Abstract
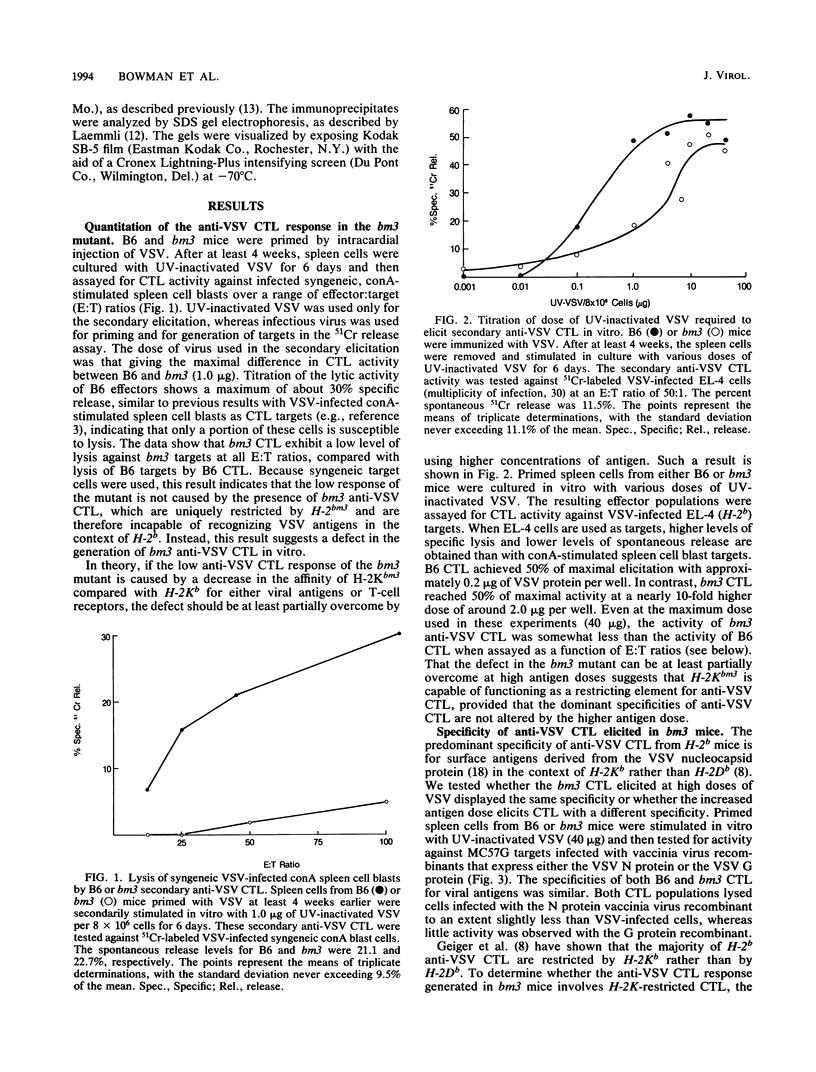
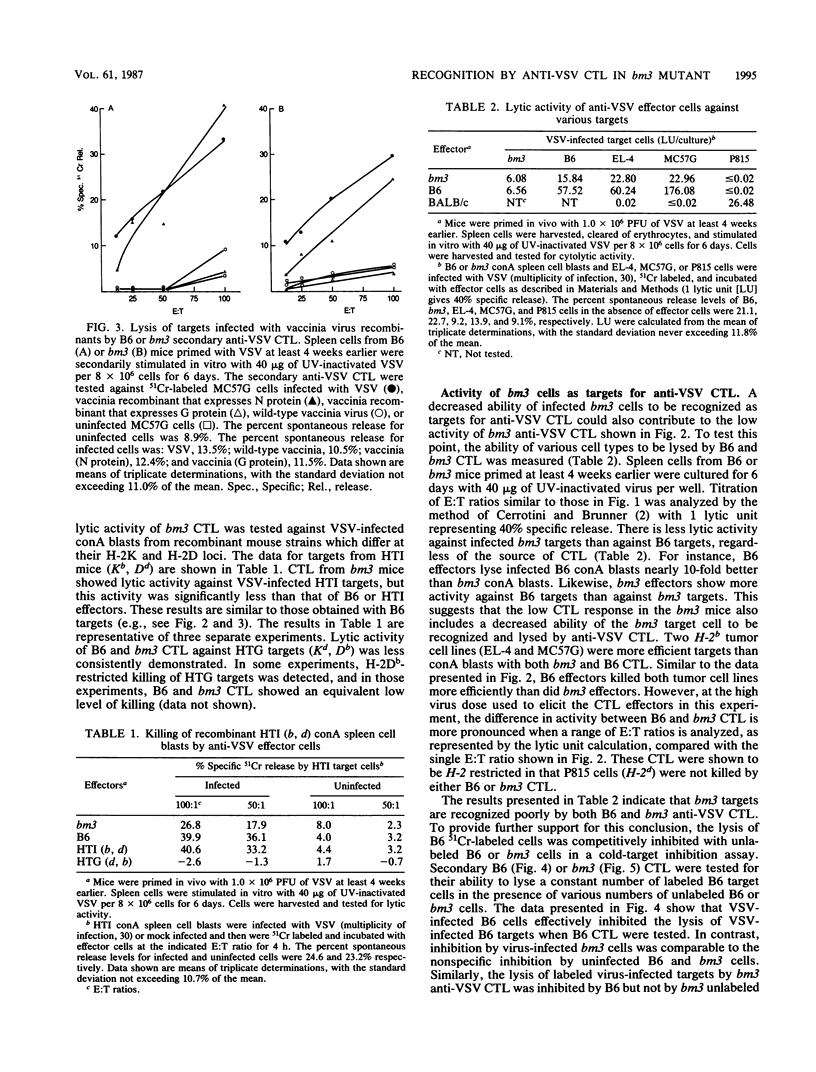
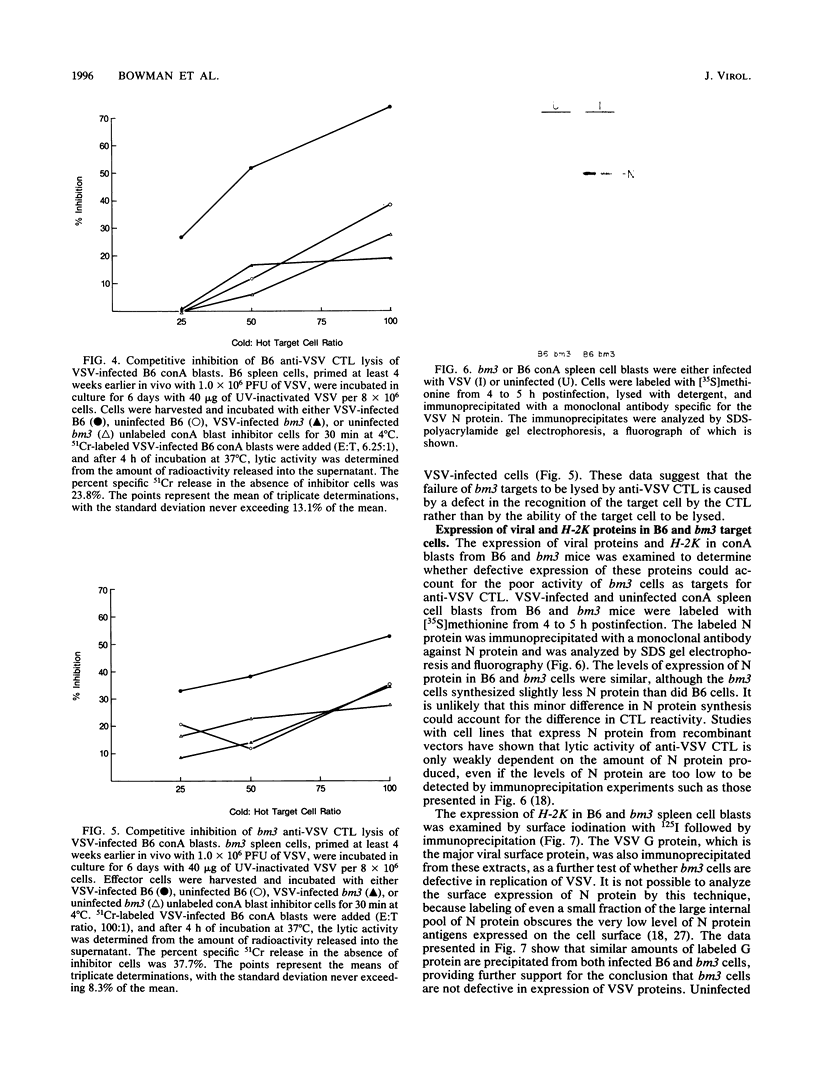
Spleen cells from C57BL/6 (B6) mice generate a strong in vitro cytotoxic T-lymphocyte (CTL) response specific for vesicular stomatitis virus (VSV). Spleen cells from VSV-primed B6-H-2bm3 (bm3) mice, which have a mutation in H-2Kb, require approximately 10-fold more UV-inactivated VSV to generate in vitro secondary anti-VSV CTL, compared with spleen cells from primed B6 mice. Anti-VSV CTL elicited in both bm3 and B6 mice are primarily specific for the viral nucleocapsid protein (N protein), as demonstrated by using recombinant vaccinia viruses that express the VSV N protein. bm3 CTL were found to exhibit only a very low level of lytic activity when tested against autologous VSV-infected concanavalin A spleen cell blasts as well as several H-2b tumor cell lines. The weak anti-VSV response of bm3 CTL was found to be the result of a combination of inefficient recognition of VSV-infected target cells and decreased elicitation of secondary effector cells. VSV-infected bm3 target cells were not killed as well as B6 targets by either bm3 or B6 effectors. This is because of the inefficient recognition of targets, as demonstrated by the fact that VSV-infected bm3 cells were unable to competitively inhibit the lysis of VSV-infected B6 target cells by either bm3 or B6 effectors. By using cells from recombinant mice, it was shown that the CTL response restricted by H-2Kb was low in the bm3 mice, compared with that of the B6 mice. However, the H-2Db-restricted CTL activity was similarly low in both the B6 and bm3 mice. The possibility that the low response to VSV-infected bm3 cells is caused by differences between the bm3 and B6 cells in expression of either viral antigens or H-2K was investigated by radiolabeling and immunoprecipitation. VSV-infected B6 and bm3 cells were found to express equivalent levels of both viral antigens and H-2K. These results indicate that the bm3 mutation alters a functional site on the H-2Kb molecule that is involved in the recognition of VSV-infected cells. The observation that elicitation of bm3 CTL can occur at high antigen doses further suggests that the bm3 mutation results in a lower affinity of H-2K either for viral antigen or for receptor sites on the CTL.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allouche M., Owen J. A., Doherty P. C. Limit-dilution analysis of weak influenza-immune T cell responses associated with H-2Kb and H-2Db. J Immunol. 1982 Aug;129(2):689–693. [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Ciavarra R., Forman J. H-2L-restricted recognition of viral antigens In the H-2d haplotype, anti-vesicular stomatitis virus cytotoxic T cells are restricted solely by H-2L. J Exp Med. 1982 Sep 1;156(3):778–790. doi: 10.1084/jem.156.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. S., Geier S., Nathenson S. G., Forman J. Analysis of associative recognition determinants on class I H-2Kb mutant molecules. Immunogenetics. 1985;22(4):391–397. doi: 10.1007/BF00430922. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Biddison W. E., Bennink J. R., Knowles B. B. Cytotoxic T-cell responses in mice infected with influenza and vaccinia viruses vary in magnitude with H-2 genotype. J Exp Med. 1978 Aug 1;148(2):534–543. doi: 10.1084/jem.148.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman J., Goodenow R. S., Hood L., Ciavarra R. Use of DNA-mediated gene transfer to analyze the role of H-2Ld in controlling the specificity of anti-vesicular stomatitis virus cytotoxic T cells. J Exp Med. 1983 Apr 1;157(4):1261–1272. doi: 10.1084/jem.157.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Rosenthal K. L., Klein J., Zinkernagel R. M., Singer S. J. Selective and unidirectional membrane redistribution of an H-2 antigen with an antibody-clustered viral antigen: relationship to mechanisms of cytotoxic T-cell interactions. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4603–4607. doi: 10.1073/pnas.76.9.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Hurwitz J. L., Pan S., Wettstein P. J., Doherty P. C. Cross-reactivity patterns of vaccinia-specific cytotoxic T lymphocytes from H-2Kb mutants. Immunogenetics. 1983;17(1):79–87. doi: 10.1007/BF00364291. [DOI] [PubMed] [Google Scholar]
- Klein J. What causes immunological nonresponsiveness? Immunol Rev. 1984 Oct;81:177–202. doi: 10.1111/j.1600-065x.1984.tb01110.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefrancios L., Lyles D. S. The interactionof antiody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982 Aug;121(1):157–167. [PubMed] [Google Scholar]
- Nathenson S. G., Geliebter J., Pfaffenbach G. M., Zeff R. A. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Pan S., Wettstein P. J., Knowles B. B. H-2Kb mutations limit the CTL response to SV40 TASA. J Immunol. 1982 Jan;128(1):243–246. [PubMed] [Google Scholar]
- Puddington L., Bevan M. J., Rose J. K., Lefrançois L. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J Virol. 1986 Nov;60(2):708–717. doi: 10.1128/jvi.60.2.708-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal K. L., Zinkernagel R. M. Inability of mice to generate cytotoxic T lymphocytes to vesicular stomatitis virus restricted to H-2Kk or H-2Dk. J Immunol. 1981 Feb;126(2):446–451. [PubMed] [Google Scholar]
- Schwartz R. H. A clonal deletion model for Ir gene control of the immune response. Scand J Immunol. 1978;7(1):3–10. doi: 10.1111/j.1365-3083.1978.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukart M. J., Boes J., Melief C. J. Recognition of H-2Kb mutant target cells by Moloney virus-specific cytotoxic T lymphocytes from bm13 (H-2Db mutant) mice. I. Full recognition of Kbm11 by Kb-restricted CTL. J Immunol. 1984 Jul;133(1):24–27. [PubMed] [Google Scholar]
- Stukart M. J., Vos A., Boes J., Melvold R. W., Bailey D. W., Melief C. J. A crucial role of the H-2 D locus in the regulation of both the D- and the K-associated cytotoxic T lymphocyte response against Moloney leukemia virus, demonstrated with two Db mutants. J Immunol. 1982 Mar;128(3):1360–1364. [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Wettstein P. J. H-2 effects on cell-cell interactions in the response to single non-H-2 alloantigens. V. Effects of H-2Kb mutations on presentation of H-4 and H-3 alloantigens. J Immunol. 1982 Jun;128(6):2629–2633. [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Mackett M., Lefrancois L., Lyles D. S., Moss B. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986 Jun 1;163(6):1529–1538. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Smith G. L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal L. P., Kast W. M., Melvold R. W., Melief C. J. Regulation of the cytotoxic T lymphocyte response against Sendai virus analyzed with H-2 mutants. J Immunol. 1983 Mar;130(3):1090–1096. [PubMed] [Google Scholar]