Abstract
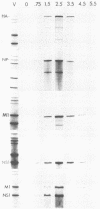
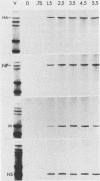
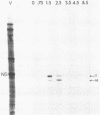
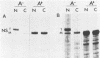
Single-stranded M13 DNAs specific for various influenza virus genomic segments were used to analyze the synthesis of virus-specific RNAs in infected cells. The results show that influenza virus infection is divided into two distinct phases. During the early phase, the syntheses of specific virion RNAs, viral mRNAs, and viral proteins were coupled. Thus, the NS (nonstructural) virion RNA was preferentially synthesized early, leading to the preferential synthesis of NS1 viral mRNA and NS1 protein; in contrast, M (matrix) RNA synthesis was delayed, leading to the delayed synthesis of M1 viral mRNA and M1 protein. This phase lasted for 2.5 h in BHK-21 cells, the time at which the rate of synthesis of all the viral mRNAs was maximal. During the second phase, the synthesis of all the virion RNAs remained at or near maximum until at least 5.5 h postinfection, whereas the rate of synthesis of all the viral mRNAs declined dramatically. By 4.5 h, the rate of synthesis of all the viral mRNAs was 5% of the maximum rate. Viral mRNA and protein syntheses were also not coupled, as the synthesis of all the viral proteins continued at maximum levels, indicating that protein synthesis during this phase was directed principally by previously synthesized viral mRNAs. Short pulses (3 min) with [3H]uridine and nonaqueous fractionation of cells were used to show that influenza virion RNA synthesis occurred in the nucleus, demonstrating that all virus-specific RNA synthesis was nuclear. Virion RNAs, like viral mRNAs, were efficiently transported to the cytoplasm at both early and late times of infection. In contrast, the full-length transcripts of the virion RNAs, which are the templates for virion RNA synthesis, were sequestered in the nucleus. Thus, the template RNAs, which were synthesized only at early times, remained in the nucleus to direct virion RNA synthesis throughout infection. These results enabled us to present an overall scheme for the control of influenza virus gene expression.
Full text
PDF



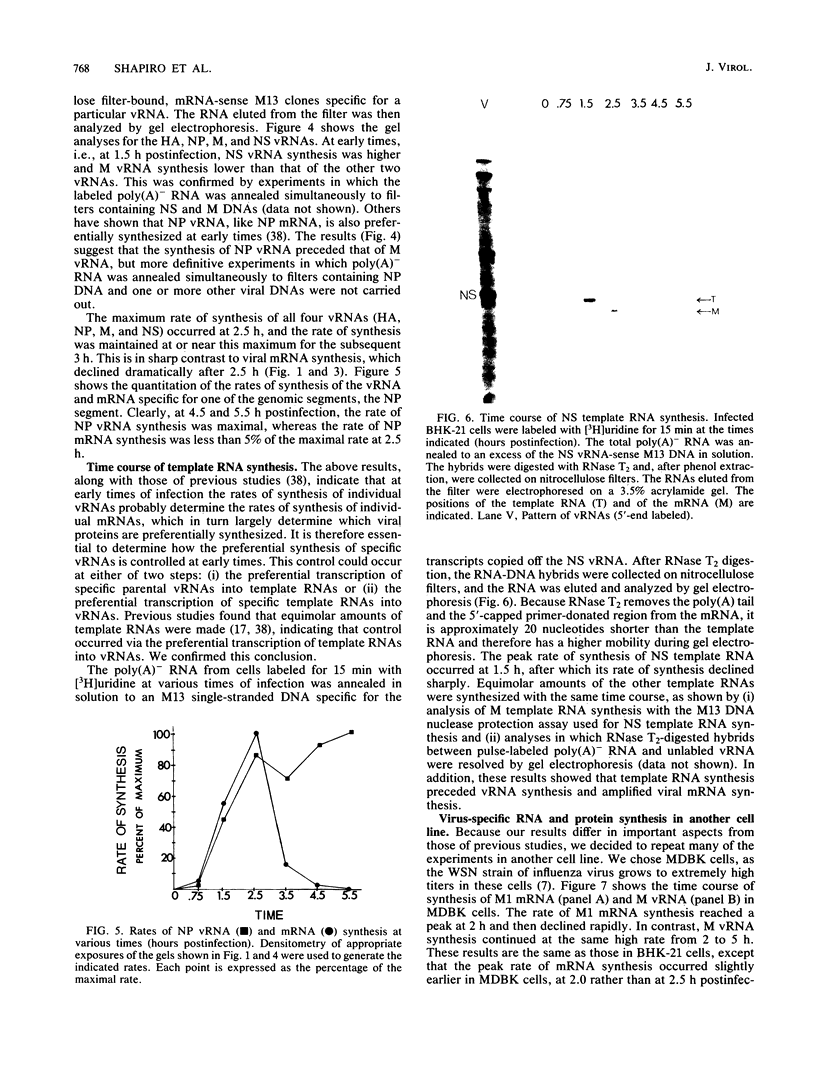

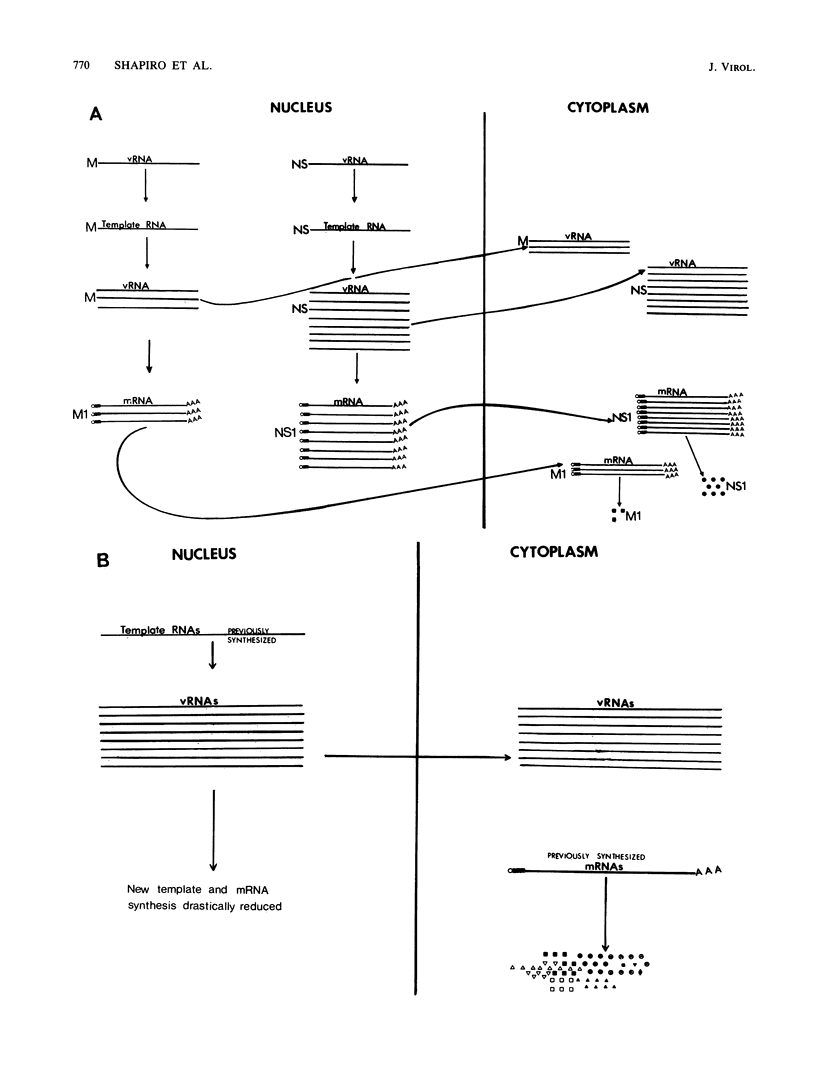



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett T., Wolstenholme A. J., Mahy B. W. Transcription and replication of influenza virus RNA. Virology. 1979 Oct 15;98(1):211–225. doi: 10.1016/0042-6822(79)90539-7. [DOI] [PubMed] [Google Scholar]
- Beaton A. R., Krug R. M. Synthesis of the templates for influenza virion RNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4682–4686. doi: 10.1073/pnas.81.15.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton A. R., Krug R. M. Transcription antitermination during influenza viral template RNA synthesis requires the nucleocapsid protein and the absence of a 5' capped end. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6282–6286. doi: 10.1073/pnas.83.17.6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Hiti A. L., Nayak D. P. Construction and characterization of a bacterial clone containing the hemagglutinin gene of the WSN strain (HON1) of influenza virus. Gene. 1980 Aug;10(3):205–218. doi: 10.1016/0378-1119(80)90050-5. [DOI] [PubMed] [Google Scholar]
- De B. P., Thornton G. B., Luk D., Banerjee A. K. Purified matrix protein of vesicular stomatitis virus blocks viral transcription in vitro. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7137–7141. doi: 10.1073/pnas.79.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami M., Fukuda R., Ishihama A. Transcription and replication of eight RNA segments of influenza virus. Virology. 1985 Apr 15;142(1):68–77. doi: 10.1016/0042-6822(85)90423-4. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Influenza viral messenger RNA. Virology. 1974 Nov;62(1):38–45. doi: 10.1016/0042-6822(74)90301-8. [DOI] [PubMed] [Google Scholar]
- Glass S. E., McGeoch D., Barry R. D. Characterization of the mRNA of influenza virus. J Virol. 1975 Dec;16(6):1435–1443. doi: 10.1128/jvi.16.6.1435-1443.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney T., Jr, Foster D. N. Nonaqueous isolation of nuclei from cultured cells. Methods Cell Biol. 1977;16:45–68. doi: 10.1016/s0091-679x(08)60091-6. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Abraham G., Skehel J. J., Smith J. C., Fellner P. Influenza virus messenger RNAs are incomplete transcripts of the genome RNAs. Nucleic Acids Res. 1977 Dec;4(12):4197–4209. doi: 10.1093/nar/4.12.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Skehel J. J., McCauley J. Structure and synthesis of influenza virus complementary RNAs. Philos Trans R Soc Lond B Biol Sci. 1980 Feb 25;288(1029):341–348. doi: 10.1098/rstb.1980.0010. [DOI] [PubMed] [Google Scholar]
- Herz C., Stavnezer E., Krug R., Gurney T., Jr Influenza virus, an RNA virus, synthesizes its messenger RNA in the nucleus of infected cells. Cell. 1981 Nov;26(3 Pt 1):391–400. doi: 10.1016/0092-8674(81)90208-7. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Mahy B. W. Polypeptides specified by the influenza virus genome. 3. Control of synthesis in infected cells. Virology. 1979 May;95(1):154–164. doi: 10.1016/0042-6822(79)90410-0. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Caton A. J., McCready S. J., Cook P. R. Influenza virus RNA is synthesized at fixed sites in the nucleus. Nature. 1982 Mar 25;296(5855):366–368. doi: 10.1038/296366a0. [DOI] [PubMed] [Google Scholar]
- Jen G., Thach R. E. Inhibition of host translation in encephalomyocarditis virus-infected L cells: a novel mechanism. J Virol. 1982 Jul;43(1):250–261. doi: 10.1128/jvi.43.1.250-261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings P. A., Finch J. T., Winter G., Robertson J. S. Does the higher order structure of the influenza virus ribonucleoprotein guide sequence rearrangements in influenza viral RNA? Cell. 1983 Sep;34(2):619–627. doi: 10.1016/0092-8674(83)90394-x. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Krug R. M. Metabolism and expression of RNA polymerase II transcripts in influenza virus-infected cells. Mol Cell Biol. 1984 Oct;4(10):2198–2206. doi: 10.1128/mcb.4.10.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M. Priming of influenza viral RNA transcription by capped heterologous RNAs. Curr Top Microbiol Immunol. 1981;93:125–149. doi: 10.1007/978-3-642-68123-3_6. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus polypeptides in cells resistant to alpha-amanitin: evidence for the involvement of cellular RNA polymerase II in virus replication. J Virol. 1977 Sep;23(3):816–819. doi: 10.1128/jvi.23.3.816-819.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The gene structure and replication of influenza virus. Annu Rev Biochem. 1983;52:467–506. doi: 10.1146/annurev.bi.52.070183.002343. [DOI] [PubMed] [Google Scholar]
- Mark G. E., Taylor J. M., Broni B., Krug R. M. Nuclear accumulation of influenza viral RNA transcripts and the effects of cycloheximide, actinomycin D, and alpha-amanitin. J Virol. 1979 Feb;29(2):744–752. doi: 10.1128/jvi.29.2.744-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D., Fellner P., Newton C. Influenza virus genome consists of eight distinct RNA species. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3045–3049. doi: 10.1073/pnas.73.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wagner R. R. Regulation of viral transcription by the matrix protein of vesicular stomatitis virus probed by monoclonal antibodies and temperature-sensitive mutants. J Virol. 1985 Nov;56(2):386–394. doi: 10.1128/jvi.56.2.386-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Krug R. M. Transfer of 5'-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1618–1622. doi: 10.1073/pnas.76.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Segments of influenza virus complementary RNA synthesized in vitro. J Virol. 1978 Feb;25(2):579–586. doi: 10.1128/jvi.25.2.579-586.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J. Polypeptide synthesis in influenza virus-infected cells. Virology. 1972 Jul;49(1):23–36. doi: 10.1016/s0042-6822(72)80004-7. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Hay A. J. Replication of the influenza virus genome. Virology. 1982 Apr 15;118(1):96–108. doi: 10.1016/0042-6822(82)90323-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zvonarjev A. Y., Ghendon Y. Z. Influence of membrane (M) protein on influenza A virus virion transcriptase activity in vitro and its susceptibility to rimantadine. J Virol. 1980 Feb;33(2):583–586. doi: 10.1128/jvi.33.2.583-586.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]