Abstract
γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian central nervous system and exerts its actions via both ionotropic (GABAA) and metabotropic (GABAB) receptors. The GABAB receptor is a dimer composed of R1 and R2 components and classically couples to the heterotrimeric Gi protein. In addition to their location on neurons, GABA and functional GABAB receptors have been detected in peripheral tissue such as airway smooth muscle. We questioned whether airway epithelium expresses receptors that could respond to GABA. We detected the mRNA encoding multiple-splice variants of the GABABR1 and GABABR2 in total RNA isolated from native human and guinea pig airway epithelium and human airway epithelial cell lines (BEAS-2B and H441). Immunoblots identified the GABABR1 and GABABR2 proteins in both guinea pig airway epithelium and BEAS-2B cells. The expression of GABABR1 protein was immunohistochemically localized to basal mucin-secreting and ciliated columnar epithelial cells in guinea pig trachea. Baclofen inhibited adenylyl cyclase activity, induced ERK phosphorylation and cross-regulated phospholipase C, leading to increased inositol phosphates in BEAS-2B cells in a pertussis toxin–sensitive manner, implicating Gi protein coupling. Thus, these receptors couple to Gi and cross-regulate the phospholipase C/inositol phosphate pathway. The second messengers of these pathways, cyclic AMP and calcium, play pivotal roles in airway epithelial cell primary functions of mucus clearance. Furthermore, the enzyme that synthesizes GABA, glutamic acid decarboxylase (GAD65/67), was also localized to airway epithelium. GABA may modulate an uncharacterized signaling cascade via GABAB receptors coupled to Gi protein in airway epithelium.
Keywords: G protein, adenylyl cyclase, mitogen-activated protein kinase, airway epithelium
CLINICAL RELEVANCE
This is a novel demonstration of the functional coupling of the GABAB receptor on airway epithelium to cyclic AMP and calcium signaling pathways, which in turn regulate a fundamental function of airway epithelial cells, mucociliary clearance.
γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian central nervous system (CNS). GABA acts at two distinct types of receptors, ligand-gated ionotropic GABAA and G protein–linked metabotropic (GABAB) receptors. Recently the expression of subunits of GABAA receptors and the enzyme that synthesizes GABA (glutamic acid decarboxylase [GAD]) were identified in airway epithelium and found to play a pivotal role in mucous overproduction in asthma (1). However, the expression and coupling of the GABAB receptor has never been described in airway epithelial cells. The GABAB receptor is composed of two subunits (GABABR1 and GABABR2) and typically functions as a Gi protein–coupled receptor. The GABAB receptor is fully functional only when both subunits are expressed and are linked in a heterodimeric assembly (2). In addition to their well-characterized expression and function on neurons, GABA and functional GABAB receptors have been detected in peripheral tissues such as adrenal medulla, islets of Langerhans, placenta, and smooth muscle cells of the urinary bladder and uterus.
Airway epithelial cells play an integral part in airway function and disease (1, 3). A novel Gi-coupled receptor could have immense importance in epithelial cell function, since Gi regulation of cyclic AMP is pivotal in the central function of airway epithelial cells, mucociliary clearance. Morever, in some cells liberated Giα or Giβγ subunits cross-regulate phospholipase Cβ1, an enzyme that liberates inositol phosphates and increases intracellular calcium, which in turn regulates ion transport, mucin secretion, and ciliary beat frequency in airway epithelial cells—all components of a fundamental role of airway epithelial cells, mucus clearance.
Studies have shown that several subunits of GABAA receptor are expressed in airway epithelium (1, 4), and activation of GABAA receptor in airway epithelium induced goblet cell hyperplasia and mucus overproduction (1). We recently described the presence of GABA in airway epithelium and of GABAB receptor in airway smooth muscle (5). In this previous study very dense immunohistochemical staining of GABABR1 was detected in the airway epithelium. These findings led us to hypothesize that there may be functional GABAB receptors that could respond to GABA in airway epithelium.
In the present study, we investigated the expression of GABAB receptors in native guinea pig and human airway epithelium and in cultured human airway epithelial cell lines (BEAS-2B and H441), and assessed the functional coupling of the GABAB receptor to the Gi protein by demonstrating adenylyl cyclase inhibition, extracellular signal–regulated kinase (ERK) activation and crosstalk activation of phospholipase C. Furthermore, the expression of glutamic acid decarboxylase (GAD65/67) in airway epithelium was confirmed to identify one potential source of the endogeneous ligand GABA in airways.
MATERIALS AND METHODS
Materials
The human bronchial epithelial cell line (BEAS-2B) (ATCC CRL-9609) and the human lung epithelial adenocarcinoma cell line (H441) (ATCC HTB-174) were obtained from ATCC (Manassas,VA). BEGM bronchial epithelial cell basal medium and growth supplements (for BEAS-2B cells) were purchased from Lonza (Walkersville, MD). RPMI basal media, fetal bovine serum, and L-glutamine (H441 cells) were purchased from Invitrogen. [32P]-α-ATP (800 Ci/mmol), [3H]cAMP (32 Ci/mmol), and [3H]myo-inositol (20 Ci/mmol) were obtained from MP Biomedicals (Irvine, CA). Lysates of human brain cerebral cortex were used as positive protein controls on immunoblots and were obtained from BD Biosciences (Palo Alto, CA). Total RNA from whole human brain was purchased from Clontech (Mountain View, CA). Pertussis toxin and protease inhibitor cocktail III were purchased from EMD Biosciences (San Diego, CA). SKF97541 (also known as CGP35024) was obtained from Tocris Bioscience (Ellisville, MO). All other chemicals were obtained from Sigma (St. Louis, MO) unless otherwise stated. We used R(+)-baclofen hydrochloride for all baclofen studies.
Cell Culture
The BEAS-2B cells were grown to confluence on 24- (adenylyl cyclase) or 6-well (immunoblotting) plates in culture medium (BEGM, supplemented with bovine pituitary extract, hydrocortisone, human epidermal growth factor, epinephrine, insulin, triiodothyronine, transferrin, gentamicin/amphotericin-B, and retinoic acid as recommended by the manufacturer [Lonza]) at 37°C in an atmosphere of 5% CO2–95% air. In all studies, cell culture medium was not changed for 72 hours (conditioned media) before initiating treatments with GABAB agonists. For analysis of ERK phosphorylation, cells were treated with GABAB agonists (baclofen or SKF97541) (100 μM) for 5 minutes in 72-hour conditioned culture medium. Pertussis toxin (100 ng/ml) was preincubated for 4 hours before the addition of GABAB agonists. After treatment, cells were rinsed with cold phosphate-buffered saline (PBS), and ice-cold lysis buffer (50 mM Tris-HCl, pH 8.0, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, 1:200 dilution of protease inhibitor cocktail III [EMD Biosciences, San Diego, CA], 1 mM Na3VO4, 1 mM NaF) was added. After the whole cell lysates were centrifuged at 15000 × g for 15 minutes at 4°C, the supernatant was saved and the protein concentration was determined. Cell lysates were solubilized by heating at 95°C for 5 minutes in sample buffer (final concentrations: 50 mM Tris-HCl, pH6.8, 2.5% SDS, 6% glycerol, 2.5% 2-mercaptoethanol, bromophenol blue) and were stored at −20°C.
Isolation of Airway Epithelium or Whole Brain from Guinea Pig
Studies were approved by the Institutional Animal Care and Use Committee (College of Physicians and Surgeons, Columbia University, New York, NY). Adult male guinea pigs were deeply anesthetized by intraperitoneal pentobarbital (100 mg/kg), the chest cavity was opened, and the animal was exsanguinated before the dissection of the trachea. The trachea or whole brain were surgically removed and placed in cold (4°C) buffer (10 mM Hepes, pH 7.4, 1 mM EDTA, 1:200 dilution of protease inhibitor cocktail III; EMD Biosciences). The exterior of guinea pig trachea was meticulously dissected free of adherent connective tissue under a dissecting microscope. Tracheas were then cut open longitudinally along the anterior border. The tracheal epithelium was removed, and tracheal epithelium or whole brain was homogenized (Tekmar Ultra Turrax T25 high-speed homogenizer [Tekmar, Mason, OH] set at top speed for 30 s) in Trizol reagent (Ambion, Austin, TX) for total RNA extraction according to the manufacturer's recommendations or in cold (4°C) buffer (10 mM Hepes, pH 7.4, 1 mM EDTA with a 1:200 dilution of protease inhibitor cocktail III). For protein samples the homogenate was filtered through 125-μm Nitex mesh and centrifuged at 1,000 × g for 10 minutes at 4°C. The supernatant was transferred into new tubes and centrifuged at 50,000 × g for 15 minutes at 4°C. The pellet was resuspended in the same buffer and centrifuged at 50,000 × g for 15 minutes at 4°C. The final pellet was resuspended in the same buffer and stored at −80°C.
Isolation of Airway Epithelium from Human Trachea
Studies were approved by Columbia University's Institutional Review Board (IRB) and deemed not human subjects research under 45 CFR 46. Discarded excess tracheal tissues from healthy donor lungs harvested for lung transplantation at Columbia University were transported to the laboratory in cold (4°C) Kreb-Henseleit buffer (in mM: NaCl 118, KCl 5.6, CaCl2 0.5, MgSO4 0.24, NaH2PO4 1.3, NaHCO3 25, glucose 5.6, pH 7.4). The exterior of the human trachea were dissected free of adhering connective tissue under a dissecting microscope. Tracheae were then cut open longitudinally along the anterior border. The thin tracheal epithelial layer was meticulously removed with forceps under a dissecting microscope and was homogenized in cold (4°C) buffer (10 mM Hepes, pH 7.4, 1 mM EDTA with a 1:200 dilution of protease inhibitor cocktail III) using a Tekmar Ultra Turrax T25 high speed homogenizer set at top speed for 30 seconds. The homogenate was filtered through 125-μm Nitex mesh and centrifuged at 1,000 × g for 10 minutes at 4°C. The supernatant was transferred into new tubes and centrifuged at 50,000 × g for 15 minutes at 4°C. The pellet was resuspended in the same buffer and centrifuged at 50,000 × g for 15 minutes at 4°C. The final pellet was resuspended in the same buffer and stored at −80°C.
RNA Isolation and RT-PCR
Total RNA was extracted from freshly dissected native human and guinea pig airway epithelium, cultured BEAS-2B cells, cultured H441 cells, and guinea pig whole brain using Trizol Reagent (Ambion) according to the manufacturer's recommendations. Total RNA from whole human brain (Clontech) was used as a positive control. Using the Advantage RT-for-PCR Kit (Clontech), 1 μg of total RNA was reverse transcribed at 42°C for 1 hour in 20 μl including 200 units of Moloney murine leukemia virus reverse transcriptase, 20 units of RNase inhibitor, 20 pmol oligo(dT) primer, and 0.5 mM each of dNTP mix in reaction buffer (50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2). PCR was performed by adding 5 μl of newly synthesized cDNA to a 45-μl reaction mixture yielding final concentrations of 0.2 mM of each dNTP, 1× Advantage 2 Polymerase Mix, PCR buffer (Clontech), and 0.4 μM of both sense and antisense primers for corresponding GABAB receptor subunits and isoforms of glutamic acid decarboxylase (GAD) (Table 1). PCR was performed using a PTC-200 Peltier thermal cycler (MJ Research, Waltham, MA) at indicated temperatures and times (Table 1). PCR products were electrophoresed on 5% nondenaturing polyacrylamide gel in 1× Tris, acetate, EDTA buffer. The gel was stained with ethidium bromide (Molecular Probes, Eugene, OR), visualized using ultraviolet illumination, and analyzed using Quantity One software (Bio-Rad, Hercules, CA).
TABLE 1.
PCR PRIMER SEQUENCES FOR GABAB RECEPTOR SUBTYPES AND GAD ISOFORMS
| Target | Sequence of Primer | Amplicon size (bp) | Denaturation (°C) | Annealing/Extension (°C) | Annealing/Extension time (min) | No. of PCR Cycles |
|---|---|---|---|---|---|---|
| GABABR1 | FP: 5′-CAAGAAGATTGGCTACTATGACAGCACCAAGGATGA-3′ | R1a/b/c: 302 | 94 | Human: 72/72 | 1/1 | 40 |
| RP: 5′-CCAGGGGGAAGACAGCAGCTAAAGCCAGTGAG-3′ | R1e: 150 | 94 | Guinea pig: 60/72 | 0.5/0.5 | 40 | |
| GABABR2 | FP: 5′-GTCCACCTCGGTCACCAGTGTGAACCAA-3′ | 357 | 94 | 68/68 | 1/1 | 38 |
| RP: 5′-AGCCGACGCTGGATGTGTTCTGGAGAGT-3′ | 357 | 94 | 68/68 | 1/1 | 38 | |
| GAD65 | FP: 5′-GAGTGGAGTGGAGAGGGCCAACTCTGTGAC-3′ | 488 | Human: 96 | Human: 68/68 | 1/1 | 45 |
| RP: 5′-TTGTGGTTCCATACTCCATCATTCTGGCTTTAATC-3′ | 488 | Guinea pig: 90 | Guinea pig: 60/70 | 0.5/0.5 | 45 | |
| GAD67 | FP: 5′-GACAATGTGATTTTGATAAAGTGCAATGAA-3′ | 375 | Human: 96 | Human: 68/68 | 1/1 | 45 |
| RP: 5′-CATCTGGTTGCATCCTTGGAGTATACCCT-3′ | 375 | Guinea pig: 90 | Guinea pig: 60/70 | 0.5/0.5 | 45 |
Western Blot Analysis
Whole cell or tissue lysates were electrophoresed (8–10% SDS-PAGE) and transferred to PVDF membranes. The PVDF membranes were blocked for 1 hour at room temperature with 5% nonfat dry milk (for antibodies against GABAB receptor) or bovine serum albumin (BSA) (for antibodies against total and phospho-ERK and β-actin) in TBS with 0.1% Tween 20, and were then probed with antibodies directed against GABABR1 (rabbit polyclonal 1:1,000, sc-14006; Santa Cruz Biotechnology, Santa Cruz, CA), GABABR2 (guinea pig polyclonal 1:5,000, AB5394; Chemicon, Temecula, CA), total ERK (rabbit polyclonal 1:1,000, #9102; Cell Signaling Technology, Danvers, MA), phospho-ERK (rabbit polyclonal 1:1,000, #9101, Cell Signaling Technology), or β-actin (mouse monoclonal 1:200, sc-8438; Santa Cruz Biotechnology) overnight at 4°C. After washing three times, membranes were incubated for 1 hour at room temperature with horseradish peroxidase (HRP)-conjugated secondary anti-rabbit antibodies (1:5,000, NA934V; Amersham Biosciences, Arlington Heights, IL), with HRP-conjugated secondary anti-mouse antibodies (1:5,000, NA931V; Amersham Biosciences) or with HRP-conjugated secondary anti-guinea pig antibodies (1:5,000, AP193P; Chemicon). The signal from the immunoreactive bands was detected by ECL Plus (Amersham Biosciences) according to the manufacturer's recommendations and developed on film (Kodak Biomax light film; Kodak, Rochester, NY). Film was developed such that band intensities were within the linear range of film responses; and band intensities were quantified using Quantity One software (Bio-Rad). Data presented were means ± SEM values.
Immunohistochemistry
Guinea pig tracheal rings were fixed using 10% formalin for 24 hours at room temperature. Tracheal rings were dehydrated, paraffin embedded, sectioned (5 μm), dewaxed in xylene, and rehydrated in a graded alcohol series to water. Endogenous peroxidase was blocked in 0.3% hydrogen peroxide in PBS. Heat-mediated antigen retrieval was performed with 10 mM sodium citrate buffer, pH 6.0 for 30 minutes. An avidin biotin blocking kit (Vector Laboratories, Peterborough, UK) and 10% serum in PBS was used to block endogenous biotin and nonspecific protein binding, respectively. Slides were rinsed with PBS and incubated overnight at 4°C in primary antibody against GABABR1 (rabbit polyclonal 1:250, sc-14006; Santa Cruz Biotechnology) or GAD65/67 (rabbit polyclonal 1:5,000, AB1511; Chemicon) in 2% serum in PBS. A parallel tracheal ring section was incubated with the appropriate isotype IgG antibody as a negative control. After overnight incubation at 4°C, slides were washed with PBS, and primary antibodies were detected using biotinylated anti-rabbit antibodies (Vector Laboratories) at a concentration of 1:100. The antigen antibody complex was then visualized by the enzymatic reduction of 3,3-diaminobenzidine tetrahydrochloride. Sections were counterstained with hematoxylin and dried, and cover slides were mounted using Poly-mount (Polysciences, Warrington, PA).
Adenylyl Cyclase Assays
Adenylyl cyclase activity was measured as previously described (6). Briefly, cells in 24-well plates were washed once with warm PBS (37°C). In some experiements cells were pretreated for 4 hours with pertussis toxin (100 ng/ml) in cell culture media before washing with PBS. One hundred microliters of warm PBS was added to each well. Subsequently, 50 μl of 3× adenylyl cyclase buffer (6) was added directly to the wells (to achieve final concentrations of 50 mM HEPES, pH 8.0, 50 mM NaCl, 0.4 mM EGTA, 1 mM cAMP, 7 mM MgCl2, 0.1 mM ATP [20 μCi/ml 32P-α-ATP], 0.1 mg/ml BSA, 50 units/ml creatine phosphoskinase, and 7 mM phosphocreatine) in the absence (basal activity) or presence of 10 μM forskolin ± 100 μM baclofen or 100 μM SKF97541 and the plates were incubated at 37°C for 15 minutes. The reactions were terminated by the addition of 100 μl stop buffer (50 mM HEPES, pH 7.5, 2 mM ATP, 0.5 mM cAMP [0.5 μCi/ml 3H-cAMP], 2% SDS) and synthesized 32P-cAMP was separated and quantitated by sequentially column chromatography over dowex and alumina as described (7).
Inositol Phosphate Assays
Synthesis of total 3H-inositol phosphates was measured in confluent BEAS-2B cells in 24-well tissue culture plates as described (6, 8). Briefly, after overnight loading with 3H-myo-inositol (10 μCi/ml, 20 Ci/mmol) in inositol-free and serum-free Dulbecco's modified Eagle's medium (Chemicon), plates were washed three times (37°C, 500 μl Hanks' balanced salt solution with 10 mM LiCl). Incubation of cells with GABAB receptor agonists (baclofen) in a final volume of 300 μl at 37°C for 30 minutes was performed in the absence or presence of pertussis toxin (PTX; 4 h before the addition of baclofen) or 5 μM U73122, a PLC inhibitor (30 min before the addition of baclofen). Reactions were terminated, and total [3H]inositol phosphates recovered by chromatography (6).
Statistical Analysis
Statistical analysis was performed using repeated measures of ANOVA, followed by Bonferroni post-test comparison using Prism 4.0 software (GraphPad, San Diego, CA). Data are presented as mean ± SEM; P < 0.05 was considered significant.
RESULTS
RT-PCR Analysis of GABAB Receptor Isoforms in Airway Epithelium
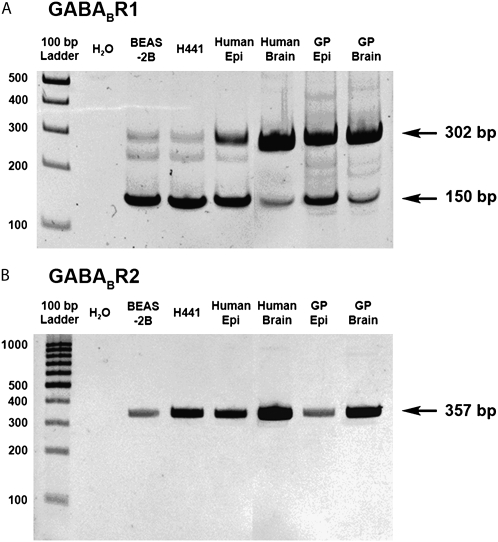
RT-PCR analysis demonstrated mRNA encoding the human GABABR1 protein (Figure 1A) and the GABABR2 protein (Figure 1B) in BEAS-2B cells, H441 cells, native human airway epithelium, guinea pig airway epithelium, and whole brain (positive controls) from both species. To date, four splice variants of the GABABR1 protein have been reported (GABABR1a, 1b, 1c, and 1e). In the present study primers for GABABR1 flanked the omitted exon of GABABR1e and therefore could distinguish 1e from other isoforms (i.e., 1a, 1b, and 1c). We observed two PCR products of predicted sizes corresponding to at least two splice variants of GABABR1, GABABR1a/b/c (302 bp) and GABABR1e (150 bp) in human airway epithelium, BEAS-2B cells, H441 cells, and guinea pig airway epithelium (Figure 1A). Messenger RNA encoding GABABR2 was also detected in human airway epithelium, BEAS-2B cells, H441 cells, and guinea pig airway epithelium (Figure 1B). At least three independent tissue or cell sources were analyzed for each mRNA studied.
Figure 1.
Representative gel images of RT-PCR analysis of total RNA using primers specific for GABAB receptor subunits. (A) Splice variants (R1a/b/c [302 bp] and R1e [150 bp]) of GABABR1 were detected in human airway epithelium (Epi), human cultured bronchial epithelial cells (BEAS-2B), human lung epithelial adenocarcinoma cells (H441), guinea pig (GP) airway epithelium, and GP and human whole brain. (B) Messenger RNA encoding GABABR2 was detected in both human epithelial cell lines and in freshly isolated epithelium or whole brain from guinea pig and human. Images are representative of at least three independent RT-PCR analyses from each cell or tissue source.
Immunoblot Analysis of GABABR Isoforms in Airway Epithelium
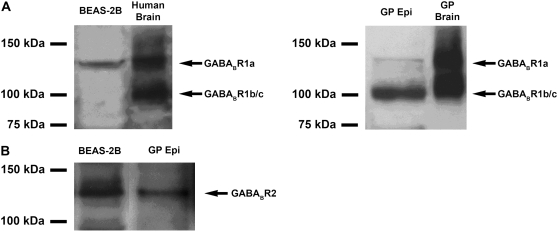
The antibody used for immunoblot analysis of GABABR1 recognizes the common COOH terminus region of three of the four known human isoforms of GABABR1 (i.e., GABABR1a, 1b, and 1c, but not 1e). Two immunoreactive bands of approximately 100 and 130 kD were identified in whole brain from both species (Figure 2A). Whereas the larger 130-kD band corresponds to the expected molecular mass of GABABR1a, the 100-kD band corresponds to the molecular mass of either GABABR1b or GABABR1c. A smaller splice variant of GABABR1 (GABABR1 b or e) was predominant in guinea pig airway epithelium, while only the larger splice variant (GABABR1a) was identified in BEAS-2B cells (Figure 2A). An antibody raised against the GABABR2 protein identified a single immunoreactive band of 120 kD in protein samples from BEAS-2B cells and guinea pig tracheal epithelium (Figure 2B), consistent with the predicted size for GABABR2 (9). Since the secondary antibody to detect the GABABR2 primary antibody was an anti–guinea pig antibody, we performed control experiments in which the primary antibody was omitted. In these negative control studies no immnunoreactive bands in the appropriate molecular weight range were detected (data not shown).
Figure 2.
Representative immunoblot analysis using protein prepared from (A, left) cultured human BEAS-2B cells (100 μg), whole human brain (25 μg), or (A, right) GP tracheal epithelium (Epi) (100 μg), and GP brain (25 μg). A larger splice variant (130 kD) corresponding to GABABR1a was detected in both brain-positive controls and in human BEAS-2B cells. A smaller splice variant (100 kD) that could represent either GABABR1b or 1c was detected in both brain-positive controls and in freshly isolated GP epithelium but not in cultured human BEAS-2B cells. (B) Representative immunoblot using antibody directed against GABABR2 identified an immunoreactive band of appropriate molecular mass (120 kD) in cultured human BEAS-2B cells and in freshly isolated GP epithelium. Images are representative of at least 3 independent immnoblot analyses from each cell or tissue source.
Immunohistochemical Analysis of GABABR1 Expression in Guinea Pig Trachea
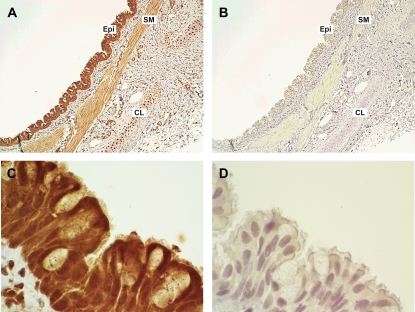
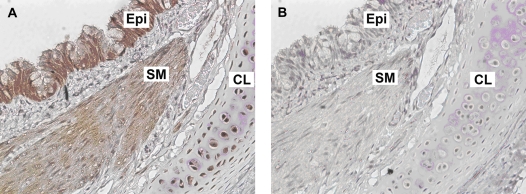
To confirm the protein localization of GABABR1 to airway epithelium in guinea pig airways, immunohistochemistry was performed. Heavy immunoreactive staining was detected in guinea pig trachea within basal mucin-secreting and ciliated columnar epithelial cells with less intense staining detected in the airway smooth muscle and tracheal cartilage chondrocytes (Figures 3A and 3C). Representative results of an isotype-specific IgG primary antibody (negative control) are shown in Figures 3B and 3D.
Figure 3.
Representative immunohistochemical staining of GABABR1 in formalin-fixed GP trachea. (A and C) GABABR1 was localized in both basal mucin-producing and ciliated columnar epithelial cells within tracheal epithelium (original magnification: ×10 and ×100 objectives, respectively). (B and D) Isotype-specific negative control primary antibody (rabbit IgG) in a parallel section of GP trachea (original magnification: ×10 and ×100 objectives, respectively). Sections were counterstained with hematoxylin. Images are representative of at least three independent immnohistochemical analyses from GP trachea.
Functional Coupling of the GABAB Receptor to Gi Signaling Pathways in BEAS-2B Cells
Demonstration of GABAB receptor mRNA and protein in airway epithelium led us to determine whether the receptor demonstrated classical coupling to the Gi protein by evaluating (1) pertussis toxin–sensitive agonist-induced inhibition of adenylyl cyclase activity, (2) pertussis toxin–sensitive agonist-induced activation of mitogen-activated protein kinase (MAPK ERK), and (3) Gi protein–mediated cross-regulation of phospholipase C activation of inositol phosphate synthesis.
Agonist-Induced Inhibition of Adenylyl Cyclase Activity in BEAS-2B Cells
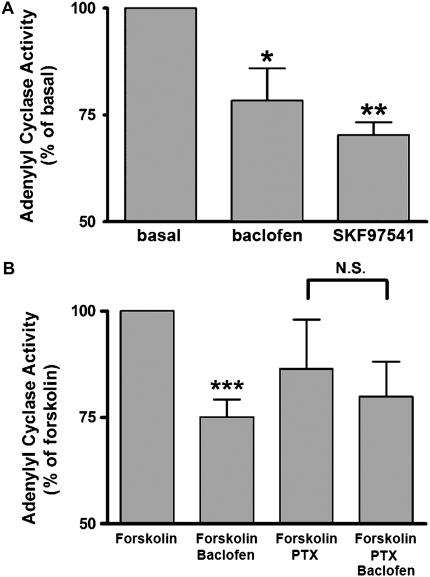
GABAB receptor–mediated inhibition of adenylyl cyclase activity via coupling to the Gi protein is well known in neurons (10). To examine whether GABAB receptor agonists inhibit adenylyl cyclase activity in cultured BEAS-2B cells, we measured adenylyl cyclase activity in the presence or absence of either baclofen (100 μM) or SKF97541 (100 μM). Both GABAB agonists significantly inhibited basal adenylyl cyclase activity (baclofen; P < 0.05, n = 6; SKF97541; P < 0.01, n = 6) (Figure 4A). Baclofen also significantly inhibited the forskolin-stimulated adenylyl cyclase activity (P < 0.001, n = 13), and this inhibition was blocked by 4 hours of pretreatment with pertussis toxin (100 ng/ml) (n = 10) (Figure 4B).
Figure 4.
(A) Basal adenylyl cyclase activity in cultured BEAS-2B cells in the presence or absence of baclofen (100 μM) or SKF97541 (100 μM) (n = 6 experiments). Data represent means ± SEM. *P < 0.05, **P < 0.01 compared with control. (B) Forskolin (10 μM)-stimulated adenylyl cyclase activity in cultured BEAS-2B cells in the presence or absence of baclofen (100 μM) (n = 13). In some experiments, cells were pretreated with pertussis toxin (PTX) (100 ng/ml for 4 h pretreatment before baclofen treatment) (n = 10). Data represent means ± SEM. ***P < 0.001 compared with forskolin. In each experiment, values were determined in triplicate.
Agonist-Induced Activation of ERK in BEAS-2B Cells
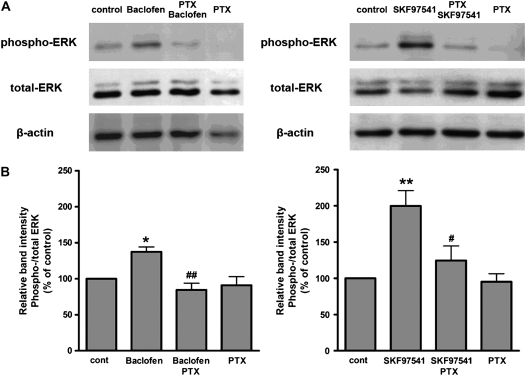
Many Gi-coupled receptors are known to activate the ERK pathway (11). ERK activation was identified by increased phospho-ERK on immunoblots and expressed as a ratio of phospho/total ERK for each sample. Baclofen (100 μM, 5 min) significantly increased phosphorylation of ERK (Figure 5, left; n = 5, P < 0.05). In separate experiments, a second selective GABAB agonist, SKF-97541 (100 μM, 5 min), also increased ERK phosphorylation (Figure 5, right; P < 0.01, n = 5). Four-hour pretreatment of cells with pertussis toxin (100 ng/ml) abrogated increases in ERK phosphorylation by either GABAB agonist (P < 0.01 and P < 0.05 for pertussis toxin + agonist versus baclofen or SKF-97541 alone, respectively, n = 5) confirming the role of Gi in GABAB receptor activation of ERK.
Figure 5.
(A) Immunoblot analyses of phospho-ERK or total ERK expression in cultured BEAS-2B cells. Cells were treated with baclofen (100 μM) (left) or SKF97541 (100 μM) (right) for 5 minutes in the presence or absence of PTX (100 ng/ml for 4 h pretreatment before GABAB selective agonist treatment). Representative immunoblots from five experiments are shown. (B) Relative band intensities of phospho-/ total ERK from five separate immunoblots. The effects of baclofen (left) and SKF97541 (right) on ERK phosphorylation are given as percentage of control. Data represent means ± SEM. *P < 0.05, **P < 0.01 compared with control. #P < 0.05, ##P < 0.01 compared with baclofen or SKF97541.
Agonist-Induced Stimulation of Inositol Phosphate Synthesis in BEAS-2B Cells
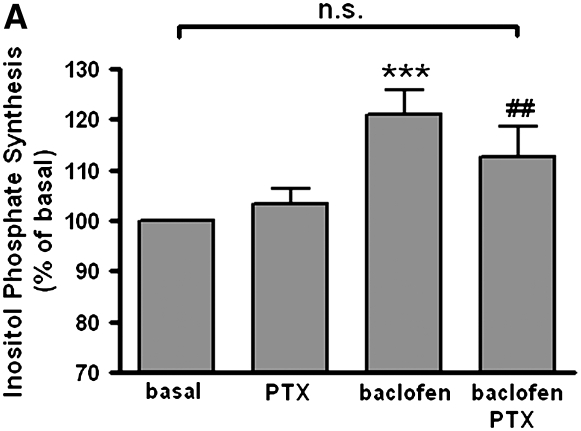
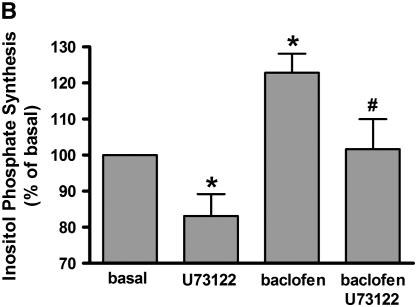
Classically receptors that couple to the Gq protein activate phospholipase C (PLC) increasing inositol phosphates and intracellular calcium. However, in some cells cross-talk to PLCβ1 from either Giα or Giβγ subunits has been described (12–15). To examine whether GABAB receptor agonists cross-talk through Gi to stimulate inositol phosphate synthesis in cultured BEAS-2B cells, we measured inositol phosphate synthesis in the presence or absence of baclofen (100 μM). Baclofen significantly increased inositol phosphate synthesis. These increases were blocked by 4 hours of pretreatment with pertussis toxin (100 ng/ml) (n = 10) (Figure 6A) or by 30 minutes of pretreatment with the PLC inhibitor U73122 (5 μM) (n = 11) (Figure 6B).
Figure 6.
(A) Effects of 4 hours of pretreatment with PTX (100 ng/ml) on 100 μM baclofen-induced inositol phosphate synthesis in BEAS-2B cells (n = 10). (B) Effects of PLC inhibitor (5 μM U73122) on 100 μM baclofen-induced inositol phosphate synthesis in BEAS-2B cells (n = 11). Data are shown as percentage of basal and represent means ± SEM. *P < 0.05 and ***P < 0.001 compared with basal. #P < 0.05 and **P < 0.01 compared with baclofen.
RT-PCR Analysis of GAD65 and GAD67 in Airway Epithelium
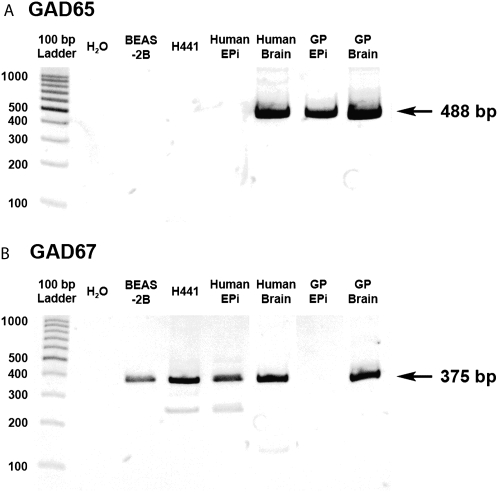
We questioned whether airway epithelium could be the source of the endogenous ligand, GABA, for the GABAB receptor by assessing the presence of the mRNA encoding the enzyme that synthesizes GABA, glutamic acid decarboxylase (GAD). RT-PCR analysis demonstrated mRNA encoding GAD in BEAS-2B cells, H441 cells, native human airway epithelium, and guinea pig airway epithelium (Figure 7). We observed PCR products of predicted sizes corresponding to GAD65 and GAD67 in both human and guinea pig brain. While GAD67 was detected in human airway epithelium, BEAS-2B cells, and H441 cells (Figure 7B), GAD65 was detected in guinea pig airway epithelium (Figure 7A).
Figure 7.
Representative gel images of RT-PCR analysis of total RNA using primers specific for GAD65 and GAD67. (A) mRNA encoding GAD65 (488 bp) was detected in whole brain from both species and freshly isolated GP epithelium (Epi). (B) mRNA encoding GAD67 [375bp] was detected in whole brain from both species and in freshly isolated human epithelium and both human cultured epithelial cell lines (BEAS-2B and H441). Images are representative of at least three independent RT-PCR analyses from each cell or tissue source.
Immunohistochemical Analysis of GAD65/67 Expression in Guinea Pig Trachea
To confirm the protein localization of GAD65/67 to airway epithelium in guinea pig airways, immunohistochemistry was performed. GAD65/67 immunoreactivity in guinea pig trachea was detected in airway epithelium (Figure 8A), with no staining in the negative control studies with rabbit IgG (Figure 8B).
Figure 8.
Representative immunohistochemical staining of GAD65/67 in formalin-fixed GP trachea. (A) GAD65/67 immunostaining detected in tracheal epithelium (Epi). (B) Isotype-specific negative control primary antibody (rabbit IgG) in a parallel section of GP trachea. Both sections were counterstained with hematoxylin (original magnification: ×200). Images are representative of at least three independent immnohistochemical analyses from GP trachea.
DISCUSSION
The present study is the first to demonstrate that functional GABAB receptors are expressed in both native human and guinea pig airway epithelium and cultured airway epithelial cells. Both GABABR1 and GABABR2 subunits were detected at the level of mRNA (by RT-PCR) and protein (by immunoblot) at appropriate molecular weights. Immunohistochemistry localized the GABABR1 subunit to airway epithelium in guinea pig tracheal rings. The GABAB receptor agonist baclofen inhibited adenylyl cyclase activity and induced ERK phosphorylation, which was abrogated by pretreatment of cultured BEAS-2B cells with pertussis toxin, confirming classical coupling of the GABAB receptor to the Gi protein signaling pathways in these cells. Furthermore, the enzyme GAD 65/67 that synthesizes GABA, the endogenous ligand for the GABAB receptor, was also detected in airway epithelium at the level of mRNA (by RT-PCR) and protein (by immunohistochemistry).
Several studies indicate the possible expression of functional GABAB receptors in peripheral and nonneuronal cells such as pancreatic β cells (16), adrenocortical cells (17), cardiomyocytes (18), chondrocytes (19), and osteoblasts (20). Furthermore, GABABR1 mRNA expression was detectable using RT-PCR analysis in many peripheral organs, including heart, spleen, lung, liver, intestine, kidney, stomach, adrenal gland, testis, ovary, and urinary bladder (21).
Recent evidence has shown that GABAB receptors must exist as a heterodimer to form a functional Gi protein–coupled receptor in the plasma membrane (2, 22). The large N-terminal extracellular domain, in particular the GABABR1 subunit, is the site for ligand binding, whereas the GABABR2 subunit is crucial for effector coupling (23). The presence of both subunits in airway epithelial cells suggests that they can assemble functional GABAB receptor heterodimers. Functional GABAB receptors coupling through Gi to the inhibition of adenylyl cyclase or ERK phosphorylation were demonstrated using the GABAB-selective agonists baclofen or SKF97541 in the absence or presence of pertussis toxin. To date, four different splice variants (GABABR1a, b, c, and e) of GABABR1 have been identified in humans. Indeed, we identified the expression of at least three splice variants in airway epithelium (GABABR1 a/b/c and e at the mRNA level and GABABR1 a or b/c at the protein level). At the present time, there are no confirmed GABABR2 splice variants (22).
Although we identified mRNA encoding of the GABABR1 subtype and the GABABR2 subtype in RNA isolated from freshly dissected human and guinea pig airway epithelium, RNA isolated from freshly dissected tissues invariably will contain some RNA from nonepithelial cells despite careful dissection. Therefore, to confirm the epithelial cell–specific expression of the GABABR1 and GABABR2 subunits, we analyzed RNA isolated from homogenous cultures of airway epithelial cells and confirmed the expression of the GABABR1 and GABABR2. Furthermore, immunohistochemistry in guinea pig tracheal rings localized the GABABR1 protein to both basal mucin-producing and ciliated columnar epithelial cells within the tracheal epithelium. The GABABR1 protein was also expressed in tracheal smooth muscle cells and chondrocytes as previously described (5, 19, 20) suggesting that multiple cell types in the airway may be responsive to endogenous GABA.
After demonstrating the mRNA and protein expression of GABAB receptors in airway epithelium, we sought to confirm its coupling to G proteins in general and its specific classical coupling to the Gi protein. Three Gi-specific coupling pathways were investigated using the GABAB agonist baclofen. Inhibition of adenylyl cyclase is a well-known effect of Gi protein activation and is known to occur in airway smooth muscle in response to activation of several Gi-coupled receptors (e.g., M2 muscarinic receptor and GABAB receptor). Activation by phosphorylation of ERK is a ubiquitous signaling pathway after Gi activation, and pertussis toxin is a widely used tool to inactivate and implicate Gi proteins in signaling events. Indeed, baclofen inhibited adenylyl cyclase and activated ERK phosphorylation in a pertussis toxin–sensitive manner in BEAS-2B cells, confirming the coupling of GABAB receptors to Gi proteins in these cells. The specificity of baclofen's effect at the GABAB receptor in the present study is supported by the finding that another GABAB agonist, SKF-97541, could mimic the baclofen's effect on ERK activation.
R-baclofen and SKF97541 (also known as CGP35024) are widely used selective agonists for the GABAB receptor. The intrinsic activity of SFK97541 at the GABAB receptor is nearly 10 times that of baclofen and 5 times that of GABA (24). These agonists do not have significant affinity for the GABAA receptor (25–29).
The physiological role of GABAB receptors in airway epithelium and of GABA-ergic modulation of intercellular cAMP or ERK activation is at present unclear. Many of the protective and anti-inflammatory functions performed by airway epithelial cells are cAMP-dependent processes activated by the β2-adrenergic receptor-adenylyl cyclase (β2AR-AC) signaling cascade via the Gsα protein (30). In particular, activation of the β2AR-AC system in human airway epithelial cells stimulates mucociliary clearance, water and electrolyte transport, and the release of bronchodilatory substances (e.g., nitric oxide) (30–34). In contrast, Giα2, which couples to the inhibition of adenylyl cyclase, was also distributed in guinea pig tracheal epithelial cells (35). In the present study, baclofen inhibited adenylyl cyclase activity in a pertussis toxin–sensitive manner, suggesting that GABAB receptor activation in airway epithelium could inhibit cAMP-mediated airway protective and anti-inflammatory functions.
Mitogenic pathways are linked to MAPK cascades that can be activated by a variety of extracellular stimuli, including agonists for G protein–coupled receptors. Giα2 plays an important role in pathways that control cell differentiation and growth via activation of MAPK. In airway epithelial cells Giα2 regulates cell growth (36), and furthermore, MAP kinases including ERK regulate the expression of chemokines such as IL-8, IL-13, and IL-17, which is increased in the airways of patients with asthma (37, 38). In the present study, we found that baclofen induced ERK phosphorylation in BEAS-2B cells. This result was consistent with observations in HEK-293 cells transfected with GABABR1 and R2 subunits (39). Furthermore, baclofen can increase cell proliferation in a GABAB receptor antagonist–sensitive manner in rat growth plate chondrocytes (19). These results suggest that activation of GABAB receptor expressed in airway epithelial cells would theoretically favor airway epithelial cells growth through MAPK.
In addition to cyclic AMP, calcium is a second messenger important in the central functional role of airway epithelial cells; mucociliary clearance (40, 41). Intracellular calcium is increased by inositol phosphates after the activation of phospholipase C (PLC) by Gq-coupled receptors. However, evidence exists in multiple cell types that activated Giα or Giβγ subunits can cross-activate PLC, thus increasing inositol phosphates and calcium (12–15). Therefore, we questioned whether GABAB agonists could cross-talk through Gi to increase inositol phosphate levels. The GABAB receptor agonist baclofen stimulated inositol phosphate synthesis, which was attenuated by pertussis toxin or an inhibitor of PLC demonstrating GABAB receptor–Gi protein–PLC crosstalk in human airway epithelial cells.
GAD65/67 was also identified in airway epithelium at the mRNA and protein level. Human airway epithelium (BEAS-2B cells, H441 cells, and freshly dissected human airway epithelium) expressed mRNA encoding GAD67 but not GAD65. In contrast, guinea pig airway epithelium expressed mRNA encoding GAD65. Previously both GAD65 and GAD67 protein were identified in cultured BEAS-2B cells and primary cultures of human airway epithelial cells (1). Identification of both isoforms in BEAS-2B cells in this previous study as opposed to the detection of only the GAD67 isoform in the present study could reflect phenotypic regulation of expression of these isoforms in cell culture. These results suggest that airway epithelium could be the source of the endogeneous ligand GABA for GABAB receptors in airway.
The demonstration of the synthetic enzymes for GABA in the current and a previous study (1), and the expression of both GABAB and GABAA (1) receptors in airway epithelial cells, suggests a previously unknown autocrine–paracrine GABAergic system exists in airway epithelial cells. The potential interplay between simultaneous activation of GABAA and GABAB receptors on the same epithelial cells is unknown. However, depolarization of membrane potential through GABAA receptor activation was suggested to promote epithelial cell proliferation and mucus production (1). Similarly, activation of Gi through GABAB receptor activation would be predicted to also favor proliferation after MAPK activation and decreased mucociliary clearance secondary to decreased cyclic AMP levels (30–34).
Further studies are required to identify the functional role of GABAB receptors in the normal physiology and perhaps pathophysiology in airway epithelial cells. GABA may modulate an uncharacterized signaling cascade via GABAB receptors expressed in airway epithelium. This signaling cascade could be a potential therapeutic target for modulation of airway epithelial cell functions.
This work was supported by National Institutes of Health grant from the Institute of General Medical Sciences GM065281 to C.E.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0414OC on April 10, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, et al. GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med 2007;13:862–867. [DOI] [PubMed] [Google Scholar]
- 2.White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 1998;396:679–682. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE. Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc 2004;1:93–98. [DOI] [PubMed] [Google Scholar]
- 4.Jin N, Narasaraju T, Kolliputi N, Chen J, Liu L. Differential expression of GABAA receptor pi subunit in cultured rat alveolar epithelial cells. Cell Tissue Res 2005;321:173–183. [DOI] [PubMed] [Google Scholar]
- 5.Osawa Y, Xu D, Sternberg D, Sonett JR, D'Armiento J, Panettieri RA, Emala CW. Functional expression of the GABAB receptor in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2006;291:L923–L931. [DOI] [PubMed] [Google Scholar]
- 6.Hotta K, Emala CW, Hirshman CA. TNF-α upregulates Giα and Gqα protein expression and function in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 1999;276:L405–L411. [DOI] [PubMed] [Google Scholar]
- 7.Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem 1974;58:541–548. [DOI] [PubMed] [Google Scholar]
- 8.Jooste E, Zhang Y, Emala CW. Rapacuronium preferentially antagonizes the function of M2 versus M3 muscarinic receptors in guinea pig airway smooth muscle. Anesthesiology 2005;102:117–124. [DOI] [PubMed] [Google Scholar]
- 9.Waldvogel HJ, Billinton A, White JH, Emson PC, Faull RL. Comparative cellular distribution of GABAA and GABAB receptors in the human basal ganglia: immunohistochemical colocalization of the alpha 1 subunit of the GABAA receptor, and the GABABR1 and GABABR2 receptor subunits. J Comp Neurol 2004;470:339–356. [DOI] [PubMed] [Google Scholar]
- 10.Hill DR. GABAB receptor modulation of adenylate cyclase activity in rat brain slices. Br J Pharmacol 1985;84:249–257. [PMC free article] [PubMed] [Google Scholar]
- 11.Gutkind JS. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem 1998;273:1839–1842. [DOI] [PubMed] [Google Scholar]
- 12.Ethier MF, Madison JM. Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol 2006;35:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abebe W, Mustafa SJ. A1 adenosine receptor-mediated Ins(1,4,5)P3 generation in allergic rabbit airway smooth muscle. Am J Physiol 1998;275:L990–L997. [DOI] [PubMed] [Google Scholar]
- 14.Dickenson JM, Hill SJ. Involvement of G-protein betagamma subunits in coupling the adenosine A1 receptor to phospholipase C in transfected CHO cells. Eur J Pharmacol 1998;355:85–93. [DOI] [PubMed] [Google Scholar]
- 15.Tomura H, Itoh H, Sho K, Sato K, Nagao M, Ui M, Kondo Y, Okajima F. Betagamma subunits of pertussis toxin-sensitive G proteins mediate A1 adenosine receptor agonist-induced activation of phospholipase C in collaboration with thyrotropin: a novel stimulatory mechanism through the cross-talk of two types of receptors. J Biol Chem 1997;272:23130–23137. [DOI] [PubMed] [Google Scholar]
- 16.Brice NL, Varadi A, Ashcroft SJ, Molnar E. Metabotropic glutamate and GABA(B) receptors contribute to the modulation of glucose-stimulated insulin secretion in pancreatic beta cells. Diabetologia 2002;45:242–252. [DOI] [PubMed] [Google Scholar]
- 17.Metzeler K, Agoston A, Gratzl M. An intrinsic gamma-aminobutyric acid (GABA)ergic system in the adrenal cortex: findings from human and rat adrenal glands and the NCI-H295R cell line. Endocrinology 2004;145:2402–2411. [DOI] [PubMed] [Google Scholar]
- 18.Lorente P, Lacampagne A, Pouzeratte Y, Richards S, Malitschek B, Kuhn R, Bettler B, Vassort G. gamma-aminobutyric acid type B receptors are expressed and functional in mammalian cardiomyocytes. Proc Natl Acad Sci USA 2000;97:8664–8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamayama T, Maemura K, Kanbara K, Hayasaki H, Yabumoto Y, Yuasa M, Watanabe M. Expression of GABA(A) and GABA(B) receptors in rat growth plate chondrocytes: activation of the GABA receptors promotes proliferation of mouse chondrogenic ATDC5 cells. Mol Cell Biochem 2005;273:117–126. [DOI] [PubMed] [Google Scholar]
- 20.Fujimori S, Hinoi E, Yoneda Y. Functional GABA(B) receptors expressed in cultured calvarial osteoblasts. Biochem Biophys Res Commun 2002;293:1445–1452. [DOI] [PubMed] [Google Scholar]
- 21.Castelli MP, Ingianni A, Stefanini E, Gessa GL. Distribution of GABA(B) receptor mRNAs in the rat brain and peripheral organs. Life Sci 1999;64:1321–1328. [DOI] [PubMed] [Google Scholar]
- 22.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 2004;84:835–867. [DOI] [PubMed] [Google Scholar]
- 23.Galvez T, Prezeau L, Milioti G, Franek M, Joly C, Froestl W, Bettler B, Bertrand HO, Blahos J, Pin JP. Mapping the agonist-binding site of GABAB type 1 subunit sheds light on the activation process of GABAB receptors. J Biol Chem 2000;275:41166–41174. [DOI] [PubMed] [Google Scholar]
- 24.Green A, Walls S, Wise A, Green RH, Martin AK, Marshall FH. Characterization of [(3)H]-CGP54626A binding to heterodimeric GABA(B) receptors stably expressed in mammalian cells. Br J Pharmacol 2000;131:1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seabrook GR, Howson W, Lacey MG. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABAB receptors on neurones in rat brain slices. Br J Pharmacol 1990;101:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bon C, Galvan M. Electrophysiological actions of GABAB agonists and antagonists in rat dorso-lateral septal neurones in vitro. Br J Pharmacol 1996;118:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karla R, Ebert B, Thorkildsen C, Herdeis C, Johansen TN, Nielsen B, Krogsgaard-Larsen P. Synthesis and pharmacology of the baclofen homologues 5-amino-4-(4-chlorophenyl)pentanoic acid and the R- and S-enantiomers of 5-amino-3-(4-chlorophenyl)pentanoic acid. J Med Chem 1999;42:2053–2059. [DOI] [PubMed] [Google Scholar]
- 28.Hollis DM, Boyd SK. Characterization of the GABA(A) receptor in the brain of the adult male bullfrog, Rana catesbeiana. Brain Res 2003;992:69–75. [DOI] [PubMed] [Google Scholar]
- 29.Ritta MN, Calamera JC, Bas DE. Occurrence of GABA and GABA receptors in human spermatozoa. Mol Hum Reprod 1998;4:769–773. [DOI] [PubMed] [Google Scholar]
- 30.Nijkamp FP, Engels F, Henricks PA, Van Oosterhout AJ. Mechanisms of beta-adrenergic receptor regulation in lungs and its implications for physiological responses. Physiol Rev 1992;72:323–367. [DOI] [PubMed] [Google Scholar]
- 31.Salathe M. Effects of beta-agonists on airway epithelial cells. J Allergy Clin Immunol 2002;110:S275–S281. [DOI] [PubMed] [Google Scholar]
- 32.Smith PL, Welsh MJ, Stoff JS, Frizzell RA. Chloride secretion by canine tracheal epithelium: I. Role of intracellular c AMP levels. J Membr Biol 1982;70:217–226. [DOI] [PubMed] [Google Scholar]
- 33.Sanderson MJ, Dirksen ER. Mechanosensitive and beta-adrenergic control of the ciliary beat frequency of mammalian respiratory tract cells in culture. Am Rev Respir Dis 1989;139:432–440. [DOI] [PubMed] [Google Scholar]
- 34.Tamaoki J, Kondo M, Takemura H, Chiyotani A, Yamawaki I, Konno K. Cyclic adenosine monophosphate-mediated release of nitric oxide from canine cultured tracheal epithelium. Am J Respir Crit Care Med 1995;152:1325–1330. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Emala CW, Hirshman CA, Proud D, Jacoby DB, Levine MA. Characterization of GTP-binding proteins coupled to inhibition of adenylyl cyclase in guinea pig tracheal epithelial cells. Am J Respir Cell Mol Biol 1994;10:665–672. [DOI] [PubMed] [Google Scholar]
- 36.Kinane TB, Komatsuzaki K, Aleixo MD, Sunday ME, Ercolani L. Regulation of the G protein Galphai2 by growth and development in fetal airway epithelium. Am J Respir Cell Mol Biol 1999;20:35–42. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Kartha S, Iasvovskaia S, Tan A, Bhat RK, Manaligod JM, Page K, Brasier AR, Hershenson MB. Regulation of human airway epithelial cell IL-8 expression by MAP kinases. Am J Physiol Lung Cell Mol Physiol 2002;283:L690–L699. [DOI] [PubMed] [Google Scholar]
- 38.Inoue D, Numasaki M, Watanabe M, Kubo H, Sasaki T, Yasuda H, Yamaya M, Sasaki H. IL-17A promotes the growth of airway epithelial cells through ERK-dependent signaling pathway. Biochem Biophys Res Commun 2006;347:852–858. [DOI] [PubMed] [Google Scholar]
- 39.Balasubramanian S, Teissere JA, Raju DV, Hall RA. Hetero-oligomerization between GABAA and GABAB receptors regulates GABAB receptor trafficking. J Biol Chem 2004;279:18840–18850. [DOI] [PubMed] [Google Scholar]
- 40.Davis CW. Regulation of mucin secretion from in vitro cellular models. Novartis Found Symp 2002;248:113–125. [PubMed] [Google Scholar]
- 41.Ribeiro CM. The role of intracellular calcium signals in inflammatory responses of polarised cystic fibrosis human airway epithelia. Drugs R D 2006;7:17–31. [DOI] [PubMed] [Google Scholar]