Abstract
Microtubules are dynamic structures whose proper rearrangement during the cell cycle is essential for the positioning of membranes during interphase and for chromosome segregation during mitosis. The previous discovery of a cyclin B/cdc2-activated microtubule-severing activity in M-phase Xenopus egg extracts suggested that a microtubule-severing protein might play an important role in cell cycle-dependent changes in microtubule dynamics and organization. However, the isolation of three different microtubule-severing proteins, p56, EF1α, and katanin, has only confused the issue because none of these proteins is directly activated by cyclin B/cdc2. Here we use immunodepletion with antibodies specific for a vertebrate katanin homologue to demonstrate that katanin is responsible for the majority of M-phase severing activity in Xenopus eggs. This result suggests that katanin is responsible for changes in microtubules occurring at mitosis. Immunofluorescence analysis demonstrated that katanin is concentrated at a microtubule-dependent structure at mitotic spindle poles in Xenopus A6 cells and in human fibroblasts, suggesting a specific role in microtubule disassembly at spindle poles. Surprisingly, katanin was also found in adult mouse brain, indicating that katanin may have other functions distinct from its mitotic role.
INTRODUCTION
Microtubules are polymers of α and β tubulin that are used to organize membranous organelles during interphase and to segregate chromosomes during mitosis. Microtubules exhibit a property called dynamic instability in which microtubules continuously switch between phases of growth by polymerization of tubulin at their ends and phases of shrinkage by loss of tubulin subunits from their ends (Mitchison and Kirschner, 1984; Horio and Hotani, 1986; Walker et al., 1988). These dynamics change dramatically upon entry into mitosis when the turnover between monomer and polymer pools of tubulin increases dramatically (Saxton et al., 1984; Zhai et al., 1996). The behavior of individual microtubules within a mitotic spindle of a living cell has not been amenable to direct observation because the microtubules are closer together than the resolution limit of the light microscope. However, the dynamics of individual microtubules can be monitored in Xenopus egg extracts, which can be converted between interphase-like and metaphase-like states. Experiments in Xenopus extracts have been used to demonstrate that the increased turnover in M-phase is at least partially due to an increase in the catastrophe frequency or frequency with which polymerizing microtubules switch to depolymerizing (Belmont et al., 1990). The increased dynamics in mitosis are thought to be important for mitotic spindle assembly and function. Thus identifying the proteins that regulate microtubule dynamics differentially in the cell cycle is an important step in elucidating the mechanisms of spindle assembly and chromosome segregation. Two proteins, OP18 (Belmont and Mitchison, 1996; Tournebize et al., 1997) and XKCM1 (Walczak et al., 1996), have been identified that directly increase the catastrophe frequency of microtubules in Xenopus extracts. However, neither protein has been demonstrated to have an increased activity in mitosis relative to interphase (Andersen et al., 1997; Tournebize et al., 1997). In contrast to OP18 and XKCM1, which promote endwise disassembly of microtubules, microtubule-severing proteins generate internal breaks within microtubules. Microtubule severing was first observed as an activity present in metaphase-like but not in interphase-like Xenopus egg extracts. This activity could be activated post-translationally by converting an interphase-like extract to a metaphase-like state by the addition of cyclin B (Vale, 1991). This observation suggested that a microtubule-severing protein might be involved in the changes in microtubule arrangement and dynamics that occur upon entry into mitosis. Thus identifying the protein responsible for the M-phase microtubule-severing activity in Xenopus egg extracts is an important step in understanding how microtubule dynamics change during the cell cycle.
Only three microtubule-severing proteins have ever been purified from any source. Two ATP-independent microtubule-severing proteins have been isolated from Xenopus eggs, p56 (Shiina et al., 1992) and EF1α (Shiina et al., 1994), and one ATP-dependent microtubule-severing protein, katanin (McNally and Vale, 1993), has been isolated from sea urchin (Strongylocentrotus purpuratus) eggs. Each of these proteins has been reported to have properties similar to those of the M-phase–activated severing activity observed in crude Xenopus extracts; however, none of the three purified proteins has been shown to be activated by cyclin B/cdc2. Thus it has been unclear which of these unrelated proteins contribute significantly to the M-phase–activated severing activity in extracts.
Of the three characterized microtubule-severing proteins, katanin is the only protein that requires ATP for its activity. Katanin isolated from sea urchin eggs is a heterodimer of 60- and 80-kDa subunits (McNally and Vale, 1993). The 60-kDa subunit is a member of the conserved AAA family of ATPases (Confalonieri and Duguet, 1995), and p60 katanin expressed in Sf-9 cells has ATP-dependent microtubule-severing activity and microtubule-stimulated ATPase activity in the absence of the 80-kDa subunit (Hartman et al., 1998). Because the microtubule-severing activity in crude Xenopus extracts also requires ATP (McNally and Vale, 1993), katanin is a strong candidate for the cyclin B-activated severing activity in Xenopus extracts. Unfortunately, p60 katanin has only been characterized in echinoderms, and there has been no molecular evidence that katanin is present in Xenopus eggs.
Identification of vertebrate homologues of sea urchin p60 katanin has been hampered by the fact that the catalytic 60-kDa subunit of the katanin heterodimer is an AAA ATPase. There are dozens of AAA ATPases (Confalonieri and Duguet, 1995) that exhibit amino acid (aa) identity with p60 katanin; however, none of these proteins show significant identity outside the 230 aa AAA domain. Thus it has been impossible to determine whether katanin homologues like the Caenorhabditis elegans mei-1 gene, an AAA ATPase required for meiotic spindle assembly (Clark-Maguire and Mains, 1994b), are microtubule-severing proteins rather than ATPases with unrelated functions. Thus identifying functional p60 katanin homologues is an important step in elucidating katanin’s function in nonechinoderms.
An important clue to katanin’s in vivo function comes from its subcellular localization. Sea urchin katanin is concentrated at centrosomes throughout the cell cycle (McNally et al., 1996). Centrosomes are composed of centrioles surrounded by the γ-tubulin–containing pericentriolar material, which does not require microtubules for assembly or maintenance (Felix et al., 1994; Stearns and Kirschner, 1994). Centrosomes comprise the mitotic spindle poles in most animal cells. In metaphase sea urchin embryos, katanin is concentrated in a unique spindle-pole matrix that surrounds the γ-tubulin–containing pericentriolar material and that requires microtubules for maintenance (McNally et al., 1996), suggesting that katanin may sever spindle microtubules from their attachment sites in the pericentriolar material. Such a severing reaction might free microtubule minus ends from their attachments to nucleation sites in the pericentriolar material and thus allow the poleward flux of tubulin (Mitchison, 1989), a process implicated in the maintenance of spindle structure (Waters et al., 1996). A human homologue of p80 katanin has been found to be concentrated at interphase centrosomes in human fibroblasts (Hartman et al., 1998); however, the localization of this homologue during mitosis was not examined. Thus it remains unclear whether the katanin-containing, microtubule-dependent spindle-pole matrix is found universally in animal cells or whether it is a specialization of echinoderm embryos.
Nonmitotic roles for a centrosomal microtubule-severing protein are also possible. For example, katanin could be responsible for the observed release of microtubules from the centrosome in epithelial cells (Keating et al., 1997), a process that may be involved in regulating the overall turnover of microtubules. A centrosome-associated microtubule-severing protein might also be involved in the release of neuronal microtubules from the centrosome, a process proposed to be involved in elaboration of axonal processes (Baas and Yu, 1996). However, there has been no molecular evidence for the presence of a microtubule-severing protein in epithelial cells or neuronal cells.
In this article we report the identification of a human homologue of p60 katanin. Antibodies specific for this p60 homologue were used to demonstrate that this vertebrate katanin is indeed a microtubule-severing protein and that katanin is responsible for the M-phase severing activity in Xenopus eggs. These antibodies were also used to demonstrate that the katanin-containing spindle-pole matrix is a conserved feature of animal cell mitotic spindles and that katanin is present in epithelial cells and neuronal cells.
MATERIALS AND METHODS
Isolation of cDNAs
Human p60.
BLAST searches with S. purpuratus p60 katanin sequences revealed human cDNA clones from the I.M.A.G.E. Consortium project (Lennon et al., 1996) that had significant identity with p60. One clone (I.M.A.G.E. clone 149526 yj22 g04.r1) had homology with a C-terminal region of p60, whereas a second (I.M.A.G.E. clone 71287 yb15h0) had homology with the N terminus of p60. Further sequence analysis indicated that clone 149526 did not extend to a stop codon; therefore sequences within clone 149526 were used to amplify a fragment encoding a C-terminal cDNA fragment from HT1080 human fibrosarcoma RNA by 3′-RACE (Frohman et al., 1988). Sequences 3′ of the predicted stop codon, determined from the 3′-RACE clones, and sequences 5′ of the predicted start codon (clone 71287) were used to amplify via PCR full-length clones with Pfu polymerase from oligo-dT–primed reverse transcription reactions using MSU1.1 cell total RNA as template. Sequence analysis of multiple independent clones confirmed that the sequence (see Figure 1) is free of PCR-derived errors.
Figure 1.
Sequence of a human homologue of p60 katanin (GenBank number AF056022). Amino acid identities between S. purpuratus p60 (Sp60) and its human homologue (Hs60) are shaded. Dashes represent gaps.
Xenopus p80.
A cDNA encoding the C terminus of Xenopus p80 katanin was obtained by PCR amplification from a Stratagene Lambda ZAP Xenopus ovary cDNA library using a degenerate primer based on identities between sea urchin and human p80 and a vector primer. An overlapping cDNA was obtained by PCR using a primer based on sequences from the Xenopus clone and a vector primer.
Antibody Production
Human p60 Antibody.
A full-length cDNA encoding the human p60 homologue was cloned into the 6-histidine fusion vector pET28. Protein expressed in E. coli BL21DE3 was mostly insoluble, so the p60 protein was purified by nickel chelate chromatography in buffers containing 8 M urea after the cells were initially solubilized in 6 M guanidine-Cl as described in the QIA expressionist handbook (Qiagen, Santa Clarita, CA). p60 was further purified by preparative SDS-PAGE before two rabbits were immunized with homogenized acrylamide slices. For affinity purification of serum, p60 protein was dialyzed from 8 M urea into 0.3% SDS and then coupled to CNBr-Sepharose (Pharmacia, Piscataway, NJ). p60 antibodies were bound and eluted successively with 4 M MgCl2 and 0.2 M glycine, pH 2.5, and desalted and concentrated as described previously (McNally et al., 1996). All antibodies added to Xenopus extracts were desalted into 50 mM K-glutamate and 5 mM K-HEPES, pH 7.5.
Human p80 Antibody.
The human p80 antibody, which has been described previously (Hartman et al., 1998), was prepared as described for the p60 antibody.
Xenopus p80 Anti-Peptide Antibodies.
Two synthetic peptides corresponding to sequences near the C terminus of Xenopus p80 (GVDISREERLSKC and CAFRELHLLMSGLE) were coupled to maleimide-activated cationized BSA (Pierce Chemical, Rockford, IL) and used to immunize rabbits. Antibodies were affinity purified from the resulting serum using peptides coupled to Sulfo-Link beads (Pierce Chemical) as described previously (McNally et al., 1996).
Control Antibodies.
Control IgG was protein A purified from p60 and p80 immune sera after the affinity adsorption of katanin-specific antibodies described above. Control IgG was desalted and concentrated as described above.
Xenopus Extract Preparation
Metaphase-like Xenopus extracts were prepared as described by Murray (1991) and frozen in liquid nitrogen after addition of sucrose to 150 mM. High-speed supernatants (for immunoprecipitations) were generated by diluting unfrozen extracts 1:1 with XB (100 mM KCl, 0.1 mM CaCl2, 1 mM MgCl2, 10 mM K-HEPES, pH 7.7, 50 mM sucrose, and 5 mM EGTA) followed by sedimentation at 250,000 × g for 30 min at 4°C.
Immunoprecipitations
Immunodepletions for analysis of severing activity in the supernatants were performed with Pansorbin cells (Calbiochem, San Diego, CA) to minimize dilution of the extract. IgG (5–10 μg) was preadsorbed to 20 μl of Pansorbin suspension and washed extensively with XB. Pansorbin cells were pelleted, and 100 μl of Xenopus extract was used to resuspend the pellet. Pansorbin–IgG complexes were removed by sedimentation after a 1 h incubation on ice. Immunoprecipitations for analysis of polypeptides in the pellet were performed with 15–20 μg of antibody covalently cross-linked to 50 μl (packed volume) of AffiPrep Protein A beads (Bio-Rad, Richmond, CA) as described in Harlow and Lane (1988) to minimize IgG contamination. Each immunoprecipitation with AffiPrep beads was performed on 3 ml of Xenopus extract high-speed supernatant.
Microtubule-severing Assays
Assays were performed essentially as described previously (McNally and Vale, 1993) with the following modifications. An ATPase-defective human kinesin cDNA (glycine 234 changed to alanine; Vale and Taylor, unpublished observations) was modified by addition of an oligonucleotide encoding the sequence QKLKKRKKKKRK at the AflII site (bp 1620). The resulting bacterially expressed protein binds microtubules in the presence of ATP and binds tightly to glass because of the engineered basic C-terminal extension. Preparations of this mutant kinesin were used to immobilize rhodamine-labeled, taxol-stabilized bovine brain microtubules to the coverslip surface of a flow cell in lieu of the previously described N-ethyl maleimide-treated Xenopus extract (McNally and Vale, 1993). Antibody-treated Xenopus extracts were perfused into these flow cells after addition of an oxygen-scavenging system (Kishino and Yanagida, 1988). Time-lapse images of microtubules were captured using a Nikon Microphot SA microscope with a 60× Plan Apo 1.4 objective, IP Lab Spectrum software (Scanalytics, Fairfax, VA), a Scion (Frederick, MD) AG5 framegrabber, a DAGE (Michigan City, IN) SIT68 camera, and a Ludl Electronics (Hawthorne, NY) MAC2000 controller and shutter to minimize exposure to light. To control for photodamage, at the end of each experiment, we moved the stage to ensure that severing had progressed to the same extent in unilluminated adjacent fields. Microtubule number in each frame was determined with a custom script using the Segmentation and Quantify Segments commands in IP Lab Spectrum. To ensure the accuracy of this method, we manually counted severing events in several sequences, and severing rates were found to be similar to those determined with the script.
Cell Culture and Immunofluorescence
MSU1.1 cells (Lin et al., 1995) were grown in Optimem media (Life Technologies, Bethesda Research Laboratories, Gaithersburg, MD) supplemented with 10% fetal bovine serum, penicillin, and streptomycin at 37°C in 5% CO2. Xenopus A6 cells were grown in 65% Optimem, 10% fetal bovine serum, 25% H2O, penicillin, and streptomycin at 22°C in air. For immunofluorescence, cells were grown on 18-mm glass coverslips, washed in PBS (137 mM NaCl, 2 mM KCl, 5 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4), fixed in −20°C methanol, rehydrated in Tris-buffered saline plus Triton X-100 (150 mM NaCl, 50 mM Tris-Cl, pH 7.5, and 0.05% Triton X-100), blocked in 4% BSA in Tris-buffered saline plus Triton X-100, and then incubated sequentially in primary antibody (usually 0.1–1 μg/ml IgG) followed by fluorescent secondary antibody. γ-Tubulin was detected with mouse monoclonal GTU88 (Sigma Chemical, St. Louis, MO), and β-tubulin was detected with mouse monoclonal E7 (Developmental Studies Hybridoma Bank, Iowa City, IA). Rabbit antibodies were detected with a Texas Red-X secondary antibody (Molecular Probes, Eugene, OR), and mouse antibodies were detected with an Oregon Green 488 secondary antibody (Molecular Probes). Images were acquired with a Nikon Microphot SA microscope equipped with Chroma Technology fluorescein/Texas Red filters, a 100× PlanFluor 1.3 objective, and a Quantix KAF1400 charge-coupled device camera (Photometrics, Tucson, AZ) operated with IP Lab Spectrum software. Areas of centrosome/spindle pole staining were determined with IP Lab Spectrum using TetraSpeck fluorescent beads (Molecular Probes) for size calibration. For nocodazole experiments, nocodazole was added to a final concentration of 20 μg/ml for 60 min at 37°C. This treatment was found to disassemble all microtubules in MSU1.1 and A6 cells as assayed by immunofluorescence labeling with the E7 antibody. Mitotic cells were identified in nocodazole-treated cultures by DAPI staining of condensed chromatin.
RESULTS
Isolation of a Human p60 Katanin Homologue
Database searches with the recently described sea urchin (S. purpuratus) p60 katanin sequence (Hartman et al., 1998) revealed many protein sequences with homology in the AAA ATPase domain but no proteins of known function showing significant aa identity at the N and C termini. Search results did, however, reveal several human expressed sequence tag sequences with homology to the N and C termini of p60 katanin. These sequences were used to isolate full-length cDNA clones from the human fibroblast cell line MSU1.1 (Lin et al., 1995) (see MATERIALS AND METHODS for details). These cDNAs encode a predicted 491 aa polypeptide with 50% aa identity in the N-terminal 80 aa and 75% identity in the C-terminal 318 aa AAA ATPase domain with S. purpuratus p60 katanin (Figure 1). This homology is interrupted by three small deletions in human p60 relative to sea urchin p60 (aa 80–110 in the human p60 sequence). An intriguing difference between human and sea urchin p60 is found at the border of one of these deletions. The consensus cyclin B/cdc2 phosphorylation site TPLK (Kennelly and Krebs, 1991) is found at aa 82–85 of human p60 (Figure 1) and is not found in sea urchin p60. This difference is interesting because the previously characterized microtubule-severing activity in Xenopus egg extracts is activated post-translationally by cyclin B (Vale, 1991), whereas katanin purified from sea urchin eggs is unaffected by cyclin B/cdc2 (McNally and Vale, 1993).
p60 Katanin Is Ubiquitous in Vertebrate Cell and Tissue Types
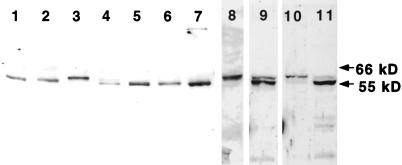
To generate antibodies that could be used for functional analysis of katanin in Xenopus egg extracts, we expressed a 6-histidine-tagged version of human p60 katanin in E. coli and purified this version by metal chelate chromatography (see MATERIALS AND METHODS). The resulting human p60 was used to generate an affinity-purified polyclonal rabbit antibody (see MATERIALS AND METHODS). Immunoblots of total SDS-soluble protein from a variety of vertebrate tissues and cell lines were probed with the polyclonal human p60 antibody that recognized a single polypeptide ranging from 55 to 60 kDa in most cell types (Figure 2). The specificity of the antibody was indicated by the recognition of a single polypeptide in the human cell lines MSU1.1 and Hela (Figure 2, lanes 1 and 2, respectively). Quantitative immunoblots revealed that p60 katanin in Hela and MSU1.1 lysates comprises 0.003% of total SDS-soluble protein, whereas β-tubulin comprises 3% of total protein. Thus there is ∼1 katanin molecule for every 1000 tubulin heterodimers in these cell lines. The presence of a single antibody-reactive species in Xenopus egg extracts (Figure 2, lane 8) was the first indication that katanin could be responsible for the cyclin B-activated severing activity in these extracts. A single p60 katanin homologue was also detected in PtK-1 cells (Figure 2, lane 5), B cells and myelomas (Figure 2, lanes 6 and 7, respectively) demonstrating that this protein is present in dividing cells with extremely different morphologies and developmental origins. Most surprisingly, the antibody recognized two polypeptides of similar molecular weight (MW) in nonmitotic adult mouse brain tissue as well as in neuroblastoma cells (Figure 2, lanes 9 and 4, respectively). Extraction experiments with mouse brain demonstrated that the lower MW polypeptide could be completely solubilized with a combination of salt and nonionic detergent (Figure 2, lane 11), whereas the higher MW polypeptide was solubilized only by SDS (Figure 2, lane 10). This result suggested that neuronal cells have a cytoplasmic isoform of katanin and an isoform that is tightly associated with a Triton-insoluble cell matrix. Overall these results indicate that p60 katanin homologues are present in a wide range of cell and tissue types.
Figure 2.
p60 katanin is ubiquitous in vertebrate cells and tissues. Total SDS-soluble proteins (lanes 1–9) from a variety of cells and tissues were subjected to immunoblot analysis with the human p60 katanin antibody. Lane 1, MSU1.1, human fibroblast; lane 2, Hela; lane 3, A6, Xenopus; lane 4, N2A, mouse neuroblastoma; lane 5, PtK-1, marsupial; lane 6, DT40, chicken B cell; lane 7, Fox NY, mouse myeloma; lane 8, CSF Xenopus egg extract; and lane 9, adult mouse brain. Mouse brains were homogenized in the presence of Triton X-100 and subjected to centrifugation. The Triton-soluble supernatant was analyzed in lane 11. The Triton-insoluble material was extracted with SDS, and the SDS-soluble fraction was analyzed in lane 10. Note that the higher molecular weight species in brain is enriched in the Triton-insoluble, SDS-soluble fraction (lane 10).
p60 Katanin Is Associated with p80 Katanin in Xenopus Egg Extracts
Because p60 katanin isolated from sea urchin eggs is always associated with p80 katanin, we sought to determine whether the p60 katanin homologue in Xenopus eggs was associated with a p80 homologue. Immunoblot analysis with a previously described polyclonal antibody specific for a human homologue of p80 katanin (Hartman et al., 1998) revealed a single 80-kDa polypeptide in immunoblots of several mammalian cell lines but a cross-reaction with both 80-kDa and 50-kDa polypeptides in Xenopus egg extracts (Figure 3). Further evidence for a Xenopus homologue of p80 katanin came from sequence analysis of a partial cDNA (GenBank number AF056021) obtained by PCR from a Xenopus ovary cDNA library using degenerate primers based on sea urchin and human p80 sequences. This partial cDNA is 79% identical with the previously described human p80 sequence over the C-terminal 210 aa and 47% identical (with extensive gaps) over the central 140 aa. In comparison, sea urchin p80 is 54% identical with human p80 in the C-terminal domain and only 23% identical in the central domain. An affinity-purified anti-peptide antibody made against a sequence found near the C terminus of the Xenopus p80 homologue recognized the same polypeptides that the human p80 antibody recognized in immunoblots of Xenopus egg extracts (our unpublished observations), indicating that a p80 katanin homologue is indeed present in Xenopus eggs.
Figure 3.
p80 katanin is ubiquitous in vertebrate cells and tissues. Total SDS-soluble proteins from a variety of cells and tissues were subjected to immunoblot analysis with the human p80 katanin antibody. Lane 1, MSU1.1; lane 2, N2A; lane 3, adult mouse brain; and lane 4, CSF Xenopus egg extract.
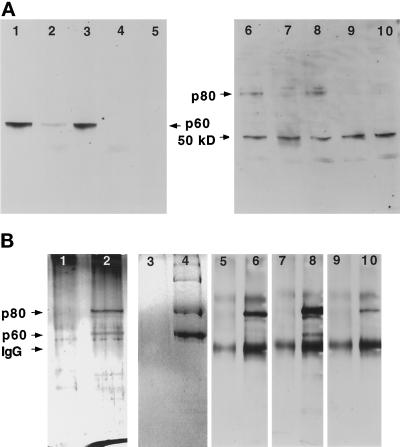
To determine whether the Xenopus egg p60 and p80 homologues are associated as they are in sea urchin eggs, we performed native immunoprecipitations with the human p60 polyclonal antibody. Immunoblot analysis of supernatants from immunoprecipitation reactions demonstrated that p60 katanin was depleted by both the p60 antibody (Figure 4A, lanes 4 and 5) and by the p80 antibody (Figure 4A, lane 2). Conversely, p80 katanin was depleted by both the p80 antibody (Figure 4A, lane 7) and by the p60 antibody (Figure 4A, lanes 9 and 10). Analysis of pellets from immunoprecipitations with the p60 antibody by silver staining (Figure 4B, lane 2) and Coomassie blue staining (Figure 4B, lane 4) revealed polypeptides of 80 and 60 kDa. Immunoblot analysis of these polypeptides was used to confirm their identities as p80 and p60 katanin. The 80-kDa protein in the immunoprecipitation pellets was recognized by the human p80 antibody (Figure 4B, lane 6) and by two different anti-peptide antibodies specific for C-terminal peptide sequences in the Xenopus p80 cDNA (Figure 4B, lanes 8 and 10). The 60-kDa protein was recognized by the human p60 antibody (our unpublished observations). These results demonstrate that the p60 katanin homologue in Xenopus eggs is associated with a p80 katanin homologue, suggesting that they form a heterodimer as they do in sea urchin eggs.
Figure 4.
p60 and p80 katanin are coimmunoprecipitated from Xenopus extracts. (A) Immunoblot analysis of supernatants from immunoprecipitations probed with the human p60 antibody (lanes 1–5) and the human p80 antibody (lanes 6–10). Lanes 1 and 6, mock immunoprecipitations with control IgG; lanes 2 and 7, immunoprecipitation with 5 μg of p80 antibody; lanes 3 and 8, immunoprecipitation with 1 μg of p80 antibody; lanes 4 and 9, immunoprecipitation with 5 μg of p60 antibody; and lanes 5 and 10, immunoprecipitation with 1 μg of p60 antibody. (B) Analysis of pellets from immunoprecipitations with control IgG (lanes 1, 3, 5, 7, and 9) or with the human p60 antibody (lanes 2, 4, 6, 8, and 10). SDS-PAGE–resolved proteins were analyzed by silver staining (lanes 1 and 2) or Coomassie blue staining (lanes 3 and 4) or by immunoblots probed with the human p80 antibody (lanes 5 and 6) or with either of two Xenopus p80 anti-peptide antibodies (lanes 7–10).
A p60 Katanin Homologue Is Responsible for the M-Phase Microtubule-severing Activity in Xenopus Egg Extracts
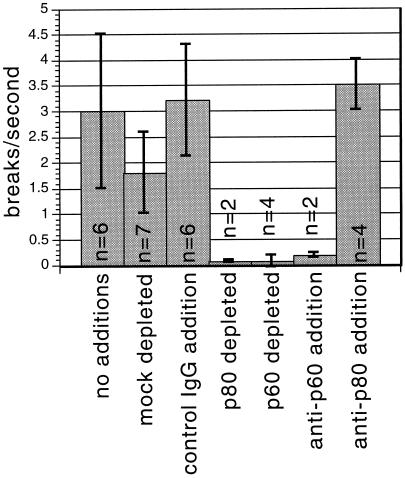
The presence of a katanin homologue in Xenopus egg extracts suggested that katanin might be responsible for the previously characterized M-phase microtubule-severing activity in Xenopus egg extracts. To test this hypothesis, we quantitated microtubule-severing activity in extracts to which the polyclonal human p60 katanin antibody had been added. A modification of the previously described assay (McNally and Vale, 1993) was used. Rhodamine-labeled, taxol-stabilized microtubules were immobilized on the coverslip of a flow cell using a bacterially expressed mutant kinesin (see MATERIALS AND METHODS). Microtubules bound to these mutant kinesin-coated coverslips do not move or release from the glass surface, allowing unambiguous determination of severing events occurring during a time-lapse experiment. Images of these immobilized microtubules were captured at 5–10 s intervals after exposure to an M-phase Xenopus egg extract (Figure 5A). Each microtubule-severing event results in an increase in the number of microtubules. Plots of the increasing number of microtubules per unit time (Figure 5B, a) displayed an initial lag period, followed by a linear phase (during which microtubule number/second is equal to breaks/second), followed by a decrease in number attributable to the complete disappearance of microtubules. Inhibition of the severing reaction by the p60 antibody can be seen qualitatively in Figure 5A, and quantitation of the inhibition in an individual experiment is shown in Figure 5B. To compare microtubule-severing rates in antibody-treated Xenopus egg extracts more accurately with those of control extracts, we averaged the rates during the linear phase of several duplicate reactions and displayed the results as bar graphs (Figure 6). Microtubule-severing activity was decreased over 10-fold by addition of the p60 antibody or by immunodepletion with either the p60 or the p80 antibodies. These results indicate that katanin rather than p56 or EF1α is responsible for the majority of the microtubule-severing activity in a Xenopus extract.
Figure 5.
Microtubule-severing activity in a Xenopus extract is inhibited by a p60 katanin antibody. (A) Images of glass-immobilized rhodamine-labeled microtubules at time points after perfusion of a Xenopus egg extract. Microtubules are rapidly severed and completely disassembled by an extract treated with a control IgG (a, b, and c). Very few severing events are observed after perfusion of an extract treated with 60 μg/ml human p60 antibody (d, e, and f). Bar, 22 μm. (B) Quantitation of the number of microtubules present at successive time points during individual microtubule-severing reactions. a, Xenopus extract treated with a control IgG. b, Xenopus extract treated with 60 μg/ml human p60 antibody.
Figure 6.
Quantitative analysis of antibody inhibition and immunodepletion of microtubule-severing activity in a Xenopus extract. The maximal rates of increase in microtubule number (e.g., Figure 5B, a, between 40 and 70 s) were taken from multiple reactions performed under the same conditions and averaged. Comparison of these average rates of severing is shown. Depleted samples are supernatants from immunoprecipitations with the indicated antibody.
p60 Katanin Is Concentrated at Centrosomes and around Spindle Poles in Cultured Vertebrate Cells
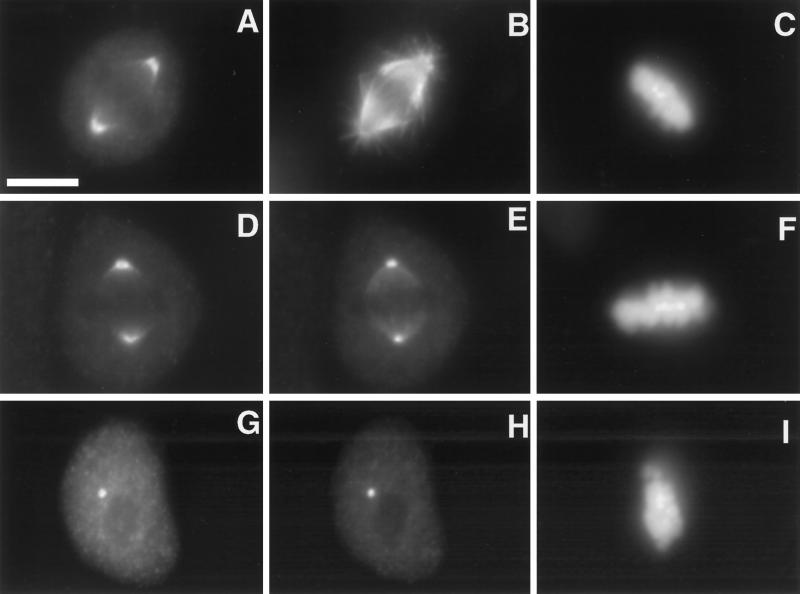
In sea urchin embryos, katanin is localized at centrosomes throughout the cell cycle in a microtubule-dependent manner, and in metaphase spindles, this centrosomal region surrounds the γ-tubulin containing pericentriolar material (McNally et al., 1996). This localization is consistent with a role in releasing spindle microtubules from their attachment points in the pericentriolar material; however, the generality of this localization has not been demonstrated. Human p80 katanin has been shown to colocalize with γ-tubulin at the centrosomes of interphase human fibroblasts through its WD40 domain (Hartman et al., 1998); however, p60 katanin’s localization and katanin’s localization during mitosis have not been reported in vertebrate cells. Immunofluorescence experiments with the human p60 antibody were used to determine whether katanin’s localization in vertebrate cells has the same cell cycle dependence and microtubule dependence observed in sea urchin embryos. When interphase MSU1.1 human fibroblasts were fixed and stained with the human p60 katanin antibody, specific staining was observed throughout the cell as well as in one or two bright foci. These foci occurred at the center of the microtubule aster (Figure 7, A and B) and colocalized with γ-tubulin (Figure 7, D and E). The foci of p60 staining remained after microtubules were completely disassembled by incubating cells in 20 μM nocodazole for 1 h (Figure 7, G and H). These results along with the previous results with p80 (Hartman et al., 1998) indicate that the katanin heterodimer is found predominantly in the cytoplasm with a concentrated subfraction that is associated with the pericentriolar material in interphase MSU1.1 cells.
Figure 7.
Immunolocalization of p60 katanin in interphase fibroblasts. MSU1.1 cells were fixed and stained with a human p60 katanin antibody (A, D, and G), a β-tubulin antibody (B), a γ-tubulin antibody (E and H), and DAPI (C, F, and I). A focus of p60 staining was observed at the center of an aster of microtubules visualized with the β-tubulin antibody (A, B, and C). This focus of p60 staining colocalized with γ-tubulin (D, E, and F) even after microtubules were depolymerized with nocodazole (G, H, and I). Bar, 5 μm.
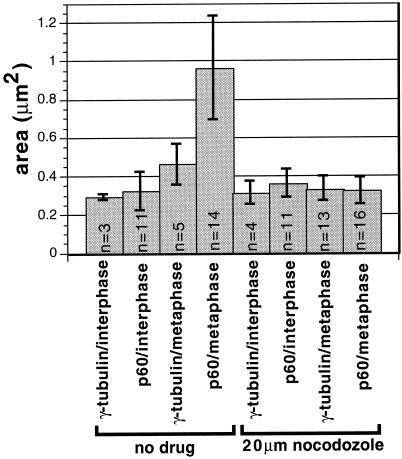
To examine whether the distribution and microtubule dependence of katanin localization changed during the cell cycle, we examined katanin-stained metaphase MSU1.1 cells. Both the human p80 antibody (Figure 8A) and the human p60 antibody (Figure 8D) labeled structures at metaphase spindle poles as judged by colocalization with β-tubulin (Figure 8B) and γ-tubulin (Figure 8E). The katanin-labeled structures, however, appeared to occupy a greater volume than did the γ-tubulin–containing structures (Figure 8, D and E). When spindle microtubules were completely disassembled by nocodazole treatment, the foci of p60 staining remained at spindle poles, but the p60-containing structures appeared to be the same size as the γ-tubulin–containing spindle-pole structures (Figure 8, G and H). To better document these observations, we quantitated the areas of p60 staining and γ-tubulin staining in a number of interphase and metaphase cells either with or without nocodazole treatment. The average area occupied by p60 increased threefold between interphase and metaphase, and all of this increase was dependent on microtubules (Figure 9). During metaphase, the area occupied by p60 was twice that occupied by γ-tubulin. These results are consistent with katanin’s association with both the nocodazole-resistant pericentriolar material and with a nocodazole-sensitive spindle-pole matrix during metaphase. Interphase MSU1.1 cells, in contrast, appear to have katanin that is restricted to the pericentriolar material, because the area occupied by p60 and γ-tubulin is the same with or without nocodazole treatment (Figure 9). (It should be noted that the diameter of these interphase structures is close to the resolution limit of the imaging system used. Thus there could be differences that were not observed.)
Figure 8.
Immunolocalization of p60 and p80 katanin at metaphase spindle poles in human fibroblasts. MSU1.1 cells were fixed and stained with a human p80 antibody (A), a human p60 antibody (D and G), a β-tubulin antibody (B), a γ-tubulin antibody (E and H), and DAPI (C, F, and I). p80 is shown concentrated at spindle poles relative to microtubules (A, B, and C), and p60 is shown colocalizing with γ-tubulin at spindle poles (D, E, and F). The concentration of p60 and γ-tubulin at spindle poles remains after microtubules are depolymerized with 20 μM nocodazole (G, H, and I). The area occupied by p60 in the presence of microtubules is greater than that occupied by γ-tubulin (D and E), whereas p60 and γ-tubulin occupy the same area after microtubules are depolymerized (G and H). Bar, 5 μm.
Figure 9.
Quantitative analysis of the cell cycle dependence and microtubule dependence of the centrosomal localization of katanin and γ-tubulin in MSU1.1 human fibroblasts. The area of concentrated p60 katanin staining and γ-tubulin staining at interphase centrosomes and metaphase spindle poles was measured (see MATERIALS AND METHODS) in several cells either untreated or treated with 20 μM nocodazole for 60 min to depolymerize microtubules. Averages of measurements in each category are shown. p60 katanin occupies an unusually large area during metaphase, and occupation of this large area requires intact microtubules.
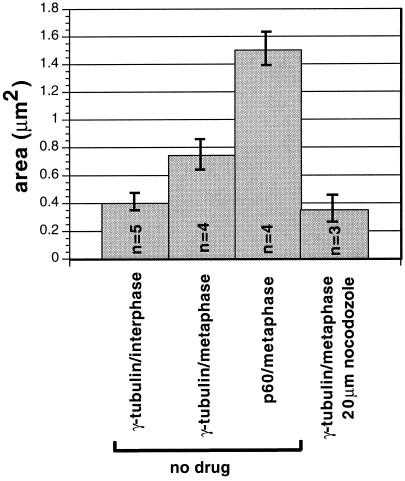
The concentration of katanin in a nocodazole-sensitive spindle-pole matrix that surrounds the γ-tubulin–containing pericentriolar material in metaphase MSU1.1 cells was similar to that observed previously in sea urchin embryos (McNally et al., 1996). However, the nocodazole-resistant association of katanin with the pericentriolar material in both interphase and metaphase MSU1.1 cells was different from that observed in sea urchin embryos. To determine how universal this localization is in vertebrate cells, we examined the localization of p60 in Xenopus A6 cells. In interphase A6 cells, p60 staining was observed throughout the cytoplasm, but no foci were observed that colocalized with γ-tubulin (our unpublished observations). In metaphase A6 cells, p60 was concentrated in large hollow structures surrounding the γ-tubulin at spindle poles (Figure 10). Quantitation of these images revealed that p60 occupies twice the area of γ-tubulin in metaphase A6 cells (Figure 11). The concentration of p60 at spindle poles was completely dispersed when microtubules were depolymerized with nocodazole (our unpublished observations), whereas γ-tubulin staining at poles remained after nocodazole treatment (Figure 11). These results indicate that katanin is concentrated in a microtubule-dependent spindle-pole matrix in both echinoderms and chordates; however, an additional association with the microtubule-independent pericentriolar material occurs only in some cell types.
Figure 10.
Comparison of katanin and γ-tubulin localization at spindle poles in Xenopus A6 cells. Xenopus A6 cells were fixed and stained with a p60 katanin antibody (A, B, and C) and a γ-tubulin antibody (D, E, and F). Metaphase cells were identified by DAPI staining. Three examples (A and D, B and E, and C and F) of spindle poles are shown. In each case, p60 occupies a large hollow area while γ-tubulin occupies a smaller area within the p60 staining area. Bar, 2.5 μm.
Figure 11.
Quantitative analysis of the cell cycle dependence and microtubule dependence of the centrosomal localization of katanin and γ-tubulin in Xenopus A6 cells. The area of concentrated p60 katanin staining and γ-tubulin staining at interphase centrosomes and metaphase spindle poles was measured (see MATERIALS AND METHODS) in several cells either untreated or treated with 20 μM nocodazole for 60 min to depolymerize microtubules. Averages of measurements in each category are shown. p60 katanin is not found at interphase centrosomes or nocodazole-treated spindle poles in these cells.
In addition to the increased area occupied by p60 during metaphase relative to interphase, an increased area of γ-tubulin staining was also observed. This increase in area was most apparent in A6 cells in which the area doubled, and the increase in area was completely dependent on intact microtubules (Figure 11). A similar increase may occur in MSU1.1 cells (Figure 9); however, the accuracy of measurements of these smaller centrosomes was limited by the resolution of the light microscope. Even when γ-tubulin spreads to twice its interphase area in metaphase A6 cells, p60 katanin occupies an even greater area, thus surrounding the γ-tubulin (Figures 10 and 11).
DISCUSSION
Correlation of M-Phase Microtubule-severing Activity with a p60 Katanin Homologue
The recently described sequence of the catalytic 60-kDa subunit of sea urchin katanin (Hartman et al., 1998) revealed that katanin is a member of the AAA ATPase family (Confalonieri and Duguet, 1995) with strong identity with the C. elegans mei-1 gene product (Clark-Maguire and Mains, 1994a,b). In this article we report the sequence of a human cDNA that has the highest degree of aa identity with sea urchin p60 katanin of any proteins currently in databases. In particular, the human p60 homologue has extensive identity with sea urchin p60 in the N-terminal 80 aa that is not found in mei-1. However, because of the similarity among AAA family members with diverse functions, it has not been possible to determine whether the human p60 homologue, mei-1, or any other protein is a functional katanin homologue by sequence comparison alone.
To test whether the human p60 homologue is a microtubule-severing protein, an antibody made against the human p60 katanin homologue was tested for its ability to inhibit and immunodeplete a previously characterized microtubule-severing activity in Xenopus egg extracts (Vale, 1991). The p60 antibody inhibited the rate and extent of microtubule severing in a Xenopus extract by 90% and nearly eliminated microtubule-severing activity by immunoprecipitating the Xenopus p60 homologue. These results indicate that a Xenopus homologue of the human p60 katanin homologue is responsible for a microtubule-severing activity. Because the p60 antibody recognized a single polypeptide in both Xenopus eggs and in cultured human cells, it is likely that the human cDNA itself encodes a microtubule-severing protein.
Much of the initial interest in microtubule-severing proteins was derived from the apparent activation of microtubule-severing activity in Xenopus extracts by cyclin B/cdc2 (Vale, 1991). This regulation suggested that microtubule severing might play a specific role in the reorganization of the mitotic spindle at the G2/M transition or in mitotic spindle assembly or function. However, there has been no direct evidence that any of the three reported microtubule-severing proteins, p56 (Shiina et al., 1992), EF1α (Shiina et al., 1994), or katanin (McNally and Vale, 1993), is actually responsible for the cyclin B-activated severing activity in Xenopus extracts. Likewise, regulation of any of the three purified severing proteins by cyclin B/cdc2 has not been demonstrated. Thus the demonstration in this article that a katanin homologue is responsible for most of the severing activity in an M-phase Xenopus extract implies that a katanin homologue is activated by cyclin B/cdc2 in these extracts. The finding of a consensus cyclin B/cdc2 phosphorylation site in the human p60 katanin sequence suggests a mechanism for this activation. When the human p60 cDNA is expressed in baculovirus, it will be possible to test whether cyclin B/cdc2 activates severing activity directly by phosphorylation of this site.
Although the experiments presented here demonstrate that a Xenopus homologue of p60 katanin is responsible for the majority of the M-phase severing activity in egg extracts, they do not preclude the existence of other microtubule-severing proteins. The slow rate of severing observed after depletion of p60 katanin could be due to another microtubule-severing protein. This question is complicated by the fact that the mutant kinesin used to immobilize microtubules in these severing assays reduces the rate of severing both by Xenopus extracts and by purified katanin (our unpublished observations). The other reported severing proteins, p56 (Shiina et al., 1992) and EF1α (Shiina et al., 1994), have only been assayed in solution. If the activities of these proteins are inhibited by the mutant kinesin, severing by these proteins might be under-represented in the experiments reported here.
Conserved Localization of Katanin at Spindle Poles
Other than katanin’s activation by cyclin B/cdc2, one of the few clues to katanin’s in vivo function is its subcellular distribution. Both subunits of sea urchin katanin were shown previously to be concentrated at interphase centrosomes and mitotic spindle poles in sea urchin embryos (McNally et al., 1996). A human homologue of p80 katanin has been shown to be concentrated at interphase centrosomes in fibroblasts, and the WD40 repeat domain of p80 has been shown to act as a centrosome-targeting domain (Hartman et al., 1998). These observations have led to the hypothesis that p80 katanin’s function is to target the catalytically active p60 subunit to centrosomes and that this targeting is conserved in animal cells. Further support for this hypothesis comes from results reported in this article that the Xenopus p60 homologue is associated with a Xenopus p80 homologue and that homologues of both p60 and p80 are concentrated at spindle poles in human and Xenopus cells.
The unusual nature of katanin’s spindle-pole localization reported here also supports the hypothesis that katanin is concentrated in a spindle-pole matrix that has not been clearly defined in the literature. It had been shown previously that during metaphase in first division sea urchin embryos, katanin is concentrated in a structure that surrounds the γ-tubulin–containing pericentriolar material and that requires microtubules for its integrity (McNally et al., 1996). The dependence on microtubules contrasts with components of the pericentriolar material such as γ-tubulin and pericentrin that do not require microtubules for assembly or maintenance (Stearns et al., 1991; Zheng et al., 1991; Doxsey et al., 1994; Felix et al., 1994; Stearns and Kirschner, 1994). This result led to the suggestion that during mitosis, katanin might be associated with a spindle-pole matrix composed of nonpericentriolar proteins such as NUMA/centrophilin, a protein that may concentrate at spindle poles via cytoplasmic dynein-mediated transport toward microtubule minus ends (Tousson et al., 1991; Merdes et al., 1996). However, NUMA has not been characterized in sea urchins, and it was not known whether katanin’s unusual spindle-pole localization was conserved in mammals and in nonembryonic cell types. The result in this article that katanin is concentrated in a nocodazole-sensitive region larger than the γ-tubulin–staining pericentriolar material in both human MSU1.1 cells and in Xenopus A6 cells indicates that this poorly defined spindle-pole matrix is indeed conserved.
Further details of katanin’s centrosome localization, however, appear to be cell-type specific. In MSU1.1 cells, katanin is localized throughout the cell cycle in a nocodazole-resistant region that colocalizes exactly with γ-tubulin. This suggests that katanin can interact with a pericentriolar protein as well as with a spindle-pole matrix protein. In contrast, in A6 cells, katanin is found associated only with the nocodazole-sensitive spindle-pole matrix resulting in the “hollow ball” staining pattern reported previously in sea urchin embryos (McNally et al., 1996). It will be interesting to determine whether the WD40 domain of p80 katanin specifically binds a pericentriolar protein as well as a distinct spindle-pole matrix protein or whether pericentriolar and spindle-pole localizations of katanin are mediated through different domains. Isolation of proteins that bind specifically to the WD40 domain of p80 katanin will allow a direct approach to this problem.
The results presented here support a model in which katanin severs microtubules predominantly near the centrosome and when cyclin B/cdc2 activity is high during mitosis. Although it is clear that the bulk of katanin is cytoplasmic, it is unlikely that the cytoplasmic pool of katanin is extremely active. Quantitative immunoblots indicate that fibroblasts contain 1 katanin heterodimer for every 1000 tubulin heterodimers. Because 40–60% of the tubulin is polymerized in an animal cell (Zhai and Borisy, 1994), there would be a ratio of 1 katanin per 500 polymerized tubulin heterodimers. Previous work demonstrated that katanin-mediated microtubule disassembly is slow even at ratios of 1:150 (McNally and Vale, 1993). More recent experiments demonstrate that katanin’s ATPase activity is inhibited by high ratios of microtubules to katanin in the range of 500:1 (Hartman et al., 1998). Inhibition by high microtubule concentration may be due to inhibition of the assembly of katanin heterodimers into multimeric rings (Hartman et al., 1998). Specific interactions between the WD40 domain of p80 katanin and a centrosomal protein may promote the assembly of katanin into the active multimeric ring structure in addition to increasing the local concentration of katanin. Katanin’s concentrated activity at spindle poles may be required to free microtubule minus ends from their attachment sites in the γ-tubulin ring complexes (Moritz et al., 1995; Zheng et al., 1995) in the pericentriolar material. This release would then allow depolymerization of the microtubule minus ends during poleward flux, a process that may be required for maintenance of spindle structure (Waters et al., 1996).
Neuronal Katanin
Although katanin’s regulation and localization suggest a role in mitosis, its presence in adult brain tissue implies a second function in nondividing cells. Because katanin is found at centrosomes in a variety of species and cell types, it is likely that katanin is concentrated around centrosomes in neurons as well. The Triton-insoluble isoform of p60 katanin reported here in brain tissue may indicate a modified centrosomal form of katanin that is specific for neuronal cells. Katanin concentrated at the centrosomes of neurons could release microtubules from their centrosomal attachment sites in the cell body to allow transport of microtubules down the axon as proposed by Baas and Yu (1996). Such a release of microtubules from interphase centrosomes has also been observed in non-neuronal cells (Kitanishi-Yumura and Fukui, 1987; Keating et al., 1997). In addition, there may be other specialized roles for katanin in specific cell types as suggested by recent work implicating katanin in flagellar excision in Chlamydomonas (Lohret et al., 1998).
ACKNOWLEDGMENTS
We thank R. Vale for the G234A kinesin mutant and R. Vale and J. Hartman for communication of results before publication. We thank L. Rose and K. McNally for critical reading of the manuscript. This work was supported by grant GM-53060 from the National Institutes of Health to F.J.M.
REFERENCES
- Andersen SSL, Ashford AJ, Tournebize R, Gavet O, Sobel A, Hyman AA, Karsenti E. Mitotic chromatin regulates phosphorylation of stathmin/OP18. Nature. 1997;389:640–643. doi: 10.1038/39382. [DOI] [PubMed] [Google Scholar]
- Baas PW, Yu W. A composite model for establishing the microtubule arrays of the neuron. Mol Neurobiol. 1996;12:145–161. doi: 10.1007/BF02740651. [DOI] [PubMed] [Google Scholar]
- Belmont LD, Hyman AA, Sawin KE, Mitchison TJ. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990;62:579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- Clark-Maguire S, Mains PE. Localization of the mei-1 gene product of Caenorhabditis elegans, a meiotic-specific spindle component. J Cell Biol. 1994a;126:199–209. doi: 10.1083/jcb.126.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Maguire S, Mains PE. mei-1, a gene required for meiotic spindle formation in Caenorhabditis elegans, is a member of a family of ATPases. Genetics. 1994b;136:533–546. doi: 10.1093/genetics/136.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confalonieri F, Duguet M. A 200-amino acid ATPase module in search of a basic function. Bioessays. 1995;17:639–650. doi: 10.1002/bies.950170710. [DOI] [PubMed] [Google Scholar]
- Doxsey S, Stein P, Evans L, Calarco P, Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Felix M, Antony C, Wright M, Maro B. Centrosome assembly in vitro: role of γ-tubulin recruitment in Xenopus sperm aster formation. J Cell Biol. 1994;124:19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman MA, Dush MK, Martin GR. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Hartman JJ, Mahr J, McNally K, Okawa K, Iwamatsu I, Thomas S, Cheesman S, Heuser J, Vale RD, McNally FJ. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell. 1998;93:277–287. doi: 10.1016/s0092-8674(00)81578-0. [DOI] [PubMed] [Google Scholar]
- Horio T, Hotani H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature. 1986;321:605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- Keating TJ, Peloquin JG, Rodionov VI, Momcilovic D, Borisy GG. Microtubule release from the centrosome. Proc Natl Acad Sci USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly PJ, Krebs EG. J. Biol Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- Kishino A, Yanagida T. Force measurements by manipulation of a single actin filament. Nature. 1988;334:74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- Kitanishi-Yumura T, Fukui Y. Reorganization of microtubules during mitosis in Dictyostelium: dissociation from MTOC and selective assembly/disassembly in situ. Cell Motil Cytoskeleton. 1987;8:106–117. [Google Scholar]
- Lennon G, Aufrray C, Polymeropoulos M, Soares MB. The I.M.A.G.E. consortium: an integrated molecular analysis of genomes and their expression. Genomics. 1996;33:151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- Lin C, Maher V, McCormick J. Malignant transformation of human fibroblast strain MSU-1.1 by v-fes requires an additional genetic change. Int J Cancer. 1995;63:140–147. doi: 10.1002/ijc.2910630125. [DOI] [PubMed] [Google Scholar]
- Lohret TA, McNally FJ, Quarmby LM. A role for katanin-mediated axonemal severing during Chlamydomonas deflagellation. Mol Biol Cell. 1998;9:1195–1207. doi: 10.1091/mbc.9.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally F, Okawa K, Iwamatsu A, Vale R. Katanin, the microtubule-severing ATPase, is concentrated at centrosomes. J Cell Sci. 1996;109:561–567. doi: 10.1242/jcs.109.3.561. [DOI] [PubMed] [Google Scholar]
- McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- Merdes A, Ramyar K, Vechio JD, Cleveland D. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol. 1989;109:637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA. Microtubule nucleation by gamma-tubulin-containing rings in the centrosome. Nature. 1995;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Saxton WM, Stemple DL, Leslie RJ, Salmon ED, Zavortink M, McIntosh RJ. Tubulin dynamics in cultured mammalian cells. J Cell Biol. 1984;99:2175–2186. doi: 10.1083/jcb.99.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina N, Gotoh Y, Kubomura N, Iwamatsu A, Nishida E. Microtubule severing by elongation factor-1α. Science. 1994;266:282–285. doi: 10.1126/science.7939665. [DOI] [PubMed] [Google Scholar]
- Shiina N, Gotoh Y, Nishida E. A novel homo-oligomeric protein responsible for an MPF-dependent microtubule-severing activity. EMBO J. 1992;11:4723–4731. doi: 10.1002/j.1460-2075.1992.tb05577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T, Evans L, Kirschner M. Gamma-tubulin is a highly conserved component of the centrosome. Cell. 1991;65:825–836. doi: 10.1016/0092-8674(91)90390-k. [DOI] [PubMed] [Google Scholar]
- Stearns T, Kirschner M. In vitro reconstitution of centrosome assembly and function: the central role of γ-tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Tournebize R, Andersen SSL, Verde F, Doree M, Karsenti E, Hyman AA. Distinct roles of PP1 and PP2A-like phosphatases in control of microtubule dynamics during mitosis. EMBO J. 1997;16:5537–5549. doi: 10.1093/emboj/16.18.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousson A, Zeng C, Brinkley B, Valdivia M. Centrophilin: a novel mitotic spindle protein involved in microtubule nucleation. J Cell Biol. 1991;112:427–440. doi: 10.1083/jcb.112.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD. Severing of stable microtubules by a mitotically activated protein in Xenopus egg extracts. Cell. 1991;64:827–839. doi: 10.1016/0092-8674(91)90511-v. [DOI] [PubMed] [Google Scholar]
- Walczak CE, Mitchison TJ, Desai A. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996;84:37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- Walker RA, O’Brien ET, Pryer NK, Soboeiro MF, Voter WA, Erickson HP, Salmon ED. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol. 1988;107:1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JC, Mitchison TJ, Rieder CL, Salmon ED. The kinetochore microtubule minus-end disassembly associated with flux produces a force that can do work. Mol Biol Cell. 1996;7:1547–1558. doi: 10.1091/mbc.7.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Borisy GG. Quantitative determination of the proportion of microtubule polymer present during the mitosis-interphase transition. J Cell Sci. 1994;107:881–890. doi: 10.1242/jcs.107.4.881. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Kronebusch PJ, Simon PM, Borisy GG. Microtubule dynamics at the G-2-M transition: abrupt breakdown of cytoplasmic microtubules at nuclear envelope breakdown and implications for spindle morphogenesis. J Cell Biol. 1996;135:201–214. doi: 10.1083/jcb.135.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YX, Jung MK, Oakley BR. Gamma-tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell. 1991;65:817–823. doi: 10.1016/0092-8674(91)90389-g. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wong ML, Alberts B, Mitchison T. Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]