Abstract
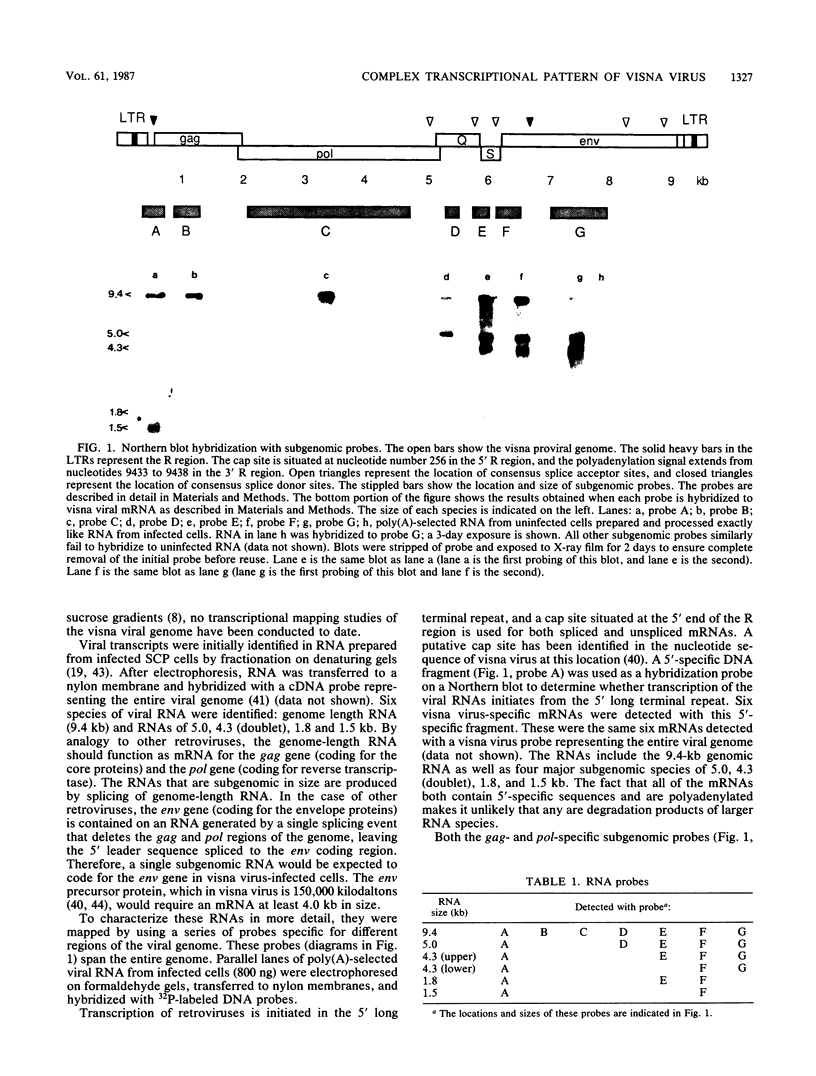
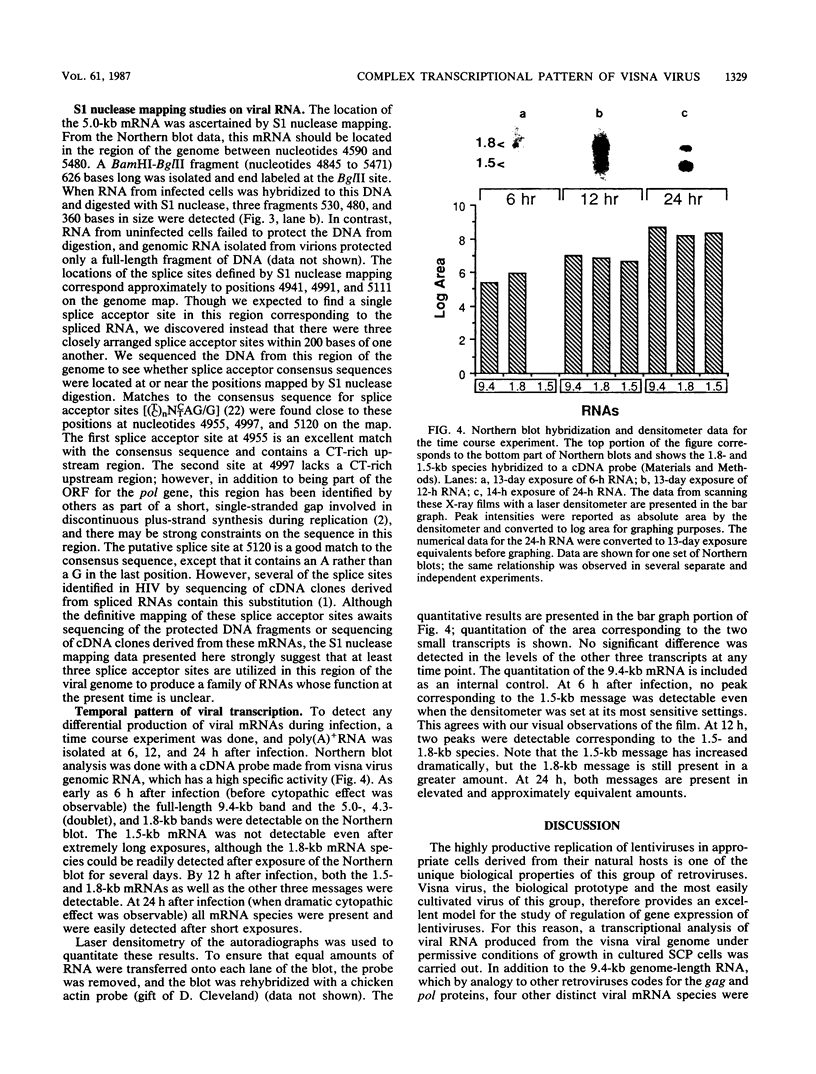
A complex pattern of gene expression was found for visna virus in a highly permissive cell culture system in vitro. In addition to the genomic RNA (9.4 kilobases [kb]), five other mRNAs were detected. The three large RNA transcripts (5.0 kb and a doublet at 4.3 kb) arise by a single splicing event joining 5' sequences to sequences located at positions 3' to the pol gene. The two smallest transcripts (1.8 and 1.5 kb) are at least doubly spliced mRNAs which contain sequences derived from the 5' end of the genome, the region between the pol and env genes, and 3' terminal sequences. In addition to this complex pattern of transcription, the mRNAs appear to be regulated temporally. The 1.5-kb mRNA appears 6 h later than the other transcripts. The significance of this complex pattern of gene expression in the unique aspects of the lentivirus life cycle and pathogenesis is considered.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Blum H. E., Harris J. D., Ventura P., Walker D., Staskus K., Retzel E., Haase A. T. Synthesis in cell culture of the gapped linear duplex DNA of the slow virus visna. Virology. 1985 Apr 30;142(2):270–277. doi: 10.1016/0042-6822(85)90335-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiu I. M., Yaniv A., Dahlberg J. E., Gazit A., Skuntz S. F., Tronick S. R., Aaronson S. A. Nucleotide sequence evidence for relationship of AIDS retrovirus to lentiviruses. 1985 Sep 26-Oct 2Nature. 317(6035):366–368. doi: 10.1038/317366a0. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Narayan O., Griffin D. E., Johnson R. T. The synthesis and structure of visna virus DNA. Virology. 1979 Mar;93(2):377–386. doi: 10.1016/0042-6822(79)90242-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Filippi P., Brahic M., Vigne R., Tamalet J. Characterization of visna virus mRNA. J Virol. 1979 Jul;31(1):25–30. doi: 10.1128/jvi.31.1.25-30.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geballe A. P., Ventura P., Stowring L., Haase A. T. Quantitative analysis of visna virus replication in vivo. Virology. 1985 Feb;141(1):148–154. doi: 10.1016/0042-6822(85)90191-6. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Clements J. E., Pezeshkpour G. H. Slow virus-macrophage interactions. Characterization of a transformed cell line of sheep alveolar macrophages that express a marker for susceptibility to ovine-caprine lentivirus infections. Lab Invest. 1984 Nov;51(5):547–555. [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Molineaux S., Clements J. E., Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda M. A., Braun M. J., Clements J. E., Pyper J. M., Wong-Staal F., Gallo R. C., Gilden R. V. Human T-cell lymphotropic virus type III shares sequence homology with a family of pathogenic lentiviruses. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4007–4011. doi: 10.1073/pnas.83.11.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda M. A., Wong-Staal F., Gallo R. C., Clements J. E., Narayan O., Gilden R. V. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science. 1985 Jan 11;227(4683):173–177. doi: 10.1126/science.2981428. [DOI] [PubMed] [Google Scholar]
- Graham D. E., Medina D., Smith G. H. Increased concentration of an indigenous proviral mouse mammary tumor virus long terminal repeat-containing transcript is associated with neoplastic transformation of mammary epithelium in C3H/Sm mice. J Virol. 1984 Mar;49(3):819–827. doi: 10.1128/jvi.49.3.819-827.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Harris J. D., Traynor B., Ventura P., Peluso R., Brahic M. Visna DNA synthesis and the tempo of infection in vitro. Virology. 1982 Jun;119(2):399–410. doi: 10.1016/0042-6822(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Haase A. T. The slow infection caused by visna virus. Curr Top Microbiol Immunol. 1975;72:101–156. doi: 10.1007/978-3-642-66289-8_4. [DOI] [PubMed] [Google Scholar]
- Hess J. L., Clements J. E., Narayan O. cis- and trans-acting transcriptional regulation of visna virus. Science. 1985 Aug 2;229(4712):482–485. doi: 10.1126/science.2990051. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineaux S., Clements J. E. Molecular cloning of unintegrated visna viral DNA and characterization of frequent deletions in the 3' terminus. Gene. 1983 Aug;23(2):137–148. doi: 10.1016/0378-1119(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Cork L. C. Lentiviral diseases of sheep and goats: chronic pneumonia leukoencephalomyelitis and arthritis. Rev Infect Dis. 1985 Jan-Feb;7(1):89–98. doi: 10.1093/clinids/7.1.89. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Chase J. Antigenic shift of visna virus in persistently infected sheep. Science. 1977 Jul 22;197(4301):376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Silverstein A. M. Slow virus infection: replication and mechanisms of persistence of visna virus in sheep. J Infect Dis. 1977 May;135(5):800–806. doi: 10.1093/infdis/135.5.800. [DOI] [PubMed] [Google Scholar]
- Narayan O., Kennedy-Stoskopf S., Sheffer D., Griffin D. E., Clements J. E. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun. 1983 Jul;41(1):67–73. doi: 10.1128/iai.41.1.67-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Nash M., Brown N. V., Wong J. L., Arlinghaus R. B., Murphy E. C., Jr S1 nuclease mapping of viral RNAs from a temperature-sensitive transformation mutant of murine sarcoma virus. J Virol. 1984 May;50(2):478–488. doi: 10.1128/jvi.50.2.478-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Ovalbumin messenger ribonucleic acid translation. Comparable rates of polypeptide initiation and elongation on ovalbumin and globin messenger ribonucleic acid in a rabbit reticulocyte lysate. J Biol Chem. 1973 Mar 25;248(6):2095–2106. [PubMed] [Google Scholar]
- Pyper J. M., Clements J. E., Gonda M. A., Narayan O. Sequence homology between cloned caprine arthritis encephalitis virus and visna virus, two neurotropic lentiviruses. J Virol. 1986 May;58(2):665–670. doi: 10.1128/jvi.58.2.665-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyper J. M., Clements J. E., Molineaux S. M., Narayan O. Genetic variation among lentiviruses: homology between visna virus and caprine arthritis-encephalitis virus is confined to the 5' gag-pol region and a small portion of the env gene. J Virol. 1984 Sep;51(3):713–721. doi: 10.1128/jvi.51.3.713-721.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson A. B., Daugherty D. F., Venkatesan S., Boulukos K. E., Benn S. I., Folks T. M., Feorino P., Martin M. A. Transcription of novel open reading frames of AIDS retrovirus during infection of lymphocytes. Science. 1985 Sep 27;229(4720):1388–1390. doi: 10.1126/science.2994220. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Seigel L. J., Ratner L., Josephs S. F., Derse D., Feinberg M. B., Reyes G. R., O'Brien S. J., Wong-Staal F. Transactivation induced by human T-lymphotropic virus type III (HTLV III) maps to a viral sequence encoding 58 amino acids and lacks tissue specificity. Virology. 1986 Jan 15;148(1):226–231. doi: 10.1016/0042-6822(86)90419-8. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Dayton A., Terwilliger E., Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature. 1986 May 22;321(6068):412–417. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Wong-Staal F., Salahuddin S. Z., Popovic M., Arya S., Gallo R. C., Haseltine W. A. Trans-acting transcriptional regulation of human T-cell leukemia virus type III long terminal repeat. Science. 1985 Jan 11;227(4683):171–173. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- Sonigo P., Alizon M., Staskus K., Klatzmann D., Cole S., Danos O., Retzel E., Tiollais P., Haase A., Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985 Aug;42(1):369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne R., Filippi P., Quérat G., Sauze N., Vitu C., Russo P., Delori P. Precursor polypeptides to structural proteins of visna virus. J Virol. 1982 Jun;42(3):1046–1056. doi: 10.1128/jvi.42.3.1046-1056.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]