Abstract
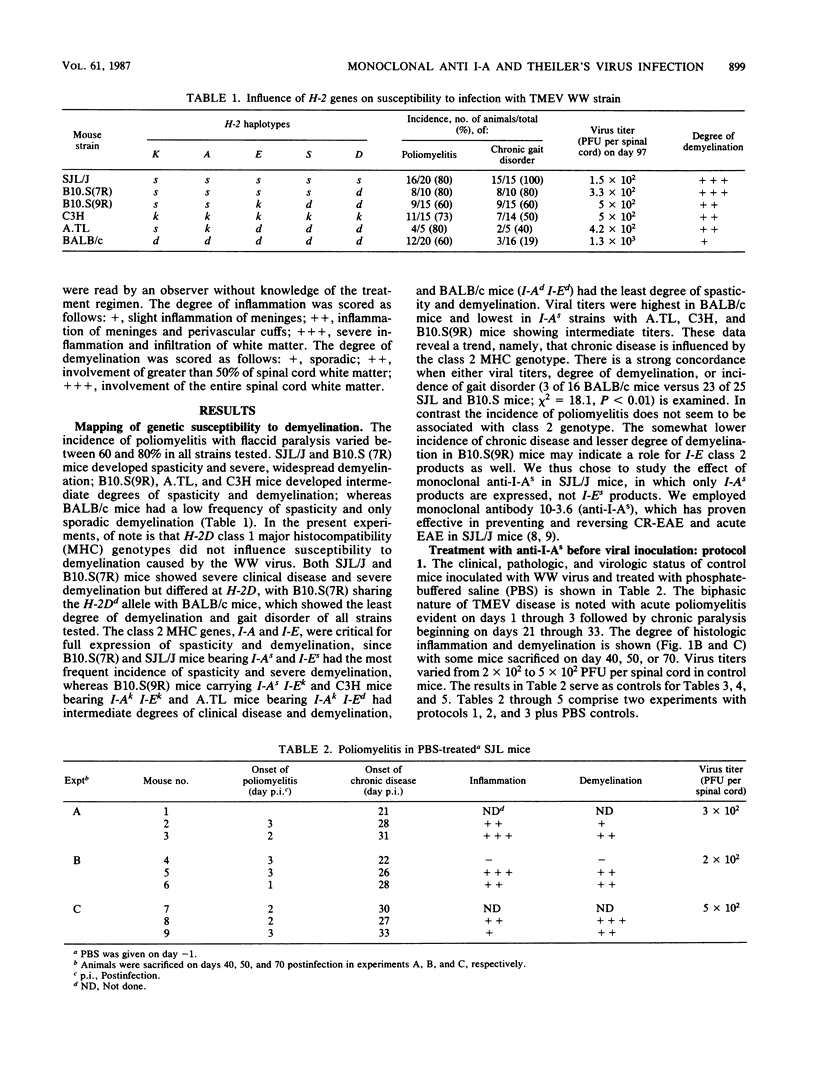
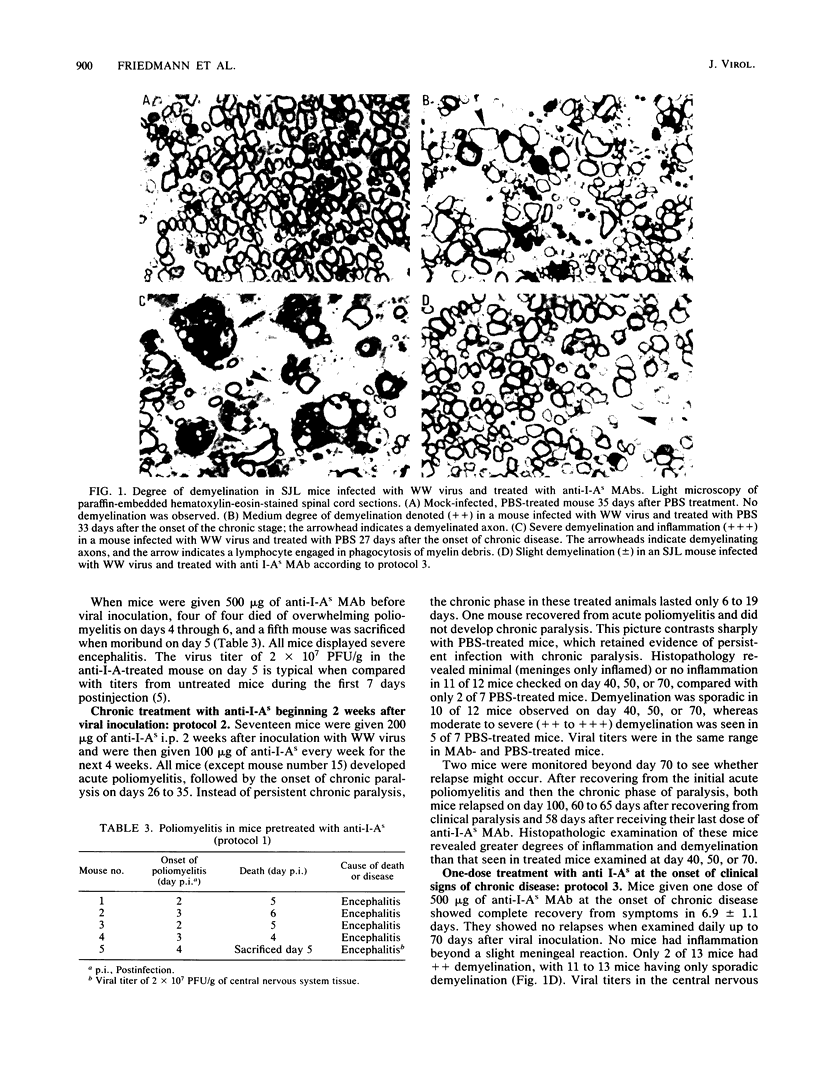
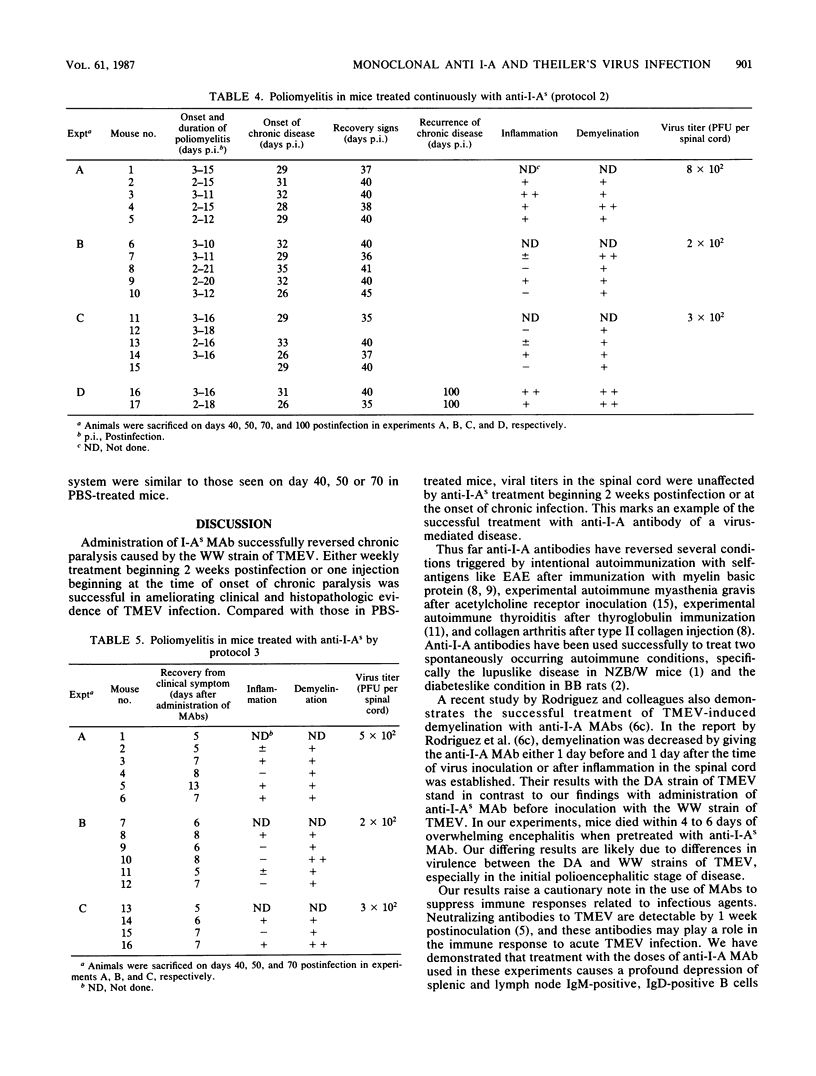
Susceptibility to demyelination caused by the WW isolate of Theiler's murine encephalomyelitis viruses is linked to class II genes of the major histocompatibility complex. SJL/J (H-2s) mice, expressing only I-As class II gene products of the major histocompatibility complex, are highly susceptible to Theiler's murine encephalomyelitis virus infection with the WW virus isolate, with chronic paralysis and severe inflammation and demyelination in the central nervous system. The effect of in vivo administration of anti-I-As monoclonal antibodies on Theiler's murine encephalomyelitis virus infection was observed. SJL/J mice were treated in various protocols pre- or postinfection. Anti-I-As monoclonal antibody reversed chronic paralysis and reduced inflammation and demyelination when given after the establishment of persistent infection. The effect was long lasting, but clinical signs, inflammation, and demyelination recurred 2 months after treatment ceased. Anti-I-As antibodies had no effect on viral titers within the central nervous system. The timing of the administration of monoclonal antibodies was critical. Administration of anti-I-As before the establishment of the persistent infection resulted in fatal encephalitis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman N. E., Watling D. L., McDevitt H. O. Treatment of (NZB x NZW)F1 disease with anti-I-A monoclonal antibodies. J Exp Med. 1983 Oct 1;158(4):1350–1355. doi: 10.1084/jem.158.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C., Michie S., Serrurier P., Butcher G. W., Larkins A. P., McDevitt H. O. In vivo prevention of thyroid and pancreatic autoimmunity in the BB rat by antibody to class II major histocompatibility complex gene products. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6627–6631. doi: 10.1073/pnas.82.19.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatch R. J., Melvold R. W., Miller S. D., Lipton H. L. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: correlation with TEMV-specific delayed-type hypersensitivity. J Immunol. 1985 Aug;135(2):1408–1414. [PubMed] [Google Scholar]
- Friedmann A., Lorch Y. Theiler's virus infection: a model for multiple sclerosis. Prog Med Virol. 1985;31:43–83. [PubMed] [Google Scholar]
- Lipton H. L., Canto C. D. Contrasting effects of immunosuppression on Theiler's virus infection in mice. Infect Immun. 1977 Mar;15(3):903–909. doi: 10.1128/iai.15.3.903-909.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Theiler's virus-induced demyelination: prevention by immunosuppression. Science. 1976 Apr 2;192(4234):62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Lafuse W. P., Leibowitz J., David C. S. Partial suppression of Theiler's virus-induced demyelination in vivo by administration of monoclonal antibodies to immune-response gene products (Ia antigens). Neurology. 1986 Jul;36(7):964–970. doi: 10.1212/wnl.36.7.964. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Leibowitz J., David C. S. Susceptibility to Theiler's virus-induced demyelination. Mapping of the gene within the H-2D region. J Exp Med. 1986 Mar 1;163(3):620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R. P., Firestone S., Wollmann R., Variakojis D., Arnason B. G. The effect of short-term and chronic immunosuppression on Theiler's virus demyelination. J Neuroimmunol. 1982 Jun;2(3-4):223–234. doi: 10.1016/0165-5728(82)90057-1. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. T., Adelman N. E., McDevitt H. O. In vivo effects of antibodies to immune response gene products. I. Haplotype-specific suppression of humoral immune responses with a monoclonal anti-I-A. J Exp Med. 1981 Nov 1;154(5):1694–1702. doi: 10.1084/jem.154.5.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram S., Steinman L. Anti I-A antibody suppresses active encephalomyelitis: treatment model for diseases linked to IR genes. J Exp Med. 1983 Oct 1;158(4):1362–1367. doi: 10.1084/jem.158.4.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L., Rosenbaum J. T., Sriram S., McDevitt H. O. In vivo effects of antibodies to immune response gene products: prevention of experimental allergic encephalitis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7111–7114. doi: 10.1073/pnas.78.11.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J., Sriram S., Rassenti L., Chou C. H., Fritz R. B., Steinman L. Characterization of T cell lines and clones from SJL/J and (BALB/c x SJL/J)F1 mice specific for myelin basic protein. J Immunol. 1985 Apr;134(4):2322–2327. [PubMed] [Google Scholar]
- Waldor M. K., Hardy R. R., Hayakawa K., Steinman L., Herzenberg L. A., Herzenberg L. A. Disappearance and reappearance of B cells after in vivo treatment with monoclonal anti-I-A antibodies. Proc Natl Acad Sci U S A. 1984 May;81(9):2855–2858. doi: 10.1073/pnas.81.9.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldor M. K., Sriram S., Hardy R., Herzenberg L. A., Herzenberg L. A., Lanier L., Lim M., Steinman L. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985 Jan 25;227(4685):415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- Waldor M. K., Sriram S., McDevitt H. O., Steinman L. In vivo therapy with monoclonal anti-I-A antibody suppresses immune responses to acetylcholine receptor. Proc Natl Acad Sci U S A. 1983 May;80(9):2713–2717. doi: 10.1073/pnas.80.9.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley P. H., Luthra H. S., Lafuse W. P., Huse A., Stuart J. M., David C. S. Type II collagen-induced arthritis in mice. III. Suppression of arthritis by using monoclonal and polyclonal anti-Ia antisera. J Immunol. 1985 Apr;134(4):2366–2374. [PubMed] [Google Scholar]
- Zamvil S. S., Mitchell D. J., Moore A. C., Kitamura K., Steinman L., Rothbard J. B. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986 Nov 20;324(6094):258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Nelson P. A., Mitchell D. J., Knobler R. L., Fritz R. B., Steinman L. Encephalitogenic T cell clones specific for myelin basic protein. An unusual bias in antigen recognition. J Exp Med. 1985 Dec 1;162(6):2107–2124. doi: 10.1084/jem.162.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil S., Nelson P., Trotter J., Mitchell D., Knobler R., Fritz R., Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. 1985 Sep 26-Oct 2Nature. 317(6035):355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]



