Abstract
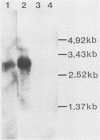
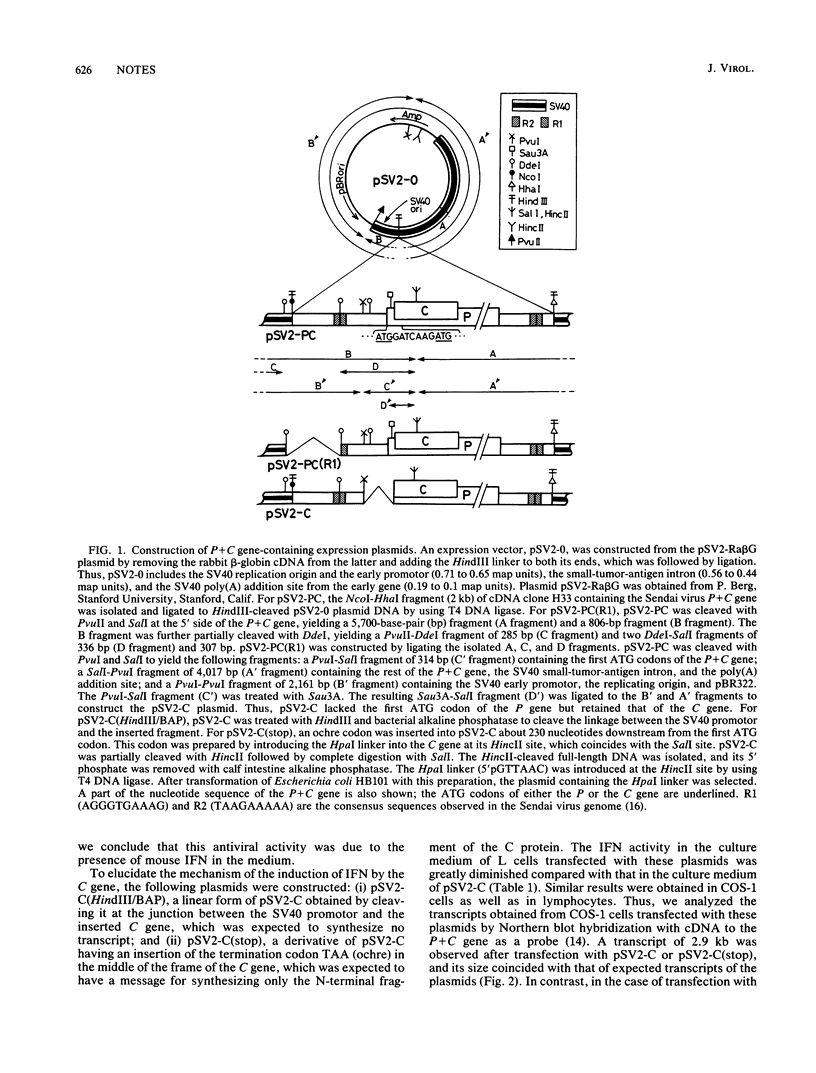
To elucidate the mechanism of interferon (IFN) induction on virus infection, we constructed two types of plasmids by inserting a part of the cDNA of the Sendai virus into a simian virus 40-derived expression vector (pSV2-0). One, pSV2-PC, contained the P + C gene, which codes for the P and C proteins in overlapping reading frames, and the other, pSV2-C, contained only the C gene. After transfecting the plasmids into mammalian cells, we determined the IFN activity in the culture medium. We found that the level obtained with pSV2-PC was significantly positive but very low, whereas that obtained with pSV2-C was as high as or even higher than that observed in the culture medium after Sendai virus infection. By cleaving pSV2-C between the simian virus 40 promotor and the C gene or by inserting a stop codon within the C gene [pSV2-C(stop)], induction of IFN was greatly diminished. In Northern blot analyses of the transcripts obtained from the cells transfected with the plasmids with cDNA to the P + C gene as a probe, the transcript having the expected size was detected with both pSV2-C and pSV2-C(stop), whereas none was detected with cleaved pSV2-C or pSV2-0. The results indicate that both transcription and translation of the C gene seem to be required for IFN induction after Sendai virus infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akashi H., Bishop D. H. Comparison of the sequences and coding of La Crosse and snowshoe hare bunyavirus S RNA species. J Virol. 1983 Mar;45(3):1155–1158. doi: 10.1128/jvi.45.3.1155-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Rozenblatt S., Arnheiter H., Richardson C. D. Measles virus P gene codes for two proteins. J Virol. 1985 Mar;53(3):908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabradilla C. D., Jr, Holloway B. P., Obijeski J. F. Molecular cloning and sequencing of the La Crosse virus S RNA. Virology. 1983 Jul 30;128(2):463–468. doi: 10.1016/0042-6822(83)90271-4. [DOI] [PubMed] [Google Scholar]
- Cashdollar L. W., Chmelo R. A., Wiener J. R., Joklik W. K. Sequences of the S1 genes of the three serotypes of reovirus. Proc Natl Acad Sci U S A. 1985 Jan;82(1):24–28. doi: 10.1073/pnas.82.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L., Kolakofsky D. In vitro synthesis of the nonstructural C protein of Sendai virus. J Virol. 1983 Apr;46(1):321–324. doi: 10.1128/jvi.46.1.321-324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst H., Shatkin A. J. Reovirus hemagglutinin mRNA codes for two polypeptides in overlapping reading frames. Proc Natl Acad Sci U S A. 1985 Jan;82(1):48–52. doi: 10.1073/pnas.82.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Conserved polyadenylation signals in two negative-strand RNA virus families. Virology. 1982 Jul 30;120(2):518–523. doi: 10.1016/0042-6822(82)90055-1. [DOI] [PubMed] [Google Scholar]
- ISAACS A., LINDENMANN J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957 Sep 12;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- Kawade Y. An analysis of neutralization reaction of interferon by antibody: a proposal on the expression of neutralization titer. J Interferon Res. 1980 Fall;1(1):61–70. doi: 10.1089/jir.1980.1.61. [DOI] [PubMed] [Google Scholar]
- Loyter A., Scangos G. A., Ruddle F. H. Mechanisms of DNA uptake by mammalian cells: fate of exogenously added DNA monitored by the use of fluorescent dyes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):422–426. doi: 10.1073/pnas.79.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk D., Sánchez A., Banerjee A. K. Messenger RNA encoding the phosphoprotein (P) gene of human parainfluenza virus 3 is bicistronic. Virology. 1986 Sep;153(2):318–325. doi: 10.1016/0042-6822(86)90036-x. [DOI] [PubMed] [Google Scholar]
- Marcus P. I. Interferon induction by viruses: one molecule of dsRNA as the threshold for interferon induction. Interferon. 1983;5:115–180. [PubMed] [Google Scholar]
- Shioda T., Hidaka Y., Kanda T., Shibuta H., Nomoto A., Iwasaki K. Sequence of 3,687 nucleotides from the 3' end of Sendai virus genome RNA and the predicted amino acid sequences of viral NP, P and C proteins. Nucleic Acids Res. 1983 Nov 11;11(21):7317–7330. doi: 10.1093/nar/11.21.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L. M., Danna K. J. Efficient infection of monkey cells with DNA of simian virus 40. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7575–7578. doi: 10.1073/pnas.78.12.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]