Abstract
The presence of porcine endogenous retroviruses presents a potential risk of transmission of infectious diseases (xenozoonosis) if tissues and organs from genetically modified pigs are to be used in xenotransplantation. Here, we report that intracellular expression of a llama single-domain antibody against p15, the matrix domain protein of the porcine endogenous retrovirus Gag polyprotein, blocks retrovirus production, providing the possibility of eliminating the risk of infection in xenotransplantation.
Since there is an evident shortage of human organs for allotransplantation, xenotransplantation (transplantation of cells and organs across species) has been considered as a possible alternative. The pig (Sus scrofa) is the preferred donor species due to its anatomical and physiological similarities with humans, breeding characteristics, lower risk of infectious disease in comparison to primates as organ donors, and ethical considerations. While most of the known exogenous pathogens can be controlled by breeding under specific-pathogen-free conditions, viruses such as porcine endogenous retroviruses (PERVs) that reside in the pig genome cannot be eliminated easily and therefore cause concern. Recent studies have shown that there are approximately 50 proviral integration sites in the pig genome (18, 25, 35), of which a number occur at the same position in different pig breeds. This makes a knockout or breeding strategy for elimination of PERVs impossible.
At least two classes of porcine retroviruses (PERV-A and PERV-B) are able to infect human cells in vitro (5, 24, 26, 34, 35). Although long-term infection after transplantation has not been found (4, 9, 14), PERVs are able to infect mouse cells in vivo (7, 39). Hence there is a possibility that PERVs lead to malignant, immunosuppressive (8), or other diseases in the recipient of a porcine transplant and that they may spread beyond the recipient into the human population, all potential risks that presently prevent the application of xenotransplantation (21).
While C-type PERVs differ significantly in the env gene encoding the envelope proteins, the genes for the polymerase (pol) and the group-specific antigens (gag) show high homology among all potentially xenotropic classes of PERVs (35). It is therefore reasonable to assume that antisera raised against Gag or Pol will react with both xenotropic and polytropic classes of PERVs (PERV-A and PERV-B) as well as the ecotropic PERV-C class.
Here, we describe the identification and selection of different llama heavy-chain-only antibodies in the form of variable heavy-chain fragments (VHH) raised against group-specific antigen Gag. VHHs, with their unique properties, such as high affinities and solubility in aqueous environments, are in principle ideal as intrabodies. We show that intracellular expression of such antibodies inhibits the production of virus particles.
MATERIALS AND METHODS
Antigen preparation and immunization.
PERV-B gag cDNA (AJ1293657) was amplified from PK15 cell RNA with the forward primer Gag forward/Asp (5′-ATAGGTACCATGGGACAGACAGTGACTACC-3′) and reverse primer Gag reverse/Hind (5′-ATAAGCTTGTCCGAACCCCGTCTCCCCTA-3′). The 1.6-kb gag cDNA was cloned into the pET-30a expression vector (Novagen, Breda, The Netherlands) and overexpressed upon isopropyl-β-d-thiogalactopyranoside (IPTG) induction in Escherichia coli BL21 DE3(pLysS). Purified 60-kDa Gag protein was used for immunization of a New Zealand rabbit that yielded a polyclonal rabbit antiserum against the PERV's Gag. The same protein was used for the immunization of a young adult male Lama glama. The immunization schedule was as previously described by van der Linden et al. (40).
Immunoelectron microscopy.
PK15 cells were fixed in 4% paraformaldehyde and prepared for the ultracryotome as previously described (37). Ultrathin cryosections (75 nm) were immunolabeled with llama polyclonal antiserum against Gag (1:250) followed by the goat anti-llama immunoglobulin G antibody (1:250; Bethyl Laboratories, Inc.) and rabbit anti-goat antibody conjugated with 10-nm colloidal gold particles (1:20; Aurion, Wageningen, The Netherlands) as described by Geuze et al. (13).
Cloning and expression of Gag cleavage products.
DNA encoding p27 capsid protein was cloned as a SmaI fragment in the pTRCB expession vector (Invitrogen). The fragment was amplified from gag cDNA with primers P27 forward/Sma (5′-TCCCCCGGGATCCGCTGCGCACCTATGGCCCT-3′) and p27 reverse/Sma (5′-TCCCCCGGGAAAATCTCTCTCTCTCTCCCT-3′).
DNA encoding p15 matrix protein was obtained by EagI fragment removal from the pET-30a-gag construct containing the full-length gag cDNA.
DNA encoding p12 was cloned as an NcoI fragment in pET-30a. The primers used for its amplification were P12 forward/Nco (5′-CATGCCATGGAGATCGAGGAGCCGCCGATC-3′) and P12 reverse/Nco (5′-CATGCCATGGGCCATAGGTGCGAGCGGTAA-3′).
P10 nucleocapsid DNA was cloned as an NcoI fragment in pET-30a. The primers used for its amplification were P10 forward/Nco (5′-CATGCCATGGCCGCACTGGTTGAAGGGAAG-3′) and P10 reverse/Nco (5′-CATGCCATGGACCCCGTCTCCCCTAATCTT-3′).
All clones were transformed into E. coli BL21 DE3(pLysS), in which Gag cleavage products were overexpressed upon IPTG induction.
Library construction and screening.
Total RNA was isolated from peripheral lymphocytes of the immunized llama with the Ultraspec RNA isolation system (Biotecx laboratories, Inc., Houston, Tex.). After purification of polyadenylated RNA (Oligotex 70022; Qiagen), cDNA was made with oligo(dT). DNA fragments encoding VHH fragments were amplified by PCR with specific primers Vh1back SfiI (23) in combination with Lam01 NotI (5′-CAGAAATGGAGCGGCCGCCTTGGGTTTTGGDGGGGAAGAKGAAGACDGATGG-3′) or Lam03 NotI (5′-CCTCGGGGTCGCGGCCGCCACRTCCACCACCACRCAYGTGACCT-3′) from exon CH2 and LH NotI (5′-GGATTGGGTTGCGGCCGCTGGTTGTGGTTGTGGTTGTGGTTTTGGTGTCTGGGGTTC-3′) from the long hinge. The amplified VHHs (≈500 bp) were separated by gel electrophoresis from the VHs of conventional antibodies containing the CH1 exon (≈800 bp) and gel purified. The isolated DNA was SfiI and NotI digested and cloned into the SfiI and NotI sites of the phagemid vector pHEN1 (15). Transformation into TG1 electrocompetent cells yielded a llama single-chain antibody library with an estimated size of 106 recombinants. Two rounds of selection were performed with panning on antigen adsorbed onto plastic (immunotubes coated with 30 μg and 10 μg of purified Gag protein per ml).
BIAcore measurements.
Experiments were carried out on a BIAcore 3000 surface plasmon resonance biosensor. Purified Gag protein was immobilized on a CM5 sensor chip to a level of 600 resonance units (RU, arbitrary binding response units) with the standard NHS-EDC kit supplied by the manufacturer. Anti-Myc tag antibody was immobilized on a separate flowcell of the same sensor chip to a level of 2,400 RU. A flowcell treated with the same procedure but with no protein was used as a control. The interaction buffer contained 20 mM HEPES, pH 7.4, 150 mM NaCl, 2 mM EDTA, and 0.005% Tween 20. Dilutions of different periplasmic fractions in the above buffer were firstly injected over the anti-Myc surface and adjusted so as to give the same response and thus ensure that the same amount of anti-Gag VHH was present. The adjusted dilutions of periplasmic fractions were then passed at the same time over the control and Gag surfaces, and the response at equilibrium was recorded. The amount of nonspecific binding was small (less than 5% of the specific) and was subtracted from the specific response. Regeneration of the surfaces was accomplished with a 5-μl pulse of 0.005% sodium dodecyl sulfate, which resulted in complete dissociation of bound protein and did not affect the binding capacity of the surface for subsequent interactions. Affinity or kinetic constants could not be obtained because of the inability to measure the exact concentration of VHH in the periplasmic fractions, and therefore results are presented as relative Gag binding at equilibrium.
Cell culture, transfections, and doxycycline induction.
The PK15 cells were grown in Dulbecco's modified Eagle's medium/Ham's F10 (Gibco-BRL) containing 10% fetal calf serum and in later stages supplemented with the appropriate selection markers. The Tet-on regulatory plasmid pUHrT 62-1 (generous gift from H. Bujard) containing the synthetic reverse tetracycline-controlled transactivator (rtTA2s) sequence was modified by introduction of the puromycin resistance gene as a eukaryotic selection marker. The resulting plasmid, pUHrT 62-1-puro, was ScaI linearized and transfected into PK15 cells with SuperFect transfection reagent (Qiagen) according to the manufacturer's instructions.
Clones were selected on puromycin at 1 μg/ml (Sigma, Zwijndrecht, The Netherlands) and screened in a transient transfection assay with the pBI-EGFP-Luc reporter plasmid (Clontech). Each clone was tested for luciferase and enhanced green fluorescent protein (EGFP) expression with and without doxycycline induction (500 ng/ml). The clone that gave the highest level of luciferase activity and EGFP expression in the “on” state and no background in the “off” state was used for the transfection experiments with the antibody coding genes. After transfection with ScaI-linearized 2xp(A)BiDi-A5-Myc plasmid, clones were selected and grown in 800 μg of G418 (Gibco) per ml. The Tet-on line and clones 13 and 17 were cultured to 40% confluency in six-well plates with 3 ml of medium. Doxycycline was added to half of the wells, all the wells were washed once after 8 h of induction to remove residual virus, and the medium was replaced for another 48 h of incubation. The cells were collected and used for Western blot, immunofluorescent staining, and immunoelectron microscopy. The supernatant was collected for reverse transcription (RT) assays or RT-PCR.
Western blot analysis.
Bacterial cell lysates (50 mM Tris, pH 8.0, 150 mM NaCl, 0.25% NP-40), PK15 viral pellets, and PK15 cell lysates were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis 12 or 15% gel, blotted, and incubated with A5 single-domain antibody followed by mouse anti-Myc (1:1,000, clone 9E10; Covance Inc., Princeton, N.J.) and goat anti-mouse immunoglobulin-alkaline phosphatase conjugate (1:1,000; Sigma, Zwijndrecht, The Netherlands).
β-Tubulin (56 kDa) was used as a loading control for cell lysates. It was detected with an anti-β-tubulin mouse monoclonal antibody (1:2,000; Sigma, Zwijndrecht, The Netherlands) followed by goat anti-mouse immunoglobulin-alkaline phosphatase conjugate (ICN Biomedicals BV, Zoetermeer, The Netherlands). Nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (Roche, Mannheim, Germany) was used as the alkaline phosphatase substrate.
For visualization of Gag and Gag cleavage products, rabbit anti-Gag polyclonal serum or llama anti-Gag polyclonal serum was used, followed by swine anti-rabbit immunoglobulin-horseradish peroxidase (1:2,000; DAKO) or goat anti-llama-horseradish peroxidase (1:2,000, Bethyl Laboratories, Inc.). Diaminobenzidine (Vector Laboratories Inc., Burlingame, Calif.) was used as a substrate.
Immunofluorescence.
Cells were grown on coverslips, fixed in 4% paraformaldehyde for 20 min, and permeabilized with 0.5% Triton X-100 for 10 min at room temperature. Gag was visualized with fluorescein isothiocyanate-coupled goat anti- rabbit immunoglobulin G (Nordic Immunological Laboratories B.V.) as a secondary antibody. VHH expression was detected with Alexa-594-coupled goat anti- mouse immunoglobulin G (Molecular Probes). For nuclear staining 4′,6′-diamino-2-phenylindole (DAPI; Sigma, Zwijndrecht, The Netherlands) was used.
RT assay.
C-type Mn2+-dependent RT activity assay was performed on the cell-free supernatants from each clone in the uninduced and induced states with the Cavidi HS-kit (Cavidi Tech AB, Uppsala, Sweden) according to the manufacturer's instructions.
RT-PCR.
Culture supernatant was harvested and filtered through a 0.45-μm-pore-size filter, and virions were pelleted by ultracentrifugation for 2 h at 150,000 × g/30,000 rpm. Viral RNA was isolated with a commercially available kit (including a DNase step; Qiagen). cDNA was synthesized with oligo(dT) and Super RT (HT Biotechnology, Cambridge, United Kingdom). A previously described env-specific set of primers for the PCR of PERV-A (pl 206/pl 205) (35), PERV-B (pl 170/pl 171) (18), and PERV-C (pl 172/pl 173) (18) were used. In addition, a pol-specific set of primers for A, B, and C type (pol forward, 5′-ATACTCCCCTGCTACCGGTT-3′, and pol reverse, 5′CAAGAGGTTATAAGGGTTCGG 3′) were used with the following cycle parameters: 92°C for 4 min, 36 cycles of 92°C for 1 min, 53°C for 1 min, and 72°C for 1 min, followed by 10 min at 72°C.
p15 epitope mapping.
Fragments of p15 DNA were generated by PCR on the pET-30a gag cDNA plasmid. Aa1 BamHI forward primer was 5′-CCGGGGATCCCCATGGGACAGACAGTGACTACC-3′. Aa46 BamHI reverse primer was 5′-GGCGGATCCCGAATGTTGGCCATTCAGAGGCAC-3′. Aa113 BamHI reverse primer was 5′-GCGGATCCAGCCAGGATTCGGGGACCTGGC-3′. Aa123 BamHI reverse primer was 5′-GGCGGATCCGTGGTGCTCGAGTGCGGCCGA-3′. Aa47 BamHI forward primer was 5′-CCGGGGATCCCCATGTTGGATGGCCATCAGAGGGG-3′.
Single-point mutations at amino acids 94 and 96 were generated by PCR on gag cDNA with primers 5′-GCAGAAGATCCT(C→G)CGCCA(T→G)GGGTTAAACCATGG-3′ forward with Aa113 reverse and 5′-CCATGGTTTAACCC(A→C)TGGCG(G→C)AGGATCTTCTGC-3′ reverse with Aa1 forward.
A second PCR was performed with the amino acid 1 forward and amino acid 113 reverse primers. The equimolar mixture of the purified fragments from the above reactions served as a template.
The seven-amino-acid peptide DPPPWVK was made by annealing the following oligonucleotides: 5′-GATCCCCGATCCTCCGCCATGGGTTAAAG-3′ and 5′-AATTCTTTAACCCATGGCGGAGGATCGGG-3′ with a BamHI overhang.
All fragments were cloned into the BamHI site of the expression vector pGEX.3X (Amersham Pharmacia) and sequenced, and proteins and peptides were expressed as fusions with glutathione S-transferase by standard protocols (33).
RESULTS
Reactivity of the Gag-specific antisera.
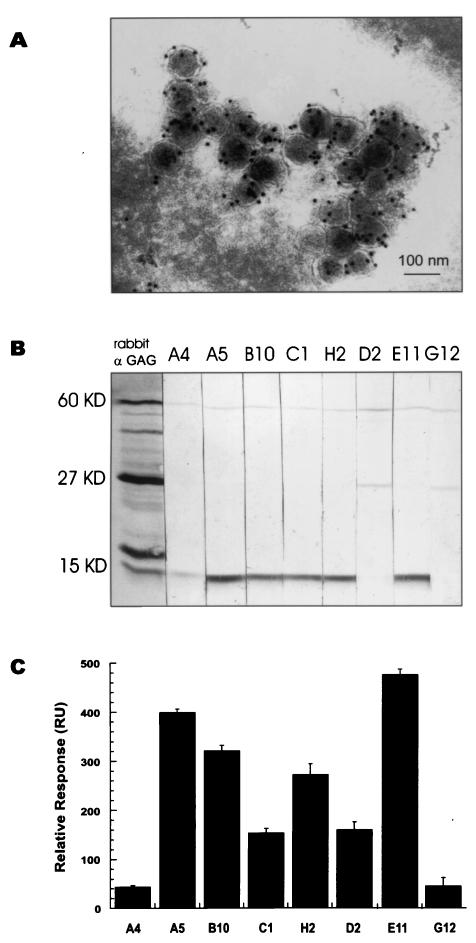
PERV-B gag cDNA was amplified from porcine PK15 cell RNA and expressed in bacteria, and the resulting protein was used for the immunization of a New Zealand rabbit that yielded a polyclonal antiserum against the PERV's Gag. The same protein was used for the immunization of a young adult male Lama glama. The specificity of both the rabbit and llama polyclonal antisera was tested on Western blots, immunocytochemistry, and immunoelectron microscopy. Both sera recognize the precursor 60-kDa Gag polyprotein, intermediate forms, and further processed matured forms of the viral structural proteins, including major capsid (p27), matrix (p15), inner coat, and nucleocapsid protein. Immunoelectron microscopy on cryosections of PK15 cells showed specific labeling of the virus particles (Fig. 1A). Based on these results, it was assumed that there would also be single-heavy-chain-only antibodies present in the llama that will react with Gag protein of PERVs.
FIG. 1.
Lama glama antiserum recognizes Gag and contains several VHHs with different affinities for viral Gag. (A) PERV detection in cryosections of PK15 cells by immunoelectron microscopy with llama anti-Gag antiserum. (B) Western blot on virus lysate showing specificity for the 60-kDa Gag polyprotein and for different Gag domains. A4, A5, B10, C1, H2, and E11 are antibodies against matrix protein p15, while D2 and G12 bind to capsid protein p27. All VHHs recognize whole Gag. (C) BIAcore affinity measurements. Equal amounts of soluble reactive VHHs from periplasmic fractions were used to measure relative binding to Gag protein immobilized on a BIAcore sensor chip.
Isolation of Gag antigen-specific llama VHH antibody fragments, their sequence analysis, and affinity determination.
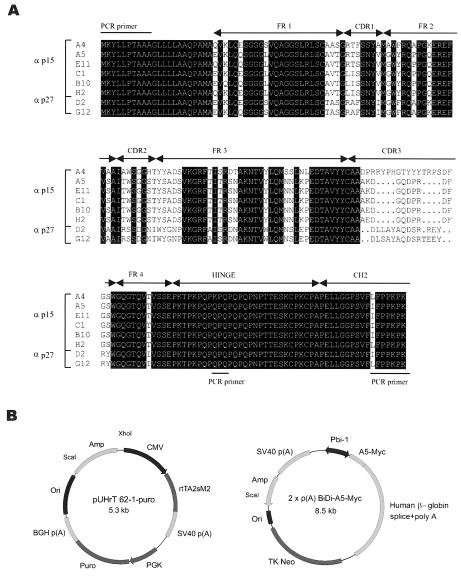
In order to isolate the genes coding for the llama heavy-chain-only antibodies, cDNA was synthesized from RNA isolated from peripheral lymphocytes of the immunized llama and cloned to yield an immune llama single-chain antibody phagemid library of 106 clones. Purified Gag protein was used to screen the library. Two rounds of selection were performed with panning on antigen adsorbed onto plastic. The clones positive in an enzyme-linked immunosorbent assay with anti-Myc antibody (9E10) for detection of VHHs were analyzed at the DNA level by HinfI fingerprinting. Eight different clones (A4, A5, E11, H2, B10, C1, D2, and G12) were obtained and sequenced (Fig. 2A). They all originated from a long hinge (IgG2) single-chain antibody of Lama glama and bind to the Gag protein used for the immunization as well as to the Gag protein from PK15 cell lysate and the cell-free virus supernatant. D2 and G12 bind to the major capsid protein p27, while A4, A5, B10, C1, H2, and E11 bind to matrix protein p15 (Fig. 1B), and not to p12 or NC, as confirmed by Western blots with separately expressed cleavage products of Gag polyprotein (data not shown).
FIG. 2.
Amino acid sequences of Gag positive binders and constructs used to express single-domain antibody A5 intracellularly. (A) Alignment of eight different llama antibodies against the PERV-B Gag protein. The VHH structural elements (complementarity-determining regions [CDRs] and framework regions [FRs]), hinge region, and CH2 exon are indicated. (B) The vectors used for transfection experiments in PK15 cells. Left: Tet-on regulatory plasmid pUHrT 62-1-puro. Right: response plasmid 2xp(A)BiDi-A5-Myc, containing A5 VHH in frame with the Myc tag cloned on one side of the bidirectional Tre-responsive promoter and the neomycin resistance gene.
For purification and mass production purposes, all eight single-domain antibodies were shortened by removal of the CH2 region and part of the hinge region. This made the histidine tag accessible for purification purposes (on Ni+ beads) and resulted in the production of satisfactory amounts of VHH fragments in the periplasm of bacteria. All eight periplasmic fractions of the VHHs were shown to be active by BIAcore analyses (16) in which the Gag antigen was immobilized on CM5 sensor chips. The binding affinity was in the order E11 > A5 > B10 > H2 > D2 > C1 > G12 > A4 (Fig. 1C).
Tet-on inducible intracellular expression of A5 single-domain antibody and its effect on virus production in PK15 cells.
The first high-affinity binder obtained, A5 antibody, was tested for its capacity to block virus production in PK15 cells. For this purpose we used the tetracycline-inducible system (Tet-on) (38). PK15 cells containing the Tet-on regulatory construct (Fig. 2B) were stably transfected with the A5 single-domain expression vector (Fig. 2B). Clones were kept on double selection with puromycin and G418. After doxycycline induction, they were screened for expression of the A5 antibody by immunofluorescence with an anti-Myc antibody. The expression of antibody differed from clone to clone, ranging from a small percentage of cells expressing in some clones up to a maximum of 90 to 95% of the cells expressing the A5 VHH (clone 13). Subcloning or new transfections (also with non-Tet systems) did not yield any clones where all cells expressed the antibody, and thus we proceeded with two clones, clone 17 (expressing the single-domain antibody in ≈30 to 35% of the cells upon doxycycline induction) and clone 13 (expressing the single-domain antibody in 90 to 95% of the cells upon induction).
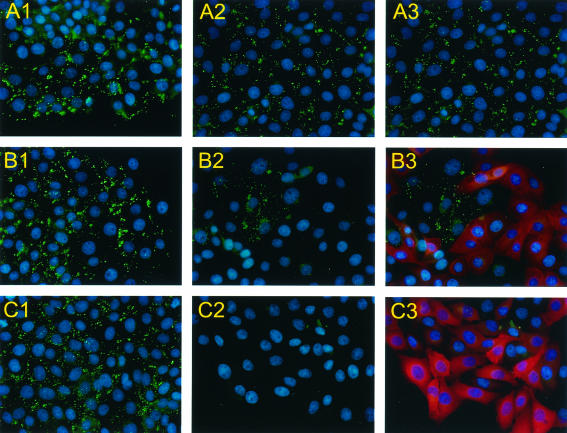
The Gag antigen could be detected by rabbit polyclonal antiserum in all of the noninduced cells (Fig. 3, A1, B1, and C1). It is also detectable in those cells that were induced but did not express single-chain antibody (Fig. 3, A2-3, B2-3, and C2-3). Gag is detected at the plasma membrane in a punctate pattern. However, when the A5 VHH is expressed, the level of Gag protein drops below the level of detection and the punctate staining pattern at the plasma membrane is lost (Fig. 3, B2-3, C2-3). The few cells that do not express the VHH after induction still show such staining. Occasionally, a faint perinuclear staining of Gag can be seen in cells expressing the antibody domain, indicating that the Gag protein that is still produced rapidly disappears early in the virus assembly process (Fig. 3, B3 and C3). This is confirmed by Western blot analysis.
FIG. 3.
Immunofluorescent staining showing doxycycline-induced production of A5 VHH and its influence on Gag expression. PK15 cells were stably transfected with the Tet-on regulatory plasmid, so-called Tet-on line (panel A), and two clones, 17 (panel B) and 13 (panel C), of Tet-on line, which were additionally stably transfected with the response plasmid containing A5 VHH. The cells were either not treated (A1, B1, and C1) or treated for 48 h with doxycycline (A2, B2, and C2). A3, B3, and C3, same fields as A2, B2, and C2, respectively, of doxycycline-induced cells showing Gag expression in green and A5 VHH expression in red.
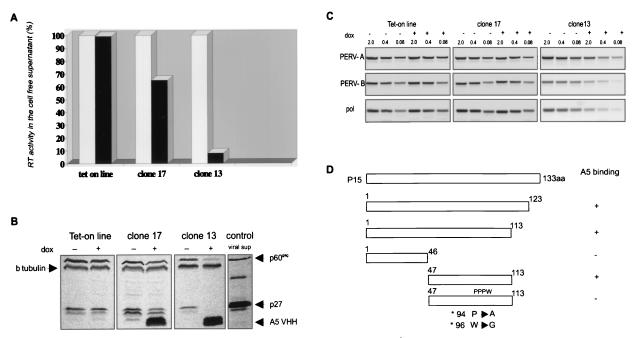
Cell lysates from the Tet-on PK15 cell line, not expressing the A5 VHH before and after induction, have the same level of expression of the Gag precursor protein and p27. In the clones that express the A5 upon doxycycline induction, the viral proteins are reduced proportionally to the number of cells expressing the antibody in each clone, with β-tubulin expression as a control (Fig. 4B). In both clones (17 and 13), the RT activity remaining in the supernatant collected from doxycycline-induced, VHH-expressing cells was greatly reduced in comparison to the supernatant collected from uninduced state of the same clone or control Tet-on line regardless of its state of induction. As expected, the largest reduction in RT activity was observed in the clone with the highest percentage of antibody-expressing cells. RT activity in this case (clone 13) was reduced to approximately 7% of the activity in uninduced state (Fig. 4A). This residual RT activity correlates very well with the number of cells in the cloned population that do not express the antibody and which still produce virus particles (Fig. 3).
FIG. 4.
Virus production (PERV-A/B) by PK15 cells is blocked upon expression of A5 VHH. (A) Relative RT activity in the cell-free supernatant. White bars represent RT activity in nontreated samples; black bars represent RT activity in supernatants from doxycycline-treated cells. The decrease in RT activity is proportional to the number of A5 VHH-expressing cells. (B) Western blot showing Gag expression in cell lysates upon doxycycline induction in the different clones. Within each clone β-tubulin is stained as a loading control. (C) RT-PCR of serially diluted viral cDNA preparations. The 2, 0.4, and 0.08 refer to microliters of template used in the reaction. The gel was stained with ethidium bromide. Both PERV-A and PERV-B are blocked (>25-fold decrease) by the expression of A5 VHH. (D) A5 epitope mapping, showing that the A5 binding site lies between amino acids 47 and 113 of the PERV matrix protein.
The same experiments were performed with two other VHHs, E11 and D2. The blocking effect of the E11 VHH was the same as that of A5, which was predictable from its sequence (only one amino acid difference in frame 1) and recognition of the same epitope. However, the D2 VHH, which binds to p27 in the PK15 cells, was not blocking virus production (data not shown).
Blocking of both PERV-A and PERV-B production.
In order to show that both PERV-A and PERV-B production was blocked, the residual viral RNA in the supernatant was reverse transcribed and amplified with PERV-A-, B-, or C-specific primers corresponding to the env sequences or with common pol primers. As expected, PERV-C particles were not present in PK15 cells (data not shown). PERV-A and PERV-B enveloped particle production by PK15 cells was inversely correlated with VHH expression based on RT-PCR of serial dilutions of viral cDNA (Fig. 4C). The results with the pol primers are in accordance with those for PERV-A and PERV-B particle production.
A5 epitope mapping.
We partially characterized the epitope responsible for the inhibitory effect of the A5 single-domain antibody. A deletional approach revealed that the binding site for the A5 VHH lies between amino acids 47 and 113 of p15 (Fig. 4D). Two-amino-acid substitution in a highly conserved PPPWVK motif abolished antibody binding completely. The A5 antibody did not recognize a short peptide of 7 amino acids containing this motif in fusion with glutathione S-transferase, nor did it recognize mouse retrovirus (Cas-Br-M murine leukemia virus) that shared the same motif (data not shown). This suggests that the motif is part of a larger or conformational epitope, maybe involving more proximal amino acids that participate in alpha helix formation.
DISCUSSION
For xenotransplantation to become a clinical reality, more than physiological compatibility between pig and human organs is required. The main problem is how to prevent immune rejection and, one step further, how to prevent a potential xenozoonosis. PERVs, residing in the pig genome and related to other retroviruses known to cause leukemias and immune deficiencies in the infected host, cause the biggest concern. Even though most of the integrated copies of the PERV genome are reported to be replication incompetent, there is still the possibility of recombination and complementation, which makes a knockout approach (limited to only functional copies of PERVs) inadequate. There are, however, other methods that could deal with inhibition of PERVs, such as antiviral chemotherapy (29), antisense (11), or ribozyme strategies (31), RNAi (20), active and passive immunization (10), or intracellular expression of inhibitory antibodies.
For therapy to succeed in clinical practice, the therapeutic agent must have a full blocking effect, be delivered efficiently to the appropriate cells and/or tissues, and remain there at the therapeutic level without harming the recipient and the xenotransplant. For immunization strategies, the fact that circulating antibodies (against PERV proteins expressed on cell membrane while budding) could contribute to transplant destruction should be taken into account. Here we describe an intracellularly expressed single-domain antibody that prevents virus release from the pig cell. Possible application in xenotransplantation would be directed towards “treatment” of the donor pig strain by means of transgenesis, rather than treatment of the human recipient. However, for the strategy to succeed, it would be necessary to express the intrabodies ubiquitously at all times. Ubiquitously acting chromatin opening elements such as hnRNPA2, for example (M. Antoniou, 21 July 1999, PCT/GB99/02357), that regulate genes that need to be switched on throughout the body could be used as regulatory elements in transgenic constructs. In PK15 cell culture experiments, with a minimal cytomegalovirus promoter, we did not achieve the desired 100% expression. Since residual RT activity correlates very well with the number of cells in the cloned population that do not express the antibody and which still produce virus particles, it is unlikely that other (as yet unknown) retroviruses present in PK15 cells contribute to the residual RT activity. We therefore conclude that our intracellularly expressed antibody against the matrix domain of PERV-B Gag polyprotein completely blocks virus production of both PERV-A and -B types of retroviruses in antibody-expressing PK15 cells.
Although it appears to be early in the process, it is not clear from our data at which step virus production is blocked. The antibody interferes with expression of both Gag protein and its cleavage products, indicating an inhibition of the maturation process. In several retroviruses analyzed to date, matrix proteins are required for the targeting and interaction of the Gag precursor with the plasma membrane involving N-terminal myristylation and a highly basic domain. They function in envelope glycoprotein incorporation into budding virions and virus particle assembly. They also play additional roles during early and late phases of the viral life cycle (12, 22, 30, 42).
At present, little work has been carried out to specifically address the function of PERV p15 matrix protein. However, reports from AIDS research suggest the importance of the human immunodeficiency virus (HIV) matrix protein (p17) as a target for antiviral therapies (19, 36). Since the majority of immunogenic sites on protein antigens are conformationally dependent and/or discontinuous, they are unlikely to be all present on short linear peptides. However, a deletional approach revealed that the binding site for the A5 VHH lies between amino acid positions 47 and 113 of p15, which excludes the involvement of the N-terminal myristylation site. The region shows high sequence conservation among all porcine gammaretroviruses, which implies that it is unlikely to be prone to mutation. However, if the virus mutates, it could escape antibody inhibition. Our results show that two base-pair mutations were sufficient to lose the antibody binding capacity. Thus, using more than one antibody simultaneously against different epitopes of the virus would be the preferential method of inhibition.
Whichever step is blocked, our data show that virus production can be inhibited by single-domain VHH, the smallest available intact antigen-binding fragment derived from the functional immunoglobulin, without cytotoxicity. The genetic modifications of pigs, such as knocking out the gene coding for α-1,3-galactosyltransferase (6, 17, 27) and expressing human complement regulatory proteins (2, 3, 28), appear to have overcome the hyperacute rejection problem. At the same time, knocking out α-1,3-galactosyltransferase from pigs might create an even greater risk of potential xenozoonosis caused by PERVs (1). Since PERV particles containing the pig cell membrane do not have the α-1,3-galactosyltransferase epitope on their surface anymore, virus emerging from the xenotransplant from knockout pigs will be less susceptible to inactivation by virus neutralizing human anti α-1,3-galactosyltransferase antibodies (32). The same is true for pigs transgenic for human CD46, CD55, or CD59. Viruses produced in such pigs would carry these molecules on their cell surface, thereby escaping complement-mediated virolysis (41). In the background of genetically modified pigs mentioned above, the ubiquitous transgenic expression of antiviral single-domain antibodies such as the one described in this paper will contribute greatly to a solution of the safety problem presented by PERVs.
Acknowledgments
We thank Rein Smid for animal care, H. Westerveld for llama immunization, and Rick Janssens for technical help during the procedure. We are grateful to R. Fouchier for provision of PK15 cells and helpful comments on the manuscript, H. Bujard for the pUHrT 62-1 plasmid, R. Delwel for the Cas-Br-M MuLV, and P. F. van Loo for technical assistance during his training period.
The Netherlands Heart Foundation supported this work.
REFERENCES
- 1.Butler, D. 2002. Xenotransplant experts express caution over knockout piglets. Nature 415:103-104. [DOI] [PubMed] [Google Scholar]
- 2.Cozzi, E., A. W. Tucker, G. A. Langford, G. Pino-Chavez, L. Wright, M. J. O'Connell, V. J. Young, R. Lancaster, M. McLaughlin, K. Hunt, M. C. Bordin, and D. J. White. 1997. Characterization of pigs transgenic for human decay-accelerating factor. Transplantation 64:1383-1392. [DOI] [PubMed] [Google Scholar]
- 3.Cozzi, E., and D. J. G. White. 1995. The generation of transgenic pigs as potential organ donors for humans. Nat. Med. 1:964-966. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham, D. A., C. Herring, X. M. Fernandez-Suarez, A. J. Whittam, K. Paradis, and G. A. Langford. 2001. Analysis of patients treated with living pig tissue for evidence of infection by porcine endogenous retroviruses. Trends Cardiovasc. Med. 11:190-196. [DOI] [PubMed] [Google Scholar]
- 5.Czauderna, F., N. Fisher, K. Boller, R. Kurth, and R. R. Tönjes. 2000. Establishment and characterization of molecular clones of porcine endogenous retroviruses replicating on human cells. J. Virol. 74:4028-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai, Y., T. D. Vaught, J. Boone, S. Chen, C. J. Phelps, S. Ball, J. A. Monahan, P. M. Jobst, K. J. McCreath, A. E. Lamborn, J. L. Cowell-Lucero, K. D. Wells, A. Colman, I. A. Polejaeva, and D. L. Ayares. 2002. Targeted disruption of the α-1, 3-galactosyltransferase gene in cloned pigs. Nat. Biotechnol. 20:251-255. [DOI] [PubMed] [Google Scholar]
- 7.Deng, Y. M., B. E. Tuch, and W. D. Rowlinson. 2000. Transmission of porcine endogenous retroviruses in severe combined immunodeficient mice xenotransplanted with fetal porcine pancreatic cells. Transplantation 70:1010-1016. [DOI] [PubMed] [Google Scholar]
- 8.Denner, J. 1998. Immunosuppression by retroviruses: implications for xenotransplantation. Ann. N. Y. Acad. Sci. 862:75-86. [DOI] [PubMed] [Google Scholar]
- 9.Dinsmore, J. H., C. Manhart, R. Raineri, D. B. Jacoby, and A. Moore. 2000. No evidence for infection of human cells with porcine endogenous retrovirus (PERV) after exposure to porcine fetal neuronal cells. Transplantation 70:1382-1389. [DOI] [PubMed] [Google Scholar]
- 10.Fiebig, U., O. Stephan, R. Kurth, and J. Denner. 2003. Neutralizing antibodies against conserved domains of p15E of porcine endogenous retroviruses: basis for a vaccine for xenotransplantation? Virology 307:406-413. [DOI] [PubMed] [Google Scholar]
- 11.Fraisier, C., D. A. Abraham, M. van Ooijen, V. Cunliffe, A. Irvine, R. Craig, and E. A. Dzierzak. 1998. Inhibition of Tat-mediated transactivation and HIV replication with Tat mutant and repressor domain fusion proteins. Gene Ther. 5:946-954. [DOI] [PubMed] [Google Scholar]
- 12.Freed, E. O. 1998. HIV-1 Gag proteins: diverse functions in the virus life cycle. Virology 251:1-15. [DOI] [PubMed] [Google Scholar]
- 13.Geuze, H. J., J. W. Slot, P. A. van der Ley, and R. C. T. Scheffer. 1981. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J. Cell Biol. 89:653-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herring, C., D. A. Cunningham, A. J. Whittam, X. M. Fernandez-Suarez, and G. A. Langford. 2001. Monitoring xenotransplant recipients for infection by PERV. Clin. Biochem. 34:23-27. [DOI] [PubMed] [Google Scholar]
- 15.Hoogenboom, H. R., A. D. Griffiths, K. S. Johnson, D. J. Chriswell, P. Hudson, and G. Winter. 1991. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 19:4133-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazemier, B., H. de Haard, P. Boender, B. van Gemen, and H. Hoogenboom. 1996. Determination of active single-chain antibody concentrations in crude periplasmic fractions. J. Immunol. Methods 194:201-209. [DOI] [PubMed] [Google Scholar]
- 17.Lai, L., D. Kolber-Simonds, K. Park, H. Cheong, J. L. Greenstein, G. Im, M. Samuel, A. Bonk, A. Rieke, B. N. Day, C. N. Murphy, D. B. Carter, R. J. Hawley, and R. S. Prather. 2002. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295:1089-1092. [DOI] [PubMed] [Google Scholar]
- 18.Le Tissier, P., J. P. Stoye, Y. Takeuchi, C. Patience, and R. A. Weiss. 1997. Two sets of human-tropic pig retroviruses. Nature 389:681-682. [DOI] [PubMed] [Google Scholar]
- 19.Levin, R., A. M. Mhashilkar, T. Dorfman, A. Bukovsky, C. Zani, J. Bagley, J. Hinkula, M. Niedrig, J. Albert, B. Wahren, H. G. Gottlinger, and W. A. Marasco. 1997. Inhibition of early and late events of the HIV-1 replication cycle by cytoplasmic Fab intrabodies against the matrix protein, p17. Mol. Med. 3:96-110. [PMC free article] [PubMed] [Google Scholar]
- 20.Novina, C. D., M. F. Murray, D. M. Dykxhoorn, P. J. Beresford, J. Riess, Sang-Kyung Lee, R. G. Collman, J. Lieberman, P. Shankar, and P. A. Sharp. 2002. SiRNA-directed inhibition of HIV-1 infection. Nat. Med. 8:681-686. [DOI] [PubMed] [Google Scholar]
- 21.Nuffield Council on Bioethics. 1996. Animal-to-human transplants: the ethics of xenotransplantation. Nuffield Council on Bioethics, London, United Kingdom.
- 22.Ono, A., J. M. Orenstein, and E. O. Freed. 2000. Role of gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J. Virol. 74:2855-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orlandi, R., D. H. Gussov, P. T. Jones, and G. Winter. 1989. Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc. Natl. Acad. Sci. USA 86:3833-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paradis, K., G. Langford, Z. Long, W. Heneine, P. Sandstrom, W. M. Switzer, L. E. Chapman, C. Lockey, D. Onions, the XEN 111 Study Group, and E. Otto. 1999. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with liver pig tissue. Science 285:1236-1241. [DOI] [PubMed] [Google Scholar]
- 25.Patience, C., W. M. Switzer, Y. Takeuchi, D. J. Griffiths, M. E. Goward, W. Heneine, J. P. Stoye, and R. A. Weiss. 2001. Multiple groups of novel retroviral genomes in pigs and related species. J. Virol. 75:2771-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patience, C., Y. Takeuchi, and R. A. Weiss. 1997. Infection of human cells by an endogenous retrovirus of pigs. Nat. Med. 3:282-286. [DOI] [PubMed] [Google Scholar]
- 27.Phelps, C. J., C. Koike, T. D. Vaught, J. Boone, K. D. Wells, S. Chen, S. Ball, S. M. Specht, I. A. Polejaeva, J. A. Monahan, P. M. Jobst, S. B. Sharma, A. E. Lamborn, A. S. Garst, M. Moore, A. J. Demetris, W. A. Rudert, R. Bottino, S. Bertera, M. Trucco, T. E. Starzl, Y. Dai, and D. L. Ayares. 2003. Production of α-1,3-galactosyltransferase-deficient pigs. Science 299:411-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Platt, J. L. 2002. Knocking out xenograft rejection. Nat. Biotechnol. 20:231-232. [DOI] [PubMed] [Google Scholar]
- 29.Powell, S. K., M. E. Gates, G. Langford, M. L. Gu, C. Lockey, Z. Long, and E. Otto. 2000. Antiretroviral agents inhibit infection of human cells by porcine endogenous retroviruses. Antimicrob. Agents Chemother. 44:3432-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rein, A., M. R. McClure, N. R. Rice, R. B. Luftig, and A. M. Schultz. 1986. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc. Natl. Acad. Sci. USA 19:7246-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi, J. J. 1999. The application of ribozymes to HIV infection. Curr. Opin. Mol. Ther. 1:316-322. [PubMed] [Google Scholar]
- 32.Rother, R. P., W. L. Fodor, J. P. Springhorn, C. W. Birks, E. Setter, M. S. Sandrin, S. P. Squinto, and S. A. Rollins. 1995. A novel mechanism of retrovirus inactivation in human serum mediated by anti-alpha-galactosyl natural antibody. J. Exp. Med. 182:1345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Specke, V., S. Rubant, and J. Denner. 2001. Productive infection of human primary cells and cell lines with porcine endogenous retroviruses. Virology 285:177-180. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi, Y., C. Patience, S. Magre, R. A. Weiss, P. T. Banerjee, P. Le Tissier, and J. P. Stoye. 1998. Host range and interference studies of three classes of pig endogenous retrovirus. J. Virol. 72:9986-9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tewari, D., S. L. Goldstein, A. L. Notkins, and P. Zhou. 1998. cDNA encoding a single-chain antibody to HIV p17 with cytoplasmic or nuclear retention signals inhibits HIV-1 replication. J. Immunol. 161:2642-2647. [PubMed] [Google Scholar]
- 37.Tokuyasu, K. T. 1978. A study of positive staining of ultrathin frozen sections. J. Ultrastruct. Res. 63:287-307. [DOI] [PubMed] [Google Scholar]
- 38.Urlinger, S., U. Baron, M. Thellmann, M. T. Hasan, H. Bujard, and W. Hillen. 2000. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. USA 97:7963-7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Laan, L. J., C. Lockey, B. C. Griffeth, F. S. Frasier, C. A. Wilson, D. E. Onions, B. J. Hering, Z. Long, E. Otto, B. E. Torbett, and D. R. Salomon. 2000. Infection by porcine endogenous retrovirus after islet xenotransplantation in SCID mice. Nature 407:90-94. [DOI] [PubMed] [Google Scholar]
- 40.van der Linden, R., B. de Geus, W. Stok, W. Bos, D. van Wassenaar, T. Verrips, and L. Frenken. 2000. Induction of immune responses and molecular cloning of the heavy chain antibody repertoire of Lama glama. J. Immunol. Methods 240:185-195. [DOI] [PubMed] [Google Scholar]
- 41.Weiss, R. A. 1998. Transgenic pigs and virus adaptation. Nature 391:327-328. [DOI] [PubMed] [Google Scholar]
- 42.Yu, X., X. Yuan, Z. Matsuda, T. H. Lee, and M. Essex. 1992. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J. Virol. 66:4966-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]