Abstract
Recently, we have shown that the rabies virus (RV) matrix (M) protein regulates the balance of virus RNA synthesis by shifting synthesis activity from transcription to replication (S. Finke, R. Mueller-Waldeck, and K. K. Conzelmann, J. Gen. Virol. 84:1613-1621, 2003). Here we describe the identification of an M residue critical for regulation of RV RNA synthesis. By analyzing the phenotype of heterotypic RV M proteins with respect to RNA synthesis of RV SAD L16, we identified the M proteins of the RV ERA and PV strains as deficient. Comparison of M sequences suggested that a single residue, arginine 58, was critical. A recombinant virus having this amino acid exchanged with a glycine, SAD M(R58G), has lost the abilities to downregulate RV transcription and to stimulate replication. This resulted in an increase in the transcription rate of more than 15-fold, as previously observed for M deletion mutants. Most importantly, the efficiencies of virus assembly and budding were equal for wild-type M and M(R58G), as determined in assays studying the transient complementation of an M- and G-deficient RV construct, NPgrL. In addition, virus particle density, protein composition, and specific infectivity of SAD L16 and SAD M(R58G) viruses were identical. Thus, we have identified mutations that affect the function of M only in regulation of RNA synthesis, but not in assembly and budding, providing evidence that these functions are genetically separable.
Matrix (M) proteins of negative-strand RNA viruses play a crucial role throughout the virus life cycle. The functions of the M proteins in virus assembly and morphogenesis and in budding off enveloped virus particles have been extensively studied and well recognized. More recently, reverse genetics experiments with rabies virus (RV), a member of the Rhabdoviridae family, revealed an additional and unexpected function of rhabdovirus M proteins apart from virus assembly, namely, the regulation of RNA synthesis and profoundly affecting the balance of replication and transcription products (13).
The viral RNA of nonsegmented negative-strand RNA viruses (order Mononegavirales) is associated with the viral nucleoprotein (N) to form a tight ribonucleoprotein (RNP) complex. Such RNPs are the template for transcription of subgenomic virus mRNAs and for replication of full-length virus RNAs. The latter probably involves concurrent encapsidation of the nascent RNA into RNPs. A constant supply of N protein is therefore a prerequisite for productive replication (1, 28). In contrast, transcription from the RNP does not need a supply of N. Both RNA replication and transcription are performed by the virus-encoded RNP-dependent RNA polymerase, which is composed of a large, catalytically active subunit (L) and a polymerase cofactor, the phosphoprotein P. P also has an important role during replication, as N-P complexes, rather than N alone, are required for proper and selective encapsidation of viral RNA (8, 20). After infection, replication is therefore not possible until the so-called primary transcription and translation provide enough N protein.
In spite of the fact that N is the most abundant viral protein in infected cells at later stages, transcription is the predominant RNA synthesis mode throughout infection, arguing against a simple and popular model in which the amount of available N protein or N-P complexes would determine the level of replication versus transcription. This is supported by reverse genetics experiments with RV (unpublished), vesicular stomatitis virus (VSV) (34), and respiratory syncytial virus (RSV) (9). The ratio of replication and transcription was found to be fixed and could not be altered by providing variable levels of N protein. Even with limiting amounts of N protein, only the overall RNA synthesis was reduced, suggesting that other trans-acting factors are responsible for balancing replication and transcription. Indeed, the RSV M2-2 protein, which is unique to members of the Pneumovirus genus and is associated with RNPs, was shown to be a trans-acting factor able to shift the balance of RNA synthesis from transcription toward RNA replication (2).
Reverse genetics experiments with RV minigenomes, M-gene deletion mutants, and recombinant full-length virus with modified M expression more recently revealed an important function of the M protein in the regulation of rhabdovirus RNA synthesis. In the absence of M, replication of RV was downregulated, while the transcription efficiency was enhanced, resulting in increased transcription rates. Moreover, M provided in trans results in a dose-dependent decrease in the transcription rate and a concurrent increase of replication products of M-deficient RVs and full-length RVs. A recombinant RV in which M expression was attenuated by rearranging the gene order again had a high-transcription phenotype. These findings indicate that the RV M protein is able to mediate a regulatory switch such that an initial high level of mRNA synthesis is followed by a shift in the RNA synthetic program in favor of genomic RNA for virion assembly. As strongly suggested, the effects of M on RNA synthesis were independent of M functions during virus assembly (13).
It has long been known that the M proteins of rhabdoviruses and other negative-strand RNA viruses are able to shut down viral gene expression. This had been attributed in general to the function of the M protein in virus assembly and budding. As shown for RV, binding of M protein to RNP results in the conversion of RNPs to highly condensed, helical skeleton-like structures, which are completely enclosed in an M layer (25). In such condensed RNPs, all RNA synthesis processes are probably shut down. The M layer around the condensed RNPs appears to be responsible for budding off virions from cell membranes, even in the absence of the single virus envelope protein G (24).
To further illuminate the functions of M proteins in regulation of RV RNA synthesis and, in particular, to dissociate these functions from the functions of M related to virus assembly, we screened for M mutants with defined transcription and/or replication phenotypes. M proteins from different RV strains were found to have distinct effects on RNA synthesis of the recombinant SAD L16 virus, both in trans or after expression from recombinant chimeric viruses. Two M proteins were identified that caused a high-transcription phenotype previously observed only for M-gene deletion mutants, suggesting that these two M proteins have lost the capacity to regulate RNA synthesis. Sequence analysis and directed mutagenesis of a full-length RV identified a single amino acid residue in the N terminus of M as critical for RNA regulation. Most importantly, the point mutation did not affect virus assembly, budding, and virion composition, demonstrating that RNA synthesis regulation and virion formation by M are two independent processes that can be genetically separated. These data further support the idea that RNA regulation by M takes place prior to virus assembly.
MATERIALS AND METHODS
Cells and viruses.
Viruses were grown on BHK-21 clone BSR cell monolayers maintained in Glasgow minimal essential medium supplemented with 10% newborn calf serum. To produce the M- and G-gene deletion virus NPgrL, a BSR cell clone (MG-on) that expresses the RV M and G proteins after induction with doxycylin was used. The MG-on cells were maintained under selection conditions by adding G418 (1 mg/ml) and hygromycin (1 mg/ml) (13). NPgrL virus is a derivative of the wild-type (wt) SAD L16 virus (29) in which the M and G genes are replaced with the enhanced green fluorescent protein (EGFP) (g) and Discosoma sp. red protein (r) reporter genes (13). The BSR T7/5 cell clone (5), which constitutively expresses bacteriophage T7 polymerase, was used for virus rescue and was maintained under selection conditions by adding 1 mg of G418 per ml of cell culture supernatant.
M expression plasmids and site-directed mutagenesis.
For transient expression of the RV SAD L16 M protein, the cells were transfected with the M expression vector pTIT-M (13), which encodes the M protein under the control of the T7 bacteriophage RNA polymerase promoter and the internal ribosome entry sequence of encephalomyocarditis virus. For rapid expression of M protein to high levels, the transfected cells were superinfected with vaccinia virus vTF7-3 expressing T7 polymerase (14). The R58G and R58E mutants were generated by site-directed mutagenesis of the pTIT-M vector by using the QuikChange site-directed mutagenesis kit (Stratagene) according to the supplier's instructions.
Full-length RV cDNA clones.
In the recombinant full-length cDNA plasmids pSAD M(PV), pSAD M(ERA), and pSAD M(CVS), the M coding sequence of pSAD L16 (29) was replaced with the M coding sequences of the RV Pasteur virus (PV), Evelyn-Rokitnicky-Abelseth (ERA), and challenge virus standard (CVS) strains, which were amplified by reverse transcription-PCR (RT-PCR) from RNA that was isolated from virus-infected cells (for amino acid sequences, see Fig. 2). The pSAD R58G and pSAD R58E cDNA clones were identical to pSAD L16, except they contained mutations in the M protein leading to the replacement of one arginine residue at position 58 with a glycine or glutamate residue, respectively. A detailed description of all cloning steps and the final sequences are available from the authors by e-mail.
FIG. 2.
Sequence comparison of the L16, ERA, PV, and CVS strains. The ERA, PV, and CVS M-protein amino acid sequences show 96, 91, and 89% identity to the L16 M protein. Amino acids that are unique in L16 and the corresponding amino acids in the other strains are indicated by bold letters. Amino acids that are identical in ERA and PV and distinct in L16 and CVS are indicated by white letters on a black background. The sequences were taken from GenBank. GenBank identifier (GI) numbers for the sequences follow: 138744 for ERA-M, 138746 for PV-M, 75239 for CVS-M, and 75237 for L16-M. The identities of the cDNAs were confirmed by sequencing. The CVS M gene amplified from the infected cells differed at two amino acid residues from the database sequence, namely, at positions 77 (G77R) and 128 (R128K).
cDNA rescue experiments.
cDNA plasmids were transfected into cells after CaPO4 precipitation (mammalian transfection kit; Stratagene) according to the supplier's instructions. To enable T7 RNA polymerase-driven expression, either the transfected cells were infected with vaccinia virus vTF7-3 expressing T7 polymerase (14) or the cDNAs were transfected in BSR T7/5 cells that constitutively express T7 polymerase (5).
Rescue of recombinant viruses was performed as described previously (11) by cotransfection of 10 μg of full-length cDNA and plasmids pTIT-N (5 μg), pTIT-P (2.5 μg), and pTIT-L (2.5 μg) in 106 BSR T7/5 cells grown in 8-cm2 culture dishes. Three days posttransfection, fresh culture medium was added, and after another 3 days, cell culture supernatants were prepared and placed on BSR cells. Two days after passage, infectious viruses were detected by immunostaining against RV N protein.
RNA analysis.
RNA from cells was isolated with the RNeasy Mini kit (Qiagen). Northern blotting and hybridization with [α-32P]dCTP-labeled cDNAs recognizing the RV N- or L-gene sequences were performed as described previously (6). To detect cellular RNA, a 28S rRNA probe (Ambion) was used. The hybridization signals were quantitated by phosphorimaging (Molecular Dynamics Storm).
RT-PCR.
cDNAs were generated by RT-PCR with RNA isolated from virus-infected cells. The first-strand cDNA was generated from 1 μg of RNA by using avian myeloblastosis virus reverse transcriptase (Stratagene). cDNA was PCR amplified using Pfu Turbo DNA polymerase (Stratagene) according to the supplier's instructions.
Density gradient centrifugation.
Supernatants from 3 × 106 virus-infected cells were prepared on day two postinfection at a multiplicity of infection (MOI) of 1, and the virions were pelleted through 20% sucrose in TEN buffer (50 mM Tris-HCl, 1 mM EDTA, 150 mM NaCl [pH 7.4]) on a 60% sucrose cushion. The interphase fraction was diluted in TEN buffer and was loaded on a 12-ml 10 to 40% iodixanol (Optiprep, Axis-Shield) density gradient. After 18 h of centrifugation (27,000 rpm on a Beckman SW28 centrifuge) at 4°C, 12 fractions (1 ml each) were collected, and the quality of the density gradients was controlled by the refraction index of each fraction. After polyacrylamide gel electrophoresis and Western blotting, each fraction was analyzed with RV N-, P-, and M-protein-specific sera as described previously (12).
RESULTS
Heterotypic M proteins shape the pattern of RV RNA synthesis.
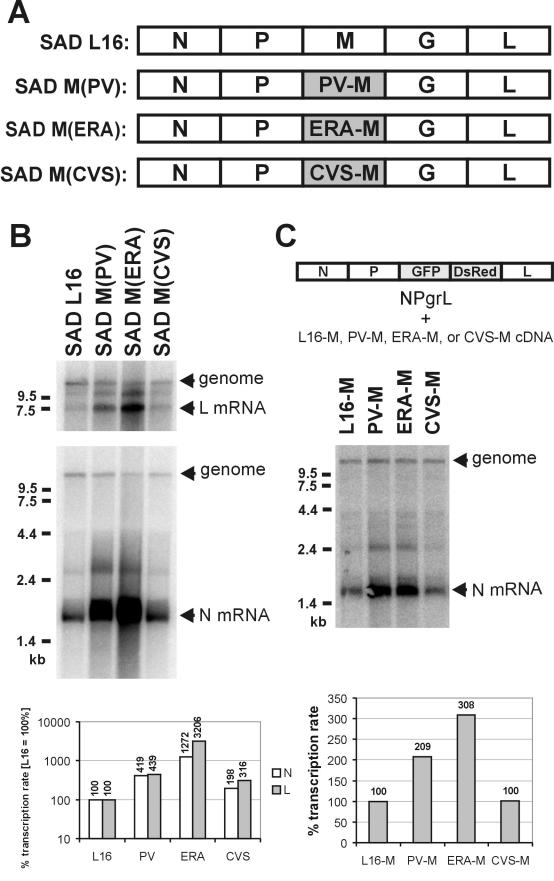
In order to identify M-protein mutants with defects in RV RNA regulation, we initially introduced single random deletions into the sequence of SAD L16 M protein. However, none of the viruses with these deletions grew, suggesting thorough structural constraints for M functions. Therefore, we decided to use functional full-length M proteins from related RV strains and generated SAD L16 cDNA in which the authentic M coding sequence was replaced by the M coding sequences of the PV, ERA, and CVS strains, respectively (Fig. 1A). Viable chimeric viruses growing in BSR cell culture were recovered from cDNA, indicating that the heterotypic M proteins did support essential functions in virus propagation. Nonetheless, striking differences in RNA synthesis were observed in Northern hybridization experiments with N- and L-gene-specific, nick-translated cDNA probes (Fig. 1B). Whereas wt RV SAD L16 and SAD M(CVS) produced similar amounts of N and L mRNAs and full-length replication products, a tremendous increase in N and L mRNAs was observed for the viruses encoding PV and ERA M. The increase in mRNA levels was accompanied by a decreased accumulation of viral full-length RNA. Quantification by phosphorimaging the hybridization signals and normalizing the mRNA levels with the full-length genome RNA levels confirmed similar transcription rates of SAD L16 and SAD M(CVS). However, the PV-M and ERA-M proteins caused increases in the transcription rate of more than 4- and 10-fold, respectively, for both N and L mRNAs (Fig. 1B, bar graph).
FIG. 1.
Increased transcription rates by PV- and ERA-M. (A) Genome organization of wt RV (SAD L16) and of the chimeric RVs SAD M(PV), SAD M(ERA), and SAD M(CVS). In the chimeric viruses, the SAD L16 M coding sequence was replaced with the M coding sequences of the RV strains indicated (shaded boxes). (B) RNA synthesis of chimeric M(PV), M(ERA), and M(CVS) viruses was compared with that of SAD L16. The RNA was isolated 24 h after infection of cells with an MOI of 1 and was analyzed in Northern hybridizations with RV L probe (top) and N probe (bottom), recognizing both positive-strand mRNAs and antigenome and negative-strand genomes. The hybridization signals were quantitated by phosphorimaging, and transcription rates (mRNA/genome) were determined (transcription rate of wt SAD L16, 100%). (C) Cells were infected with NPgrL virus (MOI of 1), and 3 days later, cDNA expression plasmids encoding the indicated M proteins were transfected into the cells. After transfection, the cells were superinfected with vaccinia virus expressing T7 polymerase. Twenty-four hours after transfection, RNA was isolated from the cells and was analyzed by Northern hybridizations with an N probe.
To exclude the possibility that clonal effects in the chimeric SAD M(PV) and SAD M(ERA) viruses were responsible for the pronounced effect on RNA synthesis, the functions of the different M proteins were assayed in transient-complementation experiments using the previously described NPgrL virus (13). In NPgrL, the M and G genes were replaced by the two reporter genes, the EGFP and Discosoma sp. red protein genes, respectively. After infection of cells with NPgrL virus for 3 days, the RV L16, PV, ERA, and CVS M proteins were expressed by transfection of T7 promoter-driven cDNA plasmids and superinfection with vaccina virus expressing T7 polymerase (14). Northern hybridization with the N probe confirmed that in the presence of the PV and ERA M proteins, the transcription rate of NPgrL is significantly increased, whereas in the presence of SAD L16 and CVS M, transcription is downregulated to a similar degree. Notably, the ERA M protein caused a higher transcription rate than the PV M protein in this transient-complementation assay, thus reflecting the situation with the recombinant chimeric viruses. Note that the incoming NPgrL virus contained only L16 M and that glycoprotein was not expressed, excluding the synthesis of infectious virions and thereby excluding the possibility that differences in uncoating of incoming RNPs were responsible for the effects on RNA synthesis.
A single amino acid substitution in M leads to the loss of the regulatory function.
The higher mRNA levels at lower genome levels in SAD M(PV)- and SAD M(ERA)-infected cells mimic the previously observed phenotypes of M deletion viruses or viruses with attenuated M expression (13). This indicated the loss of function of M proteins in transcription inhibition and replication stimulation. To identify regions in M that might be responsible for RNA regulation, the M-protein sequences of the four RV strains used in this study were compared (Fig. 2). Intriguingly, the ability of the M proteins to regulate virus RNA synthesis inversely correlated with the similarity of the four proteins to the SAD L16 M protein. With an amino acid identity of 96%, the ERA M protein yielded the highest transcription rates, followed by PV M, with 91% identity, and the most divergent, CVS M protein with 89% identity.
Of all variable residues, only two were identified to be identical in the nonregulating PV and ERA M proteins but different in the SAD L16 and CVS M proteins, namely, position 3 with an F in PV and ERA M and L or V in SAD and CVS M, respectively, and position 58 with a common G in PV and ERA instead of an R or E in SAD or CVS M, respectively. Thus, we hypothesized that one or both of these residues might be critically involved in RNA regulation. As the phenotypes of SAD M(PV) and SAD M(ERA) viruses with a leucine residue at position 3 were indistinguishable from those of the original chimeric viruses with respect to RNA synthesis (not shown), we assessed the importance of the amino acid at position 58. Two point mutations were introduced into the M gene in the SAD L16 full-length clone, such that R58 was changed either to G, resembling the ERA and PV M protein at this position (R58G), or to E, which is found in the CVS M at this position (R58E). The respective recombinant viruses SAD M(R58G) and SAD M(R58E) encoding the mutant M proteins were successfully generated from cDNAs, and virus stocks were produced. The identities of the recombinant viruses were confirmed by RT-PCR and nucleotide sequencing (not shown).
RNA synthesis of the recombinant point mutant viruses was analyzed 1 day after infection of BSR cells at an MOI of 2 by Northern hybridization. The pattern of RNAs produced by SAD M-R58G was conspicuously distinct from that produced by the parental SAD L16 virus (Fig. 3A) and mimicked that observed previously for the chimeric SAD M(ERA). The amounts of N and L mRNAs were much higher than for SAD L16-infected cells, while genome-size RNA levels were clearly lower. The transcription rates for N and L mRNAs of SAD M(R58G) as calculated from phosphorimaging were increased 16- and 34-fold (Fig. 3B). This increase in the relative transcription activity of SAD R58G was in the range of that observed previously for SAD M(ERA) (Fig. 1). Also, total RNA synthesis, as estimated by adding up all hybridization signals, was increased more than twofold compared to that of SAD L16 (Fig. 3C). Thus, the single amino acid change of R58 to G in SAD M was responsible for the negative effect on replication and the positive effect on transcription.
FIG. 3.
RNA synthesis and growth of SAD L16, SAD M(R58G), and SAD M(R58E) viruses. (A) BSR cells were infected with the viruses at an MOI of 2, and after 1 and 2 days of infection, the RNA was isolated from the cells and analyzed in Northern hybridization with N or L probes. The amount of cellular 28S rRNA at 1 day postinfection (dpi) was detected in a second hybridization step with 28S cDNA probe. n.i., not infected. (B) Transcription rates of SAD L16, SAD M(R58G), and SAD M(R58E) viruses. The transcription rates were calculated for each virus after quantitation of the N (1 and 2 dpi) and L (1 dpi) hybridization signals by phosphorimaging and normalization of the transcript levels with the genome RNA levels. The transcription rates are given as percentages (SAD L16 transcription rate, 100%). (C) Levels of virus RNAs in infected cells. The amounts of virus-specific RNAs in infected cells, detected with the N probe, were normalized to that of 28S rRNA. In SAD M(R58G)-infected cells, the level of virus-expressed RNAs increased to 239% (SAD L16 level, 100%), whereas the virus RNA levels in SAD M(R58E)-infected cells decreased to 27%. (D) One-step growth curves. The infectious virus titers were determined in supernatants of identical infection experiments at days 1, 2, and 3 postinfection.
Interestingly, the R58E mutant behaved more like wt SAD L16 in terms of the ratios of mRNAs and genome RNA. Only a moderate 41% increase in the transcription rate was noticed, similar to the chimeric virus expressing the full-length CVS M protein (Fig. 1). This indicated that replacing the positively charged residue 58 with an amino acid with a negative charge is tolerated without losing the regulatory ability of the protein. However, the total virus RNA level of SAD M(R58E) was always lower than that of SAD L16 (Fig. 3C), indicating that the R58E mutant decreases RNA synthesis in general.
To determine the effects of the mutations and the resulting transcription phenotypes of SAD M(R58G) and SAD M(R58E) on virus growth kinetics, virus production was analyzed after infection of cells at an MOI of 2 at 1, 2, and 3 days postinfection (Fig. 3D). In concordance with the observed reduced accumulation of genome RNA levels, virus production was delayed in both SAD M(R58G) and SAD M(R58E). Titers of SAD M(R58E) increased in parallel with but were approximately fivefold lower than those of SAD L16. Also, titers of SAD M(R58G) were lower than those of SAD L16 and, in contrast to SAD M(R58E), markedly decreased at the late stages of infection. This late drop in infectious titers is most likely due to severe cytopathic effects caused by SAD M(R58G) from day 2 of infection (not shown).
Virus particle formation is not affected by the R58G mutation.
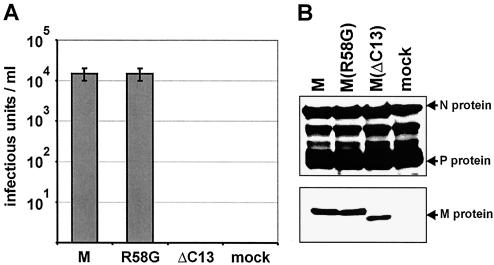
Although growth curves indicated that the R58G mutation in M protein did not substantially interfere with virus assembly, budding, and infectivity of virions, minor effects could not be excluded at this stage. To directly compare the ability of wt M and the mutant M protein in virus particle formation and release, we used a transient-complementation assay of the M- and G-deficient NPgrL virus. BSR T7/5 cells were first infected with NPgrL and were cultured for 3 days. At this stage of infection, nearly all the cells expressed GFP, indicating successful gene expression, and accumulation of RNPs. Then, the infected cells were transfected with T7 promoter-controlled plasmids encoding either wt SAD L16 M, M(R58G), or a C-terminally truncated M protein (MΔC13) together with a plasmid encoding the surface glycoprotein G to trigger formation and release of virions. To ensure rapid and high-level M-protein expression, the transfected cells were superinfected with vaccinia virus expressing T7 polymerase. Cell culture supernatants were prepared as early as 16 h posttransfection, and infectious virus titers were determined. The wt M protein and the R58G mutant reproducibly supported production of identical amounts of infectious virions with titers of 104 focus-forming units per ml. In contrast, the C-terminally truncated M protein, which served as a negative control, was completely unable to support release of infectivity into the cell culture supernatant (Fig. 4A). As comparable amounts of mutated and wt M were present in the infected and transfected cells (Fig. 4B), wt M and M(R58G) have equal capacities to support virus formation. This was also true for the R58E mutant (not shown), showing that replacing R58 with either G or the negatively charged E has no influence on the production of virus particles.
FIG. 4.
Transient complementation of NPgrL with M and G proteins. BSR T7/5 cells were infected with NPgrL at an MOI of 1, and 3 days later, 5 μg of pTIT-M, pTIT-MR58G, or pTIT-MΔC13 and 2.5 μg of pTIT-G were cotransfected into the cells. (A) Sixteen hours after transfection, the supernatants were prepared, and the infectious virus titers were determined. (B) Western blots were performed to compare NPgrL infection (N and P proteins) and plasmid-derived expression of the various M proteins.
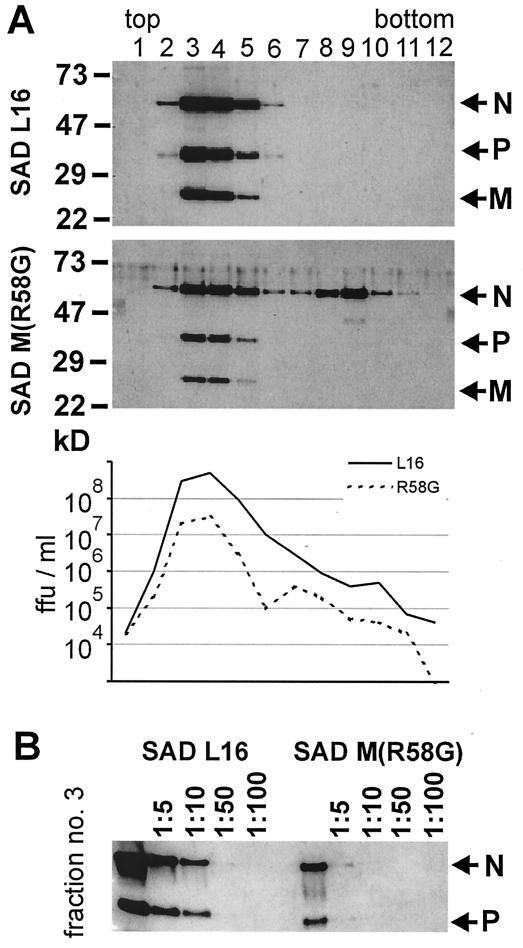
To further confirm that the M(R58G) mutation is fully functional in virus formation, the behaviors of SAD L16 and SAD M(R58G) supernatant virions in density gradients were compared. After centrifugation, common virus peaks were identified in fractions 3 and 4 by Western blotting with sera recognizing RV N, P, and M proteins (Fig. 5). No differences in viral protein content were detected. According to density and viral protein content, these fractions contain enveloped virus particles. By analyzing a dilution series of peak fraction 3, the levels of virus proteins N and P were found to be approximately 10-fold lower in supernatants from SAD M(R58G)-infected cells (Fig. 5B). Determining the infectious titer of each fraction revealed that infectivity and protein content were correlated (Fig. 5A, graph). Only in supernatants from SAD M(R58G) was a second peak detected around fraction 9 and containing mostly N protein. With respect to density and protein content, this peak represents free RNPs. The release of such RNPs into the supernatant is most likely due to the severe cytopathic effects induced by SAD M(R58G) infections described above. Taken together, these experiments show that wt M and M(R58G) function equally well in driving virus assembly, budding, and release and in sustaining virion infectivity.
FIG. 5.
Protein content and infectivity of SAD M(R58G) virions. Two days after BSR cells were infected at an MOI of 1, cell culture supernatants were prepared and purified by centrifugation through 20% sucrose. After 18 h of centrifugation in a 10 to 40% iodixanol density gradient, 12 fractions (1 ml each) were collected. (A) The infectious virus titers were determined by end point dilutions, and the protein content of each fraction was analyzed in Western blots with sera recognizing RV N, P, and M proteins. ffu, focus-forming units. (B) To compare the amount of virus protein, virus peak fraction 3 was diluted, and N and P proteins were detected by Western blotting. Nearly 10-fold-less protein was detected in the supernatant from SAD M(R58G)-infected cells.
DISCUSSION
We have previously described a major role of the RV M protein in regulating the balance of virus transcription and replication (13). At low M levels or in the absence of M, RV RNA synthesis was characterized by a very high transcription rate, allowing rapid accumulation of all virus proteins. At high M levels, a shift to replication with a concurrent downregulation of transcription was observed (13). We describe here the identification and characterization of the first RV M mutants that are unable to regulate RV RNA synthesis in the manner described above. Interestingly, a single point mutation able to completely destroy this regulatory activity was identified. Most importantly, the mutation did not destroy the essential function of M in virus assembly and particle formation, providing evidence that these functions are genetically separate.
To identify regions within the M protein critical for RNA regulation, M proteins of related RV strains were analyzed with respect to their function in RNA synthesis and virus assembly in the context of the SAD L16 virus backbone. Recombinant SAD viruses encoding the M proteins of ERA, PV, or CVS were viable, suggesting that the heterotypic M proteins supported the essential functions of M in virus formation. Unexpectedly, the closely related ERA and PV M proteins, not the most divergent CVS M protein, yielded the most pronounced transcription phenotype. The RNA synthesis pattern was virtually identical to those of M-gene-deficient viruses and similar to that of a recombinant virus in which M expression was attenuated (13). In particular, compared to wt virus, transcription rates were more than 10-fold higher, and accumulation of genome-length RNA was considerably lower.
The high similarity of M proteins and the finding that two of the M proteins behaved similarly facilitated the identification of two amino acid residues as promising candidates for causing the observed phenotype. Only these two amino acid residues were identical in the PV and ERA M proteins and distinct from those in the SAD L16 and CVS strains. By directed mutagenesis of the SAD L16 virus, the arginine residue at position 58 of the M sequence was indeed identified as crucial for regulation of RV RNA synthesis. After this positively charged residue was replaced with the small, neutral glycine residue, the resulting recombinant virus SAD M(R58G) showed increased mRNA transcription combined with reduced accumulation of full-length RNA. Indeed, this phenotype was previously observed in the absence of M or at limiting levels of M (13), suggesting that the M mutant has lost the abilities to downregulate mRNA transcription and to support accumulation of full-length RNA. The combination of these two features strongly argue in favor of a loss of function, corresponding to the complete lack of M, rather than a simultaneous gain in stimulation of transcription and inhibition of replication.
The RNA synthesis of SAD M(R58G) was characterized by an increase in transcription activity of >15-fold and was virtually indistinguishable from that of SAD M(ERA). Thus, the identity of the residue at position 58 in the ERA M protein seems to cause the loss of RNA regulation, while the other seven amino acid differences in ERA M (L3F, R11C, S19P, A22V, G46S, L110M, and V168I) do not contribute at all. Though similar, the RNA synthesis pattern of SAD M(R58G) did not exactly match that of SAD M(PV), as the latter displayed a less pronounced increase in the transcription rate of approximately fivefold. This may suggest the possibility that some of the additional 10 mutations in PV M might compensate to some degree for the effect of the 58G residue. However, with increasing dissimilarity of the M proteins, effects on virus formation and dynamics of RNP export may be expected (see below).
In the M protein of CVS, which was competent in regulating RNA synthesis of SAD M(CVS) in a manner similar to that of wt M, the critical Arg58 residue is replaced with a negatively charged glutamic acid. To determine whether this residue per se would support the regulatory function of M or whether some of the more numerous differences in the CVS M account for the unimpaired function, the single site mutant SAD M(R58E) was generated. Interestingly, in cells infected with SAD M(R58E), regulation of RNA synthesis was readily observed in terms of low transcription rates. Also, accumulation of full-length RNA was higher in cells infected with SAD M(R58E) than in cells infected with SAD M(R58G), but overall RNA synthesis was lower than in cells infected with SAD L16. This was also observed with the full-length CVS protein in SAD M(CVS). These results supported the above finding that the identity of residue 58 is the critical factor in RNA regulation, even in the most dissimilar CVS M protein, and indicated that other residues in CVS M protein do not contribute significantly. In summary, the results of experiments with the chimeric viruses and single-site mutants strongly suggested the presence of a regulatory domain within the N-terminal part of the RV SAD M protein and a particular role for Arg58.
Our previous reverse genetics experiments employing differential expression of wt M protein suggested that the regulatory function of M in RNA synthesis is independent of RV assembly, in which M also plays a crucial role (13). It is assumed that M-mediated conversion of RNPs to the typical skeleton like structures (25) completely shuts down virus RNA synthesis. This may well obscure the specific effects of M on regulation of RNA synthesis. In addition, the presence of functional M leads to export of RNPs, even in the absence of the glycoprotein G (24). The dynamics of this process makes exact comparison of template RNP and mRNAs more difficult (13). The successful rescue of virus autonomously propagating in cell culture argued against major defects in the assembly functions of the point mutants SAD M(R58G) and M(R58E). However, to verify that the mutation of this residue affects only RNA regulation and not virus formation and RNP export, it was necessary to directly compare the abilities of the M proteins to support virus assembly and release. Two different approaches confirmed that M(R58G) is fully competent with respect to the latter functions. The first approach involved transient expression of wt M and M(R58G) to compare their abilities, within a given time period, to support formation and release of infectious virus particles containing RNPs of the M- and G-deficient NPgrL virus construct. Identical infectious titers were observed with the two M proteins (Fig. 4). The second approach was the biophysical characterization of SAD L16 and SAD M(R58G) supernatant virus particles. These were indistinguishable with respect to their density in iodixanol gradients and N, P, and M content, indicating the formation of typical enveloped RV particles. Moreover, physical and infectious virus titers correlated exactly, revealing identical infectivity of virions. This was also true for the R58E mutant (not shown). Together, these results show that M(R58G) has no detected defects in virion assembly and budding but has dramatic effects on the regulation of RNA synthesis. The observed approximately 10-fold decrease in infectious virus titers of SAD M(R58G) in parallel infections can therefore be attributed to its reduced replication activity and lower genome RNP levels and, at late stages of infection, to the strong cytopathic effects induced by this virus. In summary, the above data show that residue 58 of M is involved in RNA regulation, but not in virus assembly, and provide evidence that the M RNA regulatory function is genetically dissociable from its functions in virus morphogenesis.
The N-terminal moiety of rhabdovirus M proteins appears to contain domains particularly important for their function in membrane budding and mediating the necessary interactions with cellular components. A PPXY motif known from late domains in retrovirus has been identified in RV and VSV M. This motif is involved in binding to WW domains of cellular proteins, such as ubiquitin ligase Nedd4 (7, 18, 19). Indeed, RV and VSV M proteins, like those of retroviruses or negative-strand RNA viruses such as VSV or Ebola virus, have an intrinsic budding activity (22, 33). For RV, this has been demonstrated by recombinant gene deletion viruses that were released as spikeless virions (24). Deletion of the PPXY domain in a recombinant VSV led to impaired pinching off of enveloped virus particles from the cell membrane (21). In addition, a PT/SAP motif that was originally identified in Ebola virus M protein that has been shown to bind Tsg101 (23) is also present in VSV M in close proximity to the PPXY motif (30). Also, in RV M proteins studied here, a motif similar to PT/SAP is located upstream of the PPXY box (PASAP at positions 21 to 25 [Fig. 2]). Thus, domains that are probably important for virus assembly and budding are located close to residue 58, which was identified here as crucial for regulation of RNA synthesis. Intriguingly, the recently solved three-dimensional (crystallographic) structure of the VSV M protein suggests that the N-terminal sequence of M is a flexible, unfolded region protruding from a globular core (15). This result, the location of virus budding domains, and the present identification of a nearby domain involved in RV RNA regulation suggest that both in RV and VSV, an exposed N terminus of the M proteins is the main “interactor” region of the proteins, enabling binding to and interaction with several cellular and viral factors. Despite the close proximity to the putative budding domains, the amino acid substitutions of residue 58 did not affect budding activity and the critical interactions with cellular proteins. This might argue in favor of independent functional protein domains within the N terminus of M or in favor of sequential engagement of the N terminus in RNA regulation and then in assembly.
Indeed, the importance of the VSV M protein N terminus for interactions with the RNP template has been demonstrated previously, as VSV in vitro transcription inhibition depends on the N-terminal region of M. Moreover, VSV in vitro transcription inhibition and the temperature-sensitive phenotypes are not essentially coupled (27), which might suggest that VSV also has a similar M-dependent mechanism to regulate the balance between RNA replication and transcription (26).
Indeed, the loss or modification of an existing binding site to the so far unknown molecular target of M in RNA regulation is the most probable explanation for the unrestricted transcription rates of the recombinant viruses. From the identical protein composition of the virions, it appears that binding to viral proteins is not changed in the mutant M proteins. However, as reasoned above, differential binding may occur sequentially, and binding of M to RNPs during assembly and budding may be completely different from binding of M to RNP components during regulation of RNA synthesis. Apart from cellular components, potential targets of the M regulatory activity include the RNP template and the RNP-dependent RNA polymerase.
In contrast to the obsolete model, according to which only increasing N-protein concentrations and RNA packaging into N define replication (3, 4), it is becoming increasingly clear that the polymerase may exist in the form of a replicase or transcriptase. Internal transcription initiation has been long suggested for VSV (31, 32), and more recently, VSV replication and transcription were shown to be initiated at different positions on the VSV genome (35). Polymerase complexes with different protein contents that are active either in replication or in transcription have been described (17). The existence of individual replicase and transcriptase forms of the polymerase has also been suggested for RV by analyses of gene expression of recombinant ambisense RVs (10) and for Sendai virus, whose polymerase has been proposed to have differentially regulated replicase and transcriptase activities (16). Regulation of RNA synthesis by M could directly or indirectly influence the formation of these polymerase complexes or differentially influence the activities of replicase and transcriptase.
In this study, we have described the first recombinant RVs with clearly distinguishable phenotypes of RNA synthesis regulation caused by a single point mutation in M protein and demonstrated that the role of M in regulation of intracellular RNA synthesis is genetically dissociable from morphogenesis functions. This provides a promising tool to study the mechanism of RNA polymerase regulation in detail. The availability of regulation-deficient M-protein mutants will be of use in the identification of molecular targets of the M protein in the future.
Acknowledgments
We thank N. Hagendorf for excellent technical assistance.
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft, SFB 455 A3 and FI 941/1-1.
REFERENCES
- 1.Arnheiter, H., N. L. Davis, G. Wertz, M. Schubert, and R. A. Lazzarini. 1985. Role of the nucleocapsid protein in regulating vesicular stomatitis virus RNA synthesis. Cell 41:259-267. [DOI] [PubMed] [Google Scholar]
- 2.Bermingham, A., and P. L. Collins. 1999. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc. Natl. Acad. Sci. USA 96:11259-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumberg, B. M., C. Giorgi, and D. Kolakofsky. 1983. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell 32:559-567. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg, B. M., M. Leppert, and D. Kolakofsky. 1981. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell 23:837-845. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conzelmann, K. K., J. H. Cox, and H. J. Thiel. 1991. An L (polymerase)-deficient rabies virus defective interfering particle RNA is replicated and transcribed by heterologous helper virus L proteins. Virology 184:655-663. [DOI] [PubMed] [Google Scholar]
- 7.Craven, R. C., R. N. Harty, J. Paragas, P. Palese, and J. W. Wills. 1999. Late domain function identified in the vesicular stomatitis virus M protein by use of rhabdovirus-retrovirus chimeras. J. Virol. 73:3359-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran, J., J. B. Marq, and D. Kolakofsky. 1995. An N-terminal domain of the Sendai paramyxovirus P protein acts as a chaperone for the NP protein during the nascent chain assembly step of genome replication. J. Virol. 69:849-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearns, R., M. E. Peeples, and P. L. Collins. 1997. Increased expression of the N protein of respiratory syncytial virus stimulates minigenome replication but does not alter the balance between the synthesis of mRNA and antigenome. Virology 236:188-201. [DOI] [PubMed] [Google Scholar]
- 10.Finke, S., and K. K. Conzelmann. 1997. Ambisense gene expression from recombinant rabies virus: random packaging of positive- and negative-strand ribonucleoprotein complexes into rabies virions. J. Virol. 71:7281-7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finke, S., and K. K. Conzelmann. 1999. Virus promoters determine interference by defective RNAs: selective amplification of mini-RNA vectors and rescue from cDNA by a 3′ copy-back ambisense rabies virus. J. Virol. 73:3818-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finke, S., J. H. Cox, and K. K. Conzelmann. 2000. Differential transcription attenuation of rabies virus genes by intergenic regions: generation of recombinant viruses overexpressing the polymerase gene. J. Virol. 74:7261-7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finke, S., R. Mueller-Waldeck, and K. K. Conzelmann. 2003. Rabies virus matrix protein regulates the balance of virus transcription and replication. J. Gen. Virol. 84:1613-1621. [DOI] [PubMed] [Google Scholar]
- 14.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudier, M., Y. Gaudin, and M. Knossow. 2002. Crystal structure of vesicular stomatitis virus matrix protein. EMBO J. 21:2886-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gubbay, O., J. Curran, and D. Kolakofsky. 2001. Sendai virus genome synthesis and assembly are coupled: a possible mechanism to promote viral RNA polymerase processivity. J. Gen. Virol. 82:2895-2903. [DOI] [PubMed] [Google Scholar]
- 17.Gupta, A. K., D. Shaji, and A. K. Banerjee. 2003. Identification of a novel tripartite complex involved in replication of vesicular stomatitis virus genome RNA. J. Virol. 77:732-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harty, R. N., M. E. Brown, J. P. McGettigan, G. Wang, H. R. Jayakar, J. M. Huibregtse, M. A. Whitt, and M. J. Schnell. 2001. Rhabdoviruses and the cellular ubiquitin-proteasome system: a budding interaction. J. Virol. 75:10623-10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harty, R. N., J. Paragas, M. Sudol, and P. Palese. 1999. A proline-rich motif within the matrix protein of vesicular stomatitis virus and rabies virus interacts with WW domains of cellular proteins: implications for viral budding. J. Virol. 73:2921-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horikami, S. M., J. Curran, D. Kolakofsky, and S. A. Moyer. 1992. Complexes of Sendai virus NP-P and P-L proteins are required for defective interfering particle genome replication in vitro. J. Virol. 66:4901-4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayakar, H. R., K. G. Murti, and M. A. Whitt. 2000. Mutations in the PPPY motif of vesicular stomatitis virus matrix protein reduce virus budding by inhibiting a late step in virion release. J. Virol. 74:9818-9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Y., L. Luo, M. Schubert, R. R. Wagner, and C. Y. Kang. 1993. Viral liposomes released from insect cells infected with recombinant baculovirus expressing the matrix protein of vesicular stomatitis virus. J. Virol. 67:4415-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Serrano, J., T. Zang, and P. D. Bieniasz. 2001. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 7:1313-1319. [DOI] [PubMed] [Google Scholar]
- 24.Mebatsion, T., M. Konig, and K. K. Conzelmann. 1996. Budding of rabies virus particles in the absence of the spike glycoprotein. Cell 84:941-951. [DOI] [PubMed] [Google Scholar]
- 25.Mebatsion, T., F. Weiland, and K. K. Conzelmann. 1999. Matrix protein of rabies virus is responsible for the assembly and budding of bullet-shaped particles and interacts with the transmembrane spike glycoprotein G. J. Virol. 73:242-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morita, K., R. Vanderoef, and J. Lenard. 1987. Phenotypic revertants of temperature-sensitive M protein mutants of vesicular stomatitis virus: sequence analysis and functional characterization. J. Virol. 61:256-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal, R., B. W. Grinnell, R. M. Snyder, and R. R. Wagner. 1985. Regulation of viral transcription by the matrix protein of vesicular stomatitis virus probed by monoclonal antibodies and temperature-sensitive mutants. J. Virol. 56:386-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patton, J. T., N. L. Davis, and G. W. Wertz. 1984. N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J. Virol. 49:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnell, M. J., T. Mebatsion, and K. K. Conzelmann. 1994. Infectious rabies viruses from cloned cDNA. EMBO J. 13:4195-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strack, B., A. Calistri, M. A. Accola, G. Palu, and H. G. Gottlinger. 2000. A role for ubiquitin ligase recruitment in retrovirus release. Proc. Natl. Acad. Sci. USA 97:13063-13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Testa, D., P. K. Chanda, and A. K. Banerjee. 1980. Unique mode of transcription in vitro by vesicular stomatitis virus. Cell 21:267-275. [DOI] [PubMed] [Google Scholar]
- 32.Thornton, G. B., B. P. De, and A. K. Banerjee. 1984. Interaction of L and NS proteins of vesicular stomatitis virus with its template ribonucleoprotein during RNA synthesis in vitro. J. Gen. Virol. 65:663-668. [DOI] [PubMed] [Google Scholar]
- 33.Timmins, J., S. Scianimanico, G. Schoehn, and W. Weissenhorn. 2001. Vesicular release of Ebola virus matrix protein VP40. Virology 283:1-6. [DOI] [PubMed] [Google Scholar]
- 34.Wertz, G. W., V. P. Perepelitsa, and L. A. Ball. 1998. Gene rearrangement attenuates expression and lethality of a nonsegmented negative strand RNA virus. Proc. Natl. Acad. Sci. USA 95:3501-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whelan, S. P., and G. W. Wertz. 2002. Transcription and replication initiate at separate sites on the vesicular stomatitis virus genome. Proc. Natl. Acad. Sci. USA 99:9178-9183. [DOI] [PMC free article] [PubMed] [Google Scholar]