation condition (2-[125I]iodomelatonin alone, plus 1 μm cold melatonin; plus 1 μm luzindole and 1 μm GTPγ-S), is indicated in the figure.
Fetal BAT membrane preparations displayed specific binding of 2-[125I]iodomelatonin (Fig. 2C) with a Kd of 44.2 ± 12.4 pm and Bmax of 2.0 ± 0.2 fmol (mg of tissue)−1. This Kd was also confirmed by quantitative autoradiography, which gave a Kd of 36.9 ± 6.3 (not shown). Figure 2D shows a representative example of 2-[125I]iodomelatonin binding in BAT sections incubated with 2-[125I]iodomelatonin; 2-[125I]iodomelatonin plus melatonin; 2-[125I]iodomelatonin plus luzindole and 2-[125I]iodomelatonin plus GTPγ-S obtained at 50 pm 2-[125I]iodomelatonin (concentration close to Kd). As shown in the figure, 2-[125I]iodomelatonin binding was displaced by GTPγ-S, indicating a membrane-bound site coupled to G-protein. However, the 2-[125I]iodomelatonin binding was not displaced by the MT1 and MT2 melatonin antagonist luzindole. Fetal BAT 2-[125I]iodomelatonin binding was displaced by 1 μm melatonin and 1 μm GTPγ-S, but not by luzindole (Fig. 2D).
(c) Fetal adrenal gland
Sheep fetal adrenal gland explants responded to ACTH by increasing cortisol production as reported by others (Wintour et al. 1975; Su et al. 2005) (Fig. 3A). The maximal response to ACTH was found at 10 nm ACTH with respect to basal production (P < 0.05 ANOVA and Newman–Keuls). Addition of melatonin (1.0 or 10 nm) to the culture selectively inhibited the increase in cortisol production induced by 10 nm ACTH. The inhibitory effect of melatonin on ACTH-stimulated cortisol production was reversed by 1 μm luzindole (P < 0.05 ANOVA and Newman–Keuls; Fig. 3B). Neither melatonin (not shown) nor luzindole changed basal cortisol production (Fig. 3B).
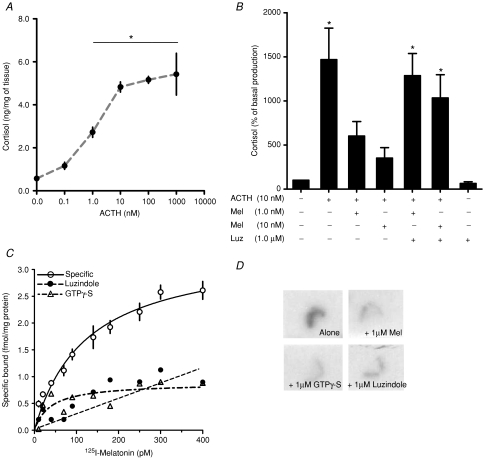
Figure 3. Detection of a functional melatonin receptor in sheep fetal adrenal gland at 90% of gestation.
A, cortisol response of adrenal explants in culture to increasing concentrations of ACTH. Values are means ±s.e.m. (ng (mg of tissue)−1). *Different to basal production (P < 0.05; ANOVA and Newman–Keuls). B, effect of melatonin on maximal response to ACTH in presence or absence of luzindole (Luz). Values are percentage of basal production. *Different to basal production (P < 0.05; ANOVA and Newman–Keuls). C, saturation isotherms of specific 2-[125I]iodomelatonin binding in adrenal membrane preparation in presence of luzindole or GTPγ-S. D, specific 125 pm 2-[125I]iodomelatonin binding in serial fetal adrenal sections by whole-mount autoradiography. Each incubation condition (2-[125I]iodomelatonin alone, plus 1 μm cold melatonin; plus 1 μm luzindole and 1 μm GTPγ-S) is indicated in the figure.
Fetal adrenal membrane preparations displayed specific binding of 2-[125I]iodomelatonin (Fig. 3C) with a Kd of 123.3 ± 22.9 pm and Bmax of 3.4 ± 0.34 fmol (mg of tissue)−1. This Kd was also confirmed by quantitative autoradiography, which gave a Kd of 98.2 ± 28.0 (not shown). Adrenal gland membrane preparations specific to 2-[125I]iodomelatonin binding were displaced by GTPγ-S and the antagonist luzindole as shown in Fig. 3C. Figure 3D shows a representative example of 2-[125I]iodomelatonin binding in adrenal sections with 2-[125I]iodomelatonin; 2-[125I]iodomelatonin plus melatonin; 2-[125I]iodomelatonin plus luzindole and 2-[125I]iodomelatonin plus GTPγ-S obtained at 125 pm 2-[125I]iodomelatonin (concentration close to Kd). Contact autoradiography shows that specific binding was restricted to the adrenal cortex and confirms that it is displaceable by GTPγ-S or luzindole (Fig. 3D).
Discussion
The unusual profile of melatonin in fetal circulation, provided by the mother during fetal life – and its absence in the early newborn – led us to formulate the hypothesis that maternal melatonin contributes to modulate functions important in fetal physiology or in preparation for competence of the newborn. Melatonin receptors have been identified in several fetal tissues of a variety of species (Williams et al. 1991, 1997; Rivkees & Reppert, 1991; Helliwell & Williams, 1994; Drew et al. 1998; Thomas et al. 2002; Jimenez-Jorge et al. 2005); with exception made of the capuchin fetal adrenal gland (Torres-Farfan et al. 2004; Torres-Farfan et al. 2006b), direct melatonin actions in fetal tissues have not been assessed to date. In the fetal sheep, circulating melatonin concentration ranges from about 0.15 nm in the day time to 0.30 nm at night time (Yellon & Longo, 1988; McMillen & Nowak, 1989; Houghton et al. 1995). In the present study, melatonin concentrations in the nanomolar range or lower have direct inhibitory effects: (a) in the middle fetal sheep cerebral arteries and the fetal sheep brown adipose tissue response to noradrenaline and (b) in the fetal sheep adrenal gland response to ACTH. We found specific melatonin binding sites in these three tissues that could be mediating the observed melatonin actions.
In vivo, cerebral arteries play an active role in the auto-regulation of blood flow to the brain in response to changes in metabolic demands such as brain growth and development, as well as changes in maternal oxygen supply to the fetus (reviewed by Pearce, 2006). In vivo and in vitro, noradrenaline concentration in the micromolar range, acting through an α1 adrenergic receptor, contracts fetal sheep cerebral arteries (Wagerle et al. 1990; Pearce et al. 1999; Bishai et al. 2002). In the present experiments, maximal cerebral artery contraction was obtained at the noradrenaline doses used in the former studies. Low picomolar melatonin concentration inhibited noradrenaline-induced contraction, an effect that was not blocked by the non-selective MT1 and MT2 melatonin receptor antagonist luzindole (Dubocovich & Markowska, 2005). Utilizing quantitative whole-mount autoradiography (Capsoni et al. 1994), we identified a specific high-affinity binding site for 2-[125I]iodomelatonin in fetal sheep cerebral arteries that was displaced by GTP-γS and not by luzindole, suggesting that this site mediates the inhibitory effect of melatonin on noradrenaline-induced contraction. Our finding is the first description of a functional melatonin receptor in fetal cerebral vessels. However, the lack of displacement by luzindole suggests that this site differs from the high-affinity melatonin receptors identified as MT1 by RT-PCR described in cerebral blood vessels from adult rats and bovines (Viswanathan et al. 1990; Capsoni et al. 1994; Chucharoen et al. 2003, 2007).
The brown adipose tissue (BAT), accrued during gestation, is a thermogenic tissue important for the survival of sheep and human newborns. The action of noradrenaline, a main stimulus for newborn thermogenesis, is inhibited in utero by placental or maternal factors (Power et al. 2004). Perirenal brown adipose tissue from fetal and newborn lambs as well as BAT from adults of other species is under sympathetic innervations (Gemmell et al. 1972; Alexander & Stevens, 1980; Cannon & Nedergaard, 2004). Noradrenaline interacting with β adrenergic receptors activates triacylglycerol lipase, releasing glycerol and free fatty acids (Cannon & Nedergaard, 2004). In late gestation, dispersed fetal sheep BAT adipocytes, noradrenaline and isoproterenol (isoprenaline) stimulate cyclic adenosine monophosphate (cAMP) accumulation and lipolysis (Fain et al. 1984) as well as mitochondrial respiration (Klein et al. 1983). In the present study, increasing concentrations of noradrenaline augmented glycerol production in fetal BAT explants in a dose-dependent manner, reaching a maximal effect with 10 μm noradrenaline, as reported for lypolysis and mitochondrial respiration (Klein et al. 1983; Fain et al. 1984). One nanomolar of melatonin (in the range of fetal night time melatonin concentration) inhibited noradrenaline-stimulated glycerol production but this effect was not reversed by luzindole. As found for fetal sheep cerebral arteries, fetal sheep BAT membranes and BAT sections showed a specific high-affinity 2-[125I]iodomelatonin binding associated to G-protein and was not displaceable by luzindole. Most probably this site mediates the inhibitory actions of melatonin in BAT, given the inability of luzindole to block melatonin inhibition of noradrenaline-stimulated lipolysis just discussed. The present study is the first description of a functional melatonin receptor in fetal BAT. However, as found for the fetal sheep cerebral vessels, this site differs from the functional melatonin receptors (in this case MT2) described in adult rat brown and white adipocytes and in adipose tissue cell lines (Zalatan et al. 2001; Brydon et al. 2001; Zwirska-Korczala et al. 2005).
Lastly, the sheep fetal adrenal gland shows a complex temporal pattern of longitudinal changes in function, being very responsive to ACTH early in gestation, then loosing this responsivity at mid-gestation to become very sensitive at term (Wintour et al. 1975; Llanos et al. 1979; Su et al. 2005), thus producing the major increase in cortisol during the last days of pregnancy, responsible for maturation of fetal organs, such as lungs in human and other species (Liggins, 1976) and induction of parturition in sheep (Liggins et al. 1973). In the present study, the fetal adrenal gland explants showed the robust response to ACTH described by others after 130 days of gestation (Wintour et al. 1975; Su et al. 2005). Melatonin inhibited ACTH-stimulated cortisol production, an effect that was completely reversed by luzindole. Fetal adrenal gland membranes and sections displayed a specific binding site for 2-[125I]iodomelatonin that was coupled to G-protein and was displaceable by luzindole, in contrast to the receptors found in the middle cerebral arteries and BAT. Autoradiography suggests that the site is located in the adrenal cortex. Using the primers reported by Migaud et al. (2005) we obtained preliminary data indicating expression of the MT1 melatonin receptor mRNA in the sheep fetal adrenal. Direct inhibitory effects of melatonin on ACTH-induced cortisol production mediated by the MT1 melatonin receptor have been shown in capuchin monkey fetal and adult adrenal and in the adult rat adrenal (Torres-Farfan et al. 2004, 2003; Richter et al. 2008). Altogether, the present data suggest the presence of a functional melatonin receptor in the fetal sheep adrenal gland cortex, with similar characteristics to that reported in fetal and adult adrenal of other species, that restrains the cortisol response to ACTH and may be involved in other aspects of fetal adrenal physiology as reported in the fetal capuchin monkey (Torres-Farfan et al. 2004, 2006b).
The inhibitory effects of melatonin in fetal cerebral arteries and fetal BAT support the presence of functional melatonin membrane receptors in these tissues. However, it is important to emphasize that the effects of melatonin in fetal middle cerebral arteries and in BAT seem to be mediated by a specific melatonin binding site of high affinity different from the classical MT1 and MT2 sites reported in the corresponding adult tissues of other species. The issue is complicated further by recent evidence that the MT2 receptor may not be expressed at all in sheep since the deduced aminoacid sequence of exon 2 of the receptor by genomic analysis suggests that the gene may encode a defective protein (Xiao et al. 2007). From the present data, we cannot establish a new type of melatonin receptor in fetal BAT and fetal cerebral arteries, which could be more appropriate to function during the fetal life in these tissues, but certainly the melatonin-binding site present in the cerebral arteries and BAT requires further investigation.
There is increasing interest in the potential therapeutic use of melatonin in perinatal medicine. Recent work shows that melatonin either given to the mother or to the fetus prevents cerebral damage induced by hypoxia in sheep and rats (Okatani et al. 2000; Miller et al. 2005; Welin et al. 2007) and the idea of melatonin treatment in pre-term babies is being put forward (Jan et al. 2007). Altogether, the results from the present study are the first demonstration of direct inhibitory actions of melatonin on noradrenaline-stimulated fetal cerebral artery contraction and release of glycerol by BAT and in ACTH-induced secretion of cortisol by the sheep fetal adrenal. In human and rats, the nocturnal concentration of maternal melatonin increases with advancing gestation (reviewed by Tamura et al. 2008). Extrapolated to fetal physiology, the daily passage of maternal melatonin to the fetal circulation would curtail contraction of cerebral vessels, release of cortisol before its time and by preventing lipolysis, would favour the accumulation of BAT during fetal life. The modulatory inhibitory responses to melatonin demonstrated in the present study support actions of melatonin that need to be investigated further to establish the full implication on fetal physiology and perinatal medicine.
Acknowledgments
We are very grateful to Renato Ebensperger (Veterinarian), and Carlos Brito for expert animal care, and to Ms Auristela Rojas for assistance in RIAs. We thank Ms Monica Prizant-Mele and Dr Joseph A. Mele III, for editorial assistance. This work was supported by Grant 1060-766 from Fondo Nacional de Desarrollo Científico y Tecnológico, Chile and a grant from The Department of Women's Health, Arrowhead Regional Medical Center (CA, USA). F.J.V. was supported by a PhD Fellowship from Comite Nacional de Ciencia y Tecnologia, Chile. M.M. was supported by a PhD Fellowship from Programa de Mejoramiento de la Calidad de la Educacion Superior, UCH313.
References
- Alexander G, Stevens D. Sympathetic innervation and the development of structure and function of brown adipose tissue: studies on lambs chemically sympathectomized in utero with 6-hydroxydopamine. J Dev Physiol. 1980;2:119–137. [PubMed] [Google Scholar]
- Bellavía SL, Carpentieri AR, Vaqué AM, Macchione AF, Vermouth NT. Pup circadian rhythm entrainment – effect of maternal ganglionectomy or pinealectomy. Physiol Behav. 2006;89:342–349. doi: 10.1016/j.physbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Bishai JM, Penninga L, Nijland R, Meulenaar R, Gheorghe CP, Zhao Y, Buchholz JN, Zhang L, Longo LD. Pre- and postjunctional alpha (2) -adrenergic receptors in fetal and adult ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1654–R1662. doi: 10.1152/ajpregu.00475.2001. [DOI] [PubMed] [Google Scholar]
- Brydon L, Petit L, Delagrange P, Strosberg AD, Jockers R. Functional expression of MT2 (Mel1b) melatonin receptors in human PAZ6 adipocytes. Endocrinology. 2001;142:4264–4271. doi: 10.1210/endo.142.10.8423. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Capsoni S, Viswanathan M, De Oliveira AM, Saavedra JM. Characterization of melatonin receptors and signal transduction system in rat arteries forming the circle of Willis. Endocrinology. 1994;135:373–378. doi: 10.1210/endo.135.1.8013371. [DOI] [PubMed] [Google Scholar]
- Challis JR, Brooks AN. Maturation and activation of hypothalamic-pituitary adrenal function in fetal sheep. Endocr Rev. 1989;10:182–204. doi: 10.1210/edrv-10-2-182. [DOI] [PubMed] [Google Scholar]
- Chucharoen P, Chetsawang B, Putthaprasart C, Srikiatkhachorn A, Govitrapong P. The presence of melatonin receptors and inhibitory effect of melatonin on hydrogen peroxide-induced endothelial nitric oxide synthase expression in bovine cerebral blood vessels. J Pineal Res. 2007;43:35–41. doi: 10.1111/j.1600-079X.2007.00440.x. [DOI] [PubMed] [Google Scholar]
- Chucharoen P, Chetsawang B, Srikiatkhachorn A, Govitrapong P. Melatonin receptor expression in rat cerebral artery. Neurosci Lett. 2003;341:259–261. doi: 10.1016/s0304-3940(03)00214-3. [DOI] [PubMed] [Google Scholar]
- Deguchi T. Ontogenesis of a biological clock for serotonin: acetyl coenzyme A N-acetyltransferase in pineal gland of rat. Proc Natl Acad Sci U S A. 1975;72:2814–2818. doi: 10.1073/pnas.72.7.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew JE, Williams LM, Hannah LT, Barrett P, Abramovich DR. Melatonin receptors in the human fetal kidney: 2-[125I]iodomelatonin binding sites correlated with expression of Mel1a and Mel1b receptor genes. J Endocrinol. 1998;156:261–267. doi: 10.1677/joe.0.1560261. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- Fain JN, Mohell N, Wallace MA, Mills I. Metabolic effects of beta, alpha 1, and alpha 2 adrenoceptor activation on brown adipocytes isolated from the perirenal adipose tissue of fetal lambs. Metabolism. 1984;33:289–294. doi: 10.1016/0026-0495(84)90052-0. [DOI] [PubMed] [Google Scholar]
- Gemmell RT, Bell AW, Alexander G. Morphology of adipose cells in lambs at birth and during subsequent transition of brown to white adipose tissue in cold and in warm conditons. Am J Anat. 1972;133:143–164. doi: 10.1002/aja.1001330203. [DOI] [PubMed] [Google Scholar]
- Helliwell RJ, Williams LM. The development of melatonin-binding sites in the ovine fetus. J Endocrinol. 1994;142:475–484. doi: 10.1677/joe.0.1420475. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Pulgar VM, Riquelme RA, Sanhueza EM, Reyes RV, Ebensperger G, Parer JT, Valdéz EA, Giussani DA, Blanco CE, Hanson MA, Llanos AJ. High-altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2234–R2240. doi: 10.1152/ajpregu.00909.2006. [DOI] [PubMed] [Google Scholar]
- Houghton DC, Young IR, McMillen IC. Evidence for hypothalamic control of the diurnal rhythms in prolactin and melatonin in the fetal sheep during late gestation. Endocrinology. 1995;136:218–223. doi: 10.1210/endo.136.1.7828534. [DOI] [PubMed] [Google Scholar]
- Jan JE, Wasdell MB, Freeman RD, Bax M. Evidence supporting the use of melatonin in short gestation infants. J Pineal Res. 2007;42:22–27. doi: 10.1111/j.1600-079X.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- Jimenez-Jorge S, Jimenez-Caliani AJ, Guerrero JM, Naranjo MC, Lardone PJ, Carrillo-Vico A, Osuna C, Molinero P. Melatonin synthesis and melatonin-membrane receptor (MT1) expression during rat thymus development: role of the pineal gland. J Pineal Res. 2005;39:77–83. doi: 10.1111/j.1600-079X.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ, Stamp GE, Goble FC. Development of melatonin production in infants and the impact of prematurity. J Clin Endocrinol Metab. 1992;75:367–369. doi: 10.1210/jcem.75.2.1639937. [DOI] [PubMed] [Google Scholar]
- Klein AH, Reviczky A, Chou P, Padbury J, Fisher DA. Development of brown adipose tissue thermogenesis in the ovine fetus and newborn. Endocrinology. 1983;112:1662–1666. doi: 10.1210/endo-112-5-1662. [DOI] [PubMed] [Google Scholar]
- Liggins GC. Adrenocortical-related maturational events in the fetus. Am J Obstet Gynecol. 1976;126:931–941. doi: 10.1016/0002-9378(76)90680-3. [DOI] [PubMed] [Google Scholar]
- Liggins GC, Fairclough RJ, Grieves SA, Kendall JZ, Knox BS. The mechanism of initiation of parturition in the ewe. Recent Prog Horm Res. 1973;29:111–159. doi: 10.1016/b978-0-12-571129-6.50007-5. [DOI] [PubMed] [Google Scholar]
- Llanos AJ, Ramachandran J, Creasy RK, Rudolph AM, Serón-Ferré M. alpha-Melanocyte-stimulating hormone and adrenocorticotropin in the regulation of glucocorticoid secretion during the perinatal period in sheep. Endocrinology. 1979;105:613–617. doi: 10.1210/endo-105-3-613. [DOI] [PubMed] [Google Scholar]
- Mcmillen IC, Nowak R. Maternal pinealectomy abolishes the diurnal rhythm in plasma melatonin concentrations in the fetal sheep and pregnant ewe during late gestation. J Endocrinol. 1989;120:459–464. doi: 10.1677/joe.0.1200459. [DOI] [PubMed] [Google Scholar]
- Migaud M, Daveau A, Malpaux B. MTNR1A melatonin receptors in the ovine premammillary hypothalamus: day-night variation in the expression of the transcripts. Biol Reprod. 2005;72:393–398. doi: 10.1095/biolreprod.104.030064. [DOI] [PubMed] [Google Scholar]
- Miller SL, Yan EB, Castillo-Meléndez M, Jenkin G, Walker DW. Melatonin provides neuroprotection in the late-gestation fetal sheep brain in response to umbilical cord occlusion. Dev Neurosci. 2005;27:200–210. doi: 10.1159/000085993. [DOI] [PubMed] [Google Scholar]
- Nowak R, Young IR, Mcmillen IC. Emergence of the diurnal rhythm in plasma melatonin concentrations in newborn lambs delivered to intact or pinealectomized ewes. J Endocrinol. 1990;125:97–102. doi: 10.1677/joe.0.1250097. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Wakatsuki A, Kaneda C. Melatonin increases activities of glutathione peroxidase and superoxide dismutase in fetal rat brain. J Pineal Res. 2000;28:89–96. doi: 10.1034/j.1600-079x.2001.280204.x. [DOI] [PubMed] [Google Scholar]
- Parraguez VH, Valenzuela GJ, Vergara M, Ducsay CA, Yellon SM, Serón-Ferré M. Effect of constant light on fetal and maternal prolactin rhythms in sheep. Endocrinology. 1996;137:2355–2361. doi: 10.1210/endo.137.6.8641186. [DOI] [PubMed] [Google Scholar]
- Pearce W. Hypoxic regulation of the fetal cerebral circulation. J Appl Physiol. 2006;100:731–738. doi: 10.1152/japplphysiol.00990.2005. [DOI] [PubMed] [Google Scholar]
- Pearce WJ, Duckles SP, Buchholz J. Effects of maturation on adrenergic neurotransmission in ovine cerebral arteries. Am J Physiol Integr Comp Physiol. 1999;277:R931–R937. doi: 10.1152/ajpregu.1999.277.4.R931. [DOI] [PubMed] [Google Scholar]
- Power GG, Blood AB, Hunter CJ. Perinatal Thermal Physiology. In: Polin RA, Fox WW, Abman SH, editors. Fetal and Neonatal Physiology. 3. Philadelphia PA USA: WB. Saunders Co; 2004. pp. 541–548. [Google Scholar]
- Richter HG, Torres-Farfan C, Garcia-Sesnich J, Abarzua-Catalan L, Henriquez MG, Alvarez-Felmer M, Gaete F, Rehren GE, Seron-Ferre M. Rhythmic expression of functional MT1 melatonin receptors in the rat adrenal gland. Endocrinology. 2008;149:995–1003. doi: 10.1210/en.2007-1009. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Reppert SM. Appearance of melatonin receptors during embryonic life in Siberian hamsters (Phodopus sungorous) Brain Res. 1991;568:345–349. doi: 10.1016/0006-8993(91)91424-y. [DOI] [PubMed] [Google Scholar]
- Ruijtenbeek K, Kessels CG, Villamor E, Blanco CE, De Mey JG. Direct effects of acute hypoxia on the reactivity of peripheral arteries of the chicken embryo. Am J Physiol Regul Integr Comp Physiol. 2002;283:R331–R338. doi: 10.1152/ajpregu.00675.2001. [DOI] [PubMed] [Google Scholar]
- Su Y, Carey LC, Valego NK, Rose JC. Developmental changes in adrenocorticotrophin (ACTH) -induced expression of ACTH receptor and steroid acute regulatory protein mRNA in ovine fetal adrenal cells. J Soc Gynecol Invest. 2005;12:416–420. doi: 10.1016/j.jsgi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Tamura H, Nakamura Y, Terron MP, Flores LJ, Manchester LC, Tan DX, Sugino N, Reiter RJ. Melatonin and pregnancy in the human. Reprod Toxicol. 2008 doi: 10.1016/j.reprotox.2008.03.005. DOI 10.1016/j.reprotox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Thomas L, Purvis CC, Drew JE, Abramovich DR, Williams LM. Melatonin receptors in human fetal brain: 2-[125, I]iodomelatonin binding and MT1 gene expression. J Pineal Res. 2002;33:218–224. doi: 10.1034/j.1600-079x.2002.02921.x. [DOI] [PubMed] [Google Scholar]
- Torres-Farfan C, Richter HG, Germain AM, Valenzuela GJ, Campino C, Rojas-García P, Forcelledo ML, Torrealba F, Serón-Ferré M. Maternal melatonin selectively inhibits cortisol production in the primate fetal adrenal gland. J Physiol. 2004;554:841–856. doi: 10.1113/jphysiol.2003.056465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Farfan C, Richter HG, Rojas-García P, Vergara M, Forcelledo ML, Valladares LE, Torrealba F, Valenzuela GJ, Serón-Ferré M. mt1 Melatonin receptor in the primate adrenal gland: inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J Clin Endocrinol Metab. 2003;88:450–458. doi: 10.1210/jc.2002-021048. [DOI] [PubMed] [Google Scholar]
- Torres-Farfan C, Rocco V, Monsó C, Valenzuela FJ, Campino C, Germain A, Torrealba F, Valenzuela GJ, Seron-Ferre M. Maternal melatonin effects on clock gene expression in a nonhuman primate fetus. Endocrinology. 2006a;147:4618–4626. doi: 10.1210/en.2006-0628. [DOI] [PubMed] [Google Scholar]
- Torres-Farfan C, Valenzuela FJ, Germain AM, Viale ML, Campino C, Torrealba F, Valenzuela GJ, Richter HG, Serón-Ferré M. Maternal melatonin stimulates growth and prevents maturation of the capuchin monkey fetal adrenal gland. J Pineal Res. 2006b;41:58–66. doi: 10.1111/j.1600-079X.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- Villamor E, Ruijtenbeek K, Pulgar V, De Mey JG, Blanco CE. Vascular reactivity in intrapulmonary arteries of chicken embryos during transition to ex ovo life. Am J Physiol Regul Integr Comp Physiol. 2002;282:R917–R927. doi: 10.1152/ajpregu.00369.2001. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Laitinen JT, Saavedra JM. Expression of melatonin receptors in arteries involved in thermoregulation. Proc Natl Acad Sci U S A. 1990;87:6200–6203. doi: 10.1073/pnas.87.16.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagerle LC, Kurth CD, Roth RA. Sympathetic reactivity of cerebral arteries in developing fetal lamb and adult sheep. Am J Physiol. 1990;258:H1432–H1438. doi: 10.1152/ajpheart.1990.258.5.H1432. [DOI] [PubMed] [Google Scholar]
- Welin AK, Svedin P, Lapatto R, Sultan B, Hagberg H, Gressens P, Kjellmer I, Mallard C. Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr Res. 2007;61:153–158. doi: 10.1203/01.pdr.0000252546.20451.1a. [DOI] [PubMed] [Google Scholar]
- Williams LM, Hannah LT, Adam CL, Bourke DA. Melatonin receptors in red deer fetuses (Cervus elaphus) J Reprod Fertil. 1997;110:145–151. doi: 10.1530/jrf.0.1100145. [DOI] [PubMed] [Google Scholar]
- Williams LM, Martinoli MG, Titchener LT, Pelletier G. The ontogeny of central melatonin binding sites in the rat. Endocrinology. 1991;128:2083–2090. doi: 10.1210/endo-128-4-2083. [DOI] [PubMed] [Google Scholar]
- Wintour EM, Brown EH, Denton DA, Hardy KJ, McDougall JG, Oddie CJ, Whipp GT. The ontogeny and regulation of corticosteroid secretion by the ovine foetal adrenal. Acta Endocrinol (Copenh) 1975;79:301–316. doi: 10.1530/acta.0.0790301. [DOI] [PubMed] [Google Scholar]
- Xiao CT, Chu MX, Fu Y, Fang L, Ye SC. Analysis of polymorphism, structure and function of exon 2 of ovine melatonin receptor 1b gene: a clue as to why it lacks expression in sheep. J Pineal Res. 2007;42:97–104. doi: 10.1111/j.1600-079X.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Longo LD. Effect of maternal pinealectomy and reverse photoperiod on the circadian melatonin rhythm in the sheep and fetus during the last trimester of pregnancy. Biol Reprod. 1988;39:1093–1099. doi: 10.1095/biolreprod39.5.1093. [DOI] [PubMed] [Google Scholar]
- Zalatan F, Krause JA, Blask DE. Inhibition of isoproterenol-induced lipolysis in rat inguinal adipocytes in vitro by physiological melatonin via a receptor-mediated mechanism. Endocrinology. 2001;142:3783–3790. doi: 10.1210/endo.142.9.8378. [DOI] [PubMed] [Google Scholar]
- Zwirska-Korczala K, Jochem J, Adamczyk-Sowa M, Sowa P, Polaniak R, Birkner E, Latocha M, Pilc K, Suchanek R. Influence of melatonin on cell proliferation, antioxidative enzyme activities and lipid peroxidation in 3T3-L1 preadipocytes – an in vitro study. J Physiol Pharmacol. 2005;56:91–99. [PubMed] [Google Scholar]