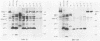Abstract
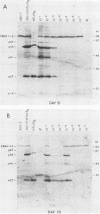
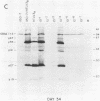
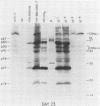
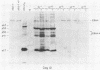
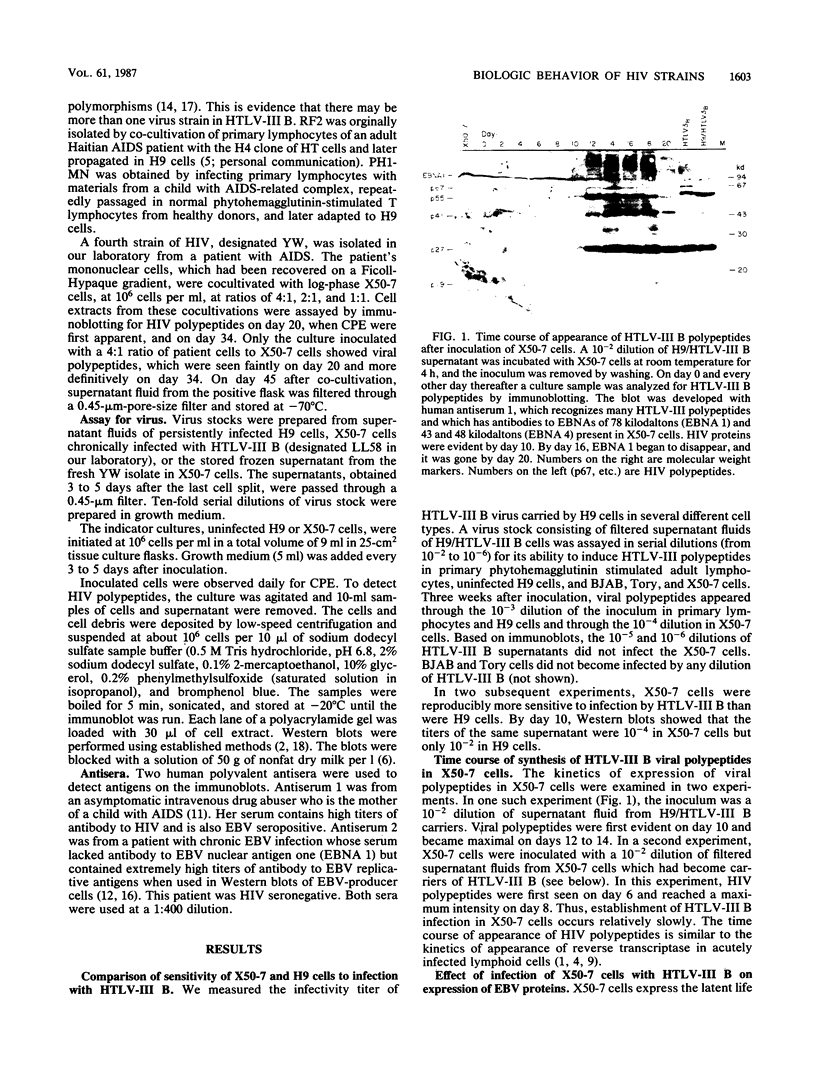
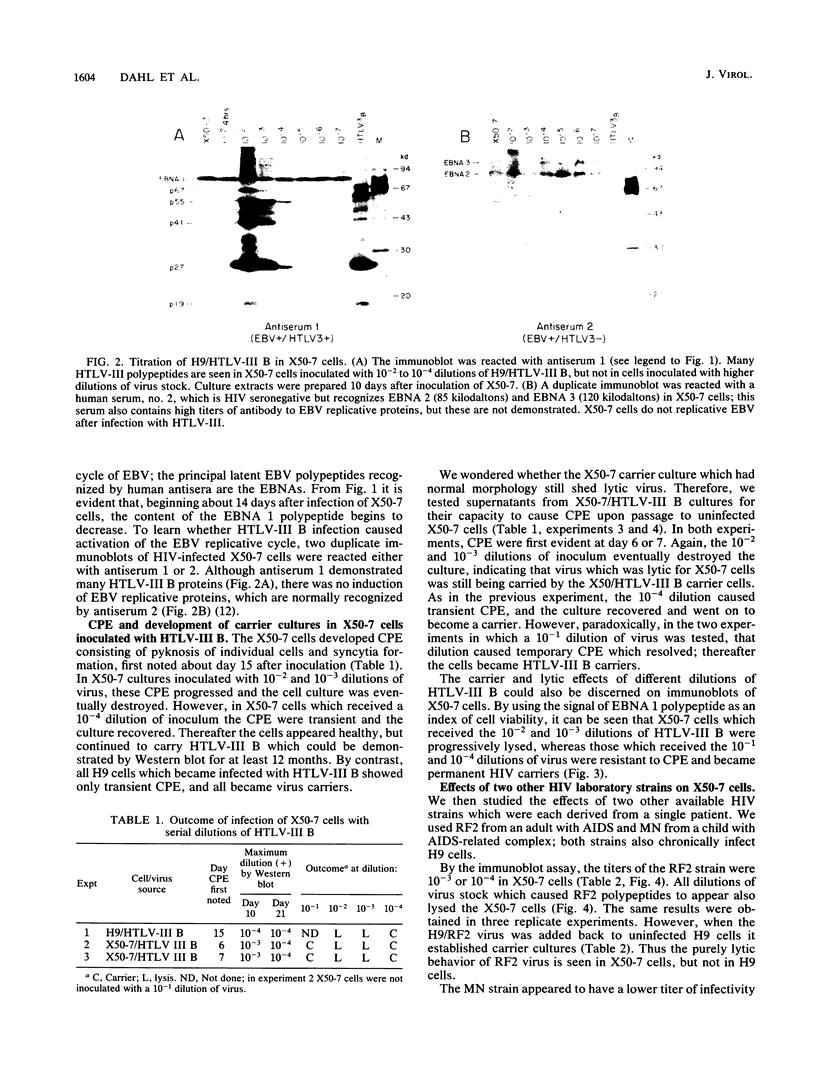
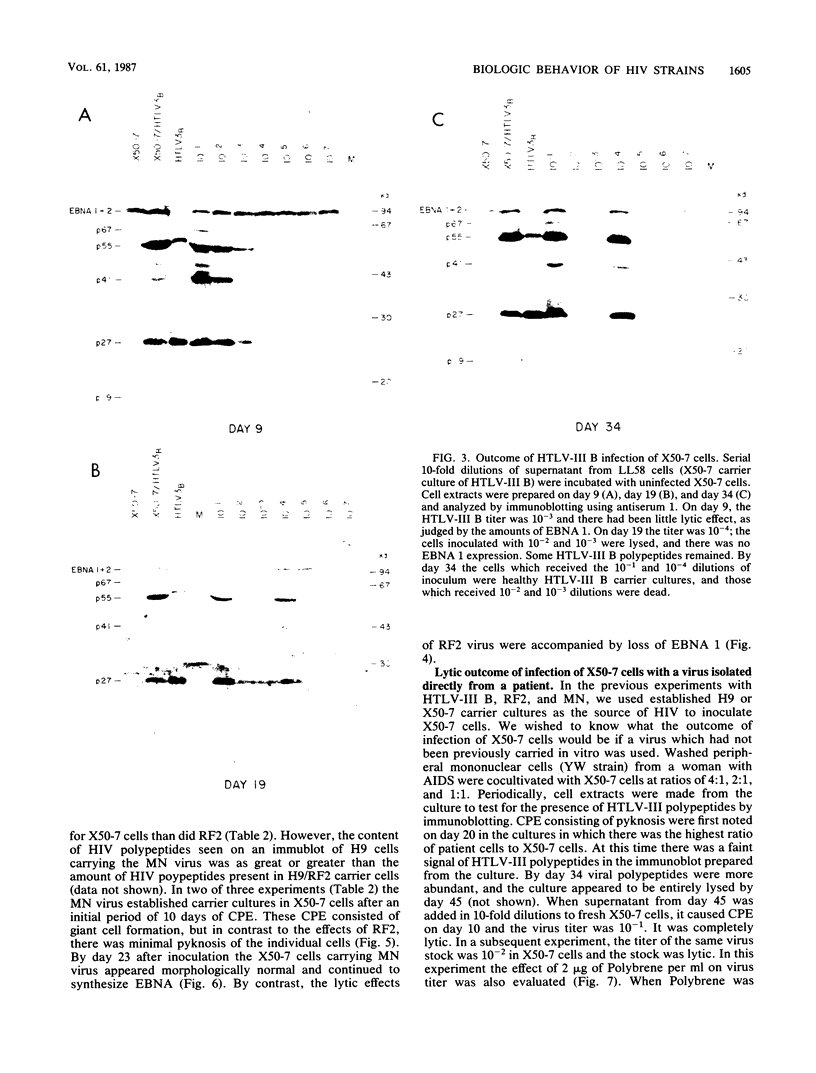
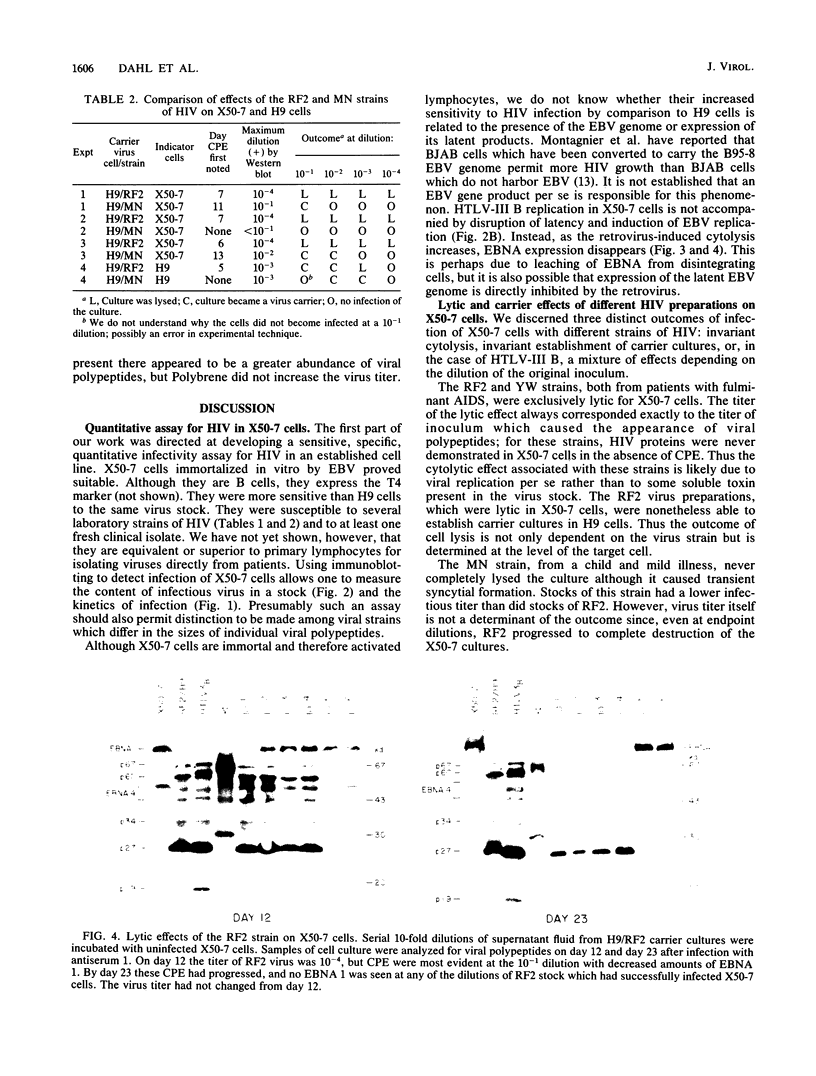
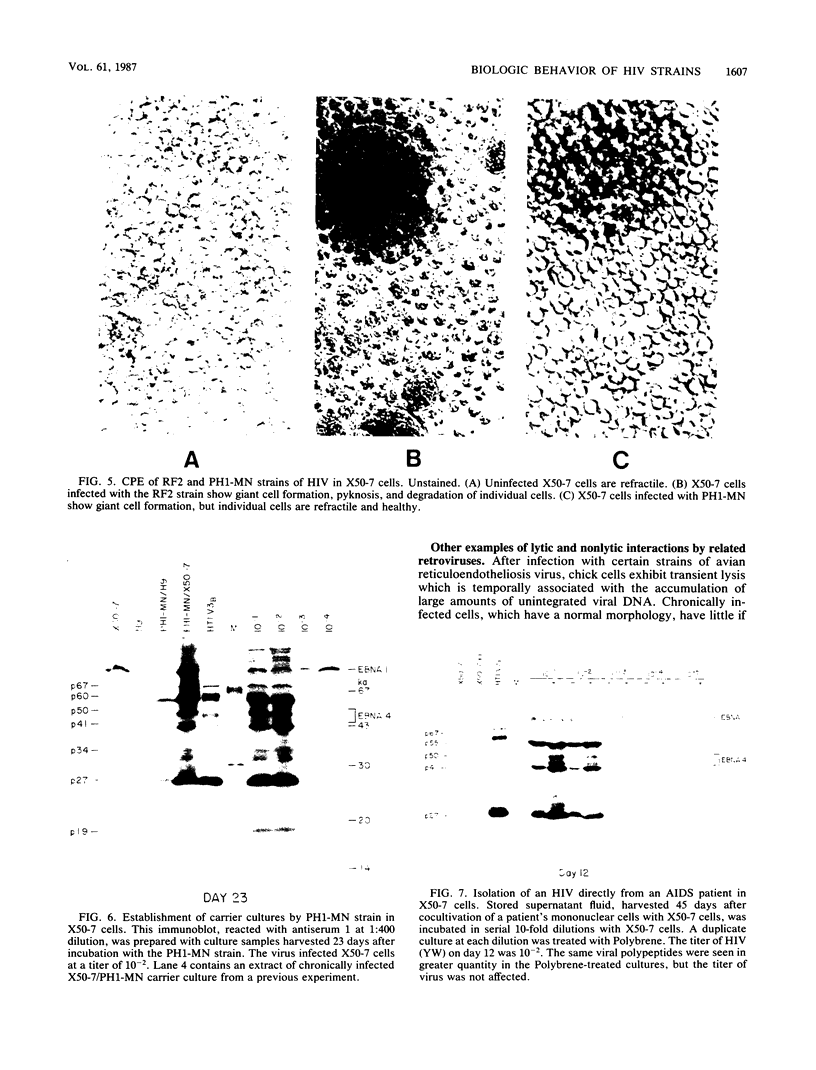
Four strains of human immunodeficiency virus (HIV) manifest consistent differences in biologic behavior after infection of the X50-7 line of human umbilical cord lymphocytes immortalized by Epstein-Barr virus (EBV). Some dilutions of the first strain examined, human T-cell lymphotropic virus type III B, which is derived from a pool of patient isolates propagated in H9 cells, caused transient cytopathic effects (CPE) followed by recovery of a subpopulation of X50-7 cells which became virus carrier cultures. Other dilutions of the same virus stock completely lysed X50-7 cells. Two other strains, RF2 and YW, both from individual patients with acquired immune deficiency syndrome, always induced complete cytolysis of X50-7 cells at all dilutions which infected the cells. However, RF2 did establish persistent infection of H9 cells. A fourth strain, PH1-MN, from a child with acquired immune deficiency syndrome-related complex, induced only transient CPE in X50-7 and H9 cells, which thereafter always recovered to form carrier cultures. For all four strains, the dilutions of HIV stocks which caused CPE corresponded to dilutions which resulted in the detection of HIV polypeptides by immunoblot. Cytolysis in HIV-infected X50-7 cells was accompanied by a decrease in the amount of EBV nuclear antigen; however, HIV infection did not induce EBV replication. Thus CPE in X50-7 cells is due to replication of HIV per se and not to activation of EBV. The observations indicate that there are differences in the cytolytic properties of HIVs and that these differences are influenced by the target cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Ratner L., Mitsuya H., Marselle L. M., Harper M. E., Broder S., Gallo R. C., Wong-Staal F. Infectious mutants of HTLV-III with changes in the 3' region and markedly reduced cytopathic effects. Science. 1986 Aug 8;233(4764):655–659. doi: 10.1126/science.3014663. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Arya S. K., Popovic M., Gallo R. C., Wong-Staal F. Molecular cloning and characterization of the HTLV-III virus associated with AIDS. Nature. 1984 Nov 8;312(5990):166–169. doi: 10.1038/312166a0. [DOI] [PubMed] [Google Scholar]
- Keshet E., Temin H. M. Cell killing by spleen necrosis virus is correlated with a transient accumulation of spleen necrosis virus DNA. J Virol. 1979 Aug;31(2):376–388. doi: 10.1128/jvi.31.2.376-388.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Kaminsky L. S., Morrow W. J., Steimer K., Luciw P., Dina D., Hoxie J., Oshiro L. Infection by the retrovirus associated with the acquired immunodeficiency syndrome. Clinical, biological, and molecular features. Ann Intern Med. 1985 Nov;103(5):694–699. doi: 10.7326/0003-4819-103-5-694. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J., McHugh T., Casavant C., Stites D., Oshiro L. AIDS-associated retroviruses (ARV) can productively infect other cells besides human T helper cells. Virology. 1985 Dec;147(2):441–448. doi: 10.1016/0042-6822(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J. Recovery of AIDS-associated retroviruses from patients with AIDS or AIDS-related conditions and from clinically healthy individuals. J Infect Dis. 1985 Oct;152(4):734–738. doi: 10.1093/infdis/152.4.734. [DOI] [PubMed] [Google Scholar]
- Martin K., Katz B. Z., Miller G. AIDS and antibodies to human immunodeficiency virus (HIV) in children and their families. J Infect Dis. 1987 Jan;155(1):54–63. doi: 10.1093/infdis/155.1.54. [DOI] [PubMed] [Google Scholar]
- Miller G., Grogan E., Fischer D. K., Niederman J. C., Schooley R. T., Henle W., Lenoir G., Liu C. R. Antibody responses to two Epstein-Barr virus nuclear antigens defined by gene transfer. N Engl J Med. 1985 Mar 21;312(12):750–755. doi: 10.1056/NEJM198503213121204. [DOI] [PubMed] [Google Scholar]
- Montagnier L., Gruest J., Chamaret S., Dauguet C., Axler C., Guétard D., Nugeyre M. T., Barré-Sinoussi F., Chermann J. C., Brunet J. B. Adaptation of lymphadenopathy associated virus (LAV) to replication in EBV-transformed B lymphoblastoid cell lines. Science. 1984 Jul 6;225(4657):63–66. doi: 10.1126/science.6328661. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Schooley R. T., Carey R. W., Miller G., Henle W., Eastman R., Mark E. J., Kenyon K., Wheeler E. O., Rubin R. H. Chronic Epstein-Barr virus infection associated with fever and interstitial pneumonitis. Clinical and serologic features and response to antiviral chemotherapy. Ann Intern Med. 1986 May;104(5):636–643. doi: 10.7326/0003-4819-104-5-636. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Hahn B. H., Arya S. K., Groopman J. E., Gallo R. C., Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984 Dec 7;226(4679):1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Joy A. E., Temin H. M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980 Jan;33(1):494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G., Miller G. Recovery of Epstein-Barr virus from nonproducer neonatal human lymphoid cell transformants. Virology. 1979 Jun;95(2):351–358. doi: 10.1016/0042-6822(79)90490-2. [DOI] [PubMed] [Google Scholar]