Abstract
The epigenetic repression of FLOWERING LOCUS C (FLC) in winter-annual ecotypes of Arabidopsis by prolonged cold ensures that plants flower in spring and not during winter. Resetting of the FLC expression level in progeny is an important step in the life cycle of the plant. We show that both the paternally derived and the maternally derived FLC:GUS genes are reset to activity but that the timing of their first expression differs. The paternal FLC:GUS gene in vernalized plants is expressed in the male reproductive organs, the anthers, in both somatic tissue and in the sporogenous pollen mother cells, but there is no expression in mature pollen. In the progeny generation, the paternally derived FLC:GUS gene is expressed in the single-celled zygote (fertilized egg cell) and through embryo development, but not in the fertilized central cell, which generates the endosperm of the progeny seed. FLC:GUS is not expressed during female gametogenesis, with the maternally derived FLC:GUS being first expressed in the early multicellular embryo. We show that FLC activity during late embryo development is a prerequisite for the repressive action of FLC on flowering.
Keywords: Arabidopsis, embryo, gametogenesis
Flowering of many plants is induced by the environmental cues photoperiod and prolonged low temperature (vernalization). With photoperiod control, flowering is induced in response to a critical day length, either long or short days depending on the species. In Arabidopsis, a long-day plant, the gene CONSTANS (CO) is triggered by long days to activate FLOWERING LOCUS T (FT) and is antagonistic to the action of the FLOWERING LOCUS C (FLC) protein (1), which binds to FT to repress its activity and prevent flowering (2, 3). FLC is repressed by vernalization (4, 5), so that after the cold exposure FLC activity is low, releasing the repression of FT and allowing CO-mediated activation of FT in the long days of spring. The repression of FLC by vernalization occurs because of changes in FLC chromatin. The low-temperature exposure induces expression of VERNALIZATION INSENSITIVE3 (6), which interacts with the VERNALIZATION2 polycomb-like complex (7). This complex binds to FLC chromatin (6), modifying histone residues, including the trimethylation of lysine-27 of histone H3, and expression is repressed (6, 8, 9).
The chromatin control of FLC activity is an example of epigenetic control of gene expression. The repressed state is retained through successive mitotic divisions throughout the development of the plant after the period of low temperature ends, and the gene is then reset to an active transcriptional state in the next sexual generation (10). This mode of control of FLC activity ensures that FT is repressed before winter so that the long-day photoperiod of spring is able to induce FT activity, with flowering occurring at an optimal time.
Nothing is known about the mechanism of resetting FLC gene activity or of the timing of this event. In this article we show that the activities of both paternally and maternally derived FLC:GUS reporter genes are reset after vernalization, but the timing of their initial expression differs. The paternal gene copy is active during early gametogenesis and in the single-celled zygote, whereas the maternal copy is not expressed until the early multicellular embryo stage.
Results
FLC:GUS Is Expressed in the Somatic and Sporogenous Tissues of Anthers After Vernalization.
In nonvernalized C24 ecotype plants, which carry an active FRIGIDA (FRI) allele, FLC and a reporter construct with the C24 FLC allele linked to the GUS coding region (FLC-C24:GUS) were expressed in the vegetative plant, and their expression was repressed in vernalized plants [supporting information (SI) Fig. 6] (4). After vernalization, repression of FLC and FLC-C24:GUS is maintained during and after the transition to flowering, but activity is reset in progeny plants (SI Fig. 6) (10). To determine when resetting occurs, we have used the FLC-C24:GUS reporter to follow activity during gametogenesis and embryogenesis.
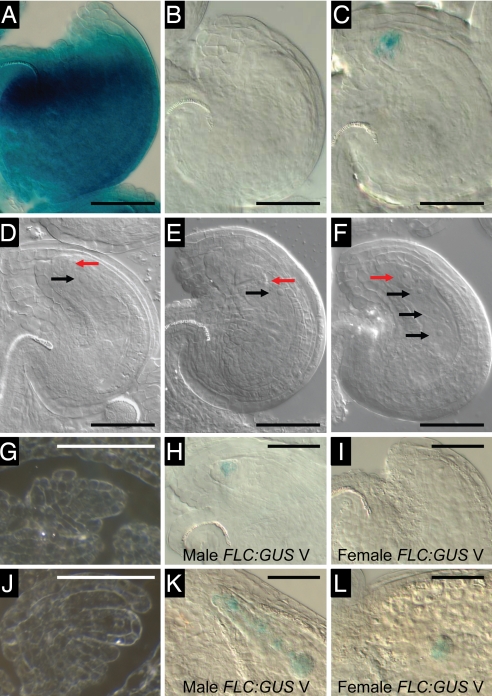
In flower buds from nonvernalized plants, FLC-C24:GUS was expressed in both the male and female reproductive structures, the anthers and carpels (Fig. 1A), whereas expression in vernalized flower buds was restricted to anthers (Fig. 1 B and E). The behavior of the reporter gene is consistent with the expression pattern of the endogenous FLC gene (Fig. 1C).
Fig. 1.
FLC-C24:GUS is reset in the somatic and sporogenous tissues in the anther. (A and B) GUS-stained buds from nonvernalized (A) and vernalized (B) C24 + FLC-C24:GUS plants. (C) Quantitative RT-PCR comparing FLC expression in nonvernalized (NV) and vernalized (V) unopened buds and comparing expression in stamens from young buds (S) and remaining bud tissue (B), indicating that expression in young vernalized buds is largely limited to stamens. (D–M) Transverse sections through consecutive buds from vernalized C24 + FLC-C24:GUS plants viewed under dark-field conditions in which the GUS crystals appear pink (F and G are higher-magnification images of D and E, respectively). (D and F) Flower bud at late stage 8, anther stage 4 with sporogenous cells. (E and G) Flower bud at early stage 9, anthers at stage 5 with premeiotic or early meiotic PMC. (H) Stage-6 anther with PMC in meiosis. (I) Stage-7 anther containing tetrads. (J and K) Stage-8 anther with haploid microspores showing induction of expression in tapetum. (L) Stage-10 to -11 anther with degenerating tapetum. (M) Stage-12 anther. The tapetum has degenerated, and two pollen mitoses are complete. a, anther; m, microspore; p, pistil; s, sporogenous cells; t, tapetum; te, tetrad. (Scale bars: 50 μm.) Flower stages are from ref. 11, and anther stages are from ref. 12.
In vernalized plants, there was no FLC-C24:GUS activity in stage-8 flower buds (flower stages from ref. 11) that contained stage-4 anthers (anther stages from ref. 12) (Fig. 1 D and F) or in earlier bud stages. GUS activity was first detected in stage-5 anthers and was present in pollen mother cells (PMC), in the tapetum surrounding the PMC, in other cell layers of the anther wall, and in the anther connective tissue (Fig. 1 E and G). GUS activity was also present within PMC undergoing meiosis in stage-6 anthers (Fig. 1H) and at the tetrad stage in stage-7 anthers (Fig. 1I). In stage-8 anthers, there was a low level of GUS activity in the haploid microspores released from the tetrads and in other tissues (Fig. 1J). Before and during deposition of the pollen coat, there was a high level of FLC:GUS activity in the tapetum (Fig. 1 J and K). FLC:GUS activity decreased markedly as the tapetum degenerated in stage-10 to -11 anthers, but occasional GUS crystals were still evident in mitotic pollen grains (Fig. 1L). No GUS activity was detectable in tricellular pollen (Fig. 1M). A similar pattern of expression was obtained with a second FLC:GUS construct that contained the Columbia (Col) FLC sequence in Landsberg erecta carrying the active FRI-H51 allele (FLC-Col:GUS in Ler FRI). These observations indicate that FLC:GUS activity has been reset in the male reproductive structure in both sporogenous and somatic tissues and that the gene becomes inactive in maturing pollen.
FLC:GUS Is Expressed in the Next Generation Zygote.
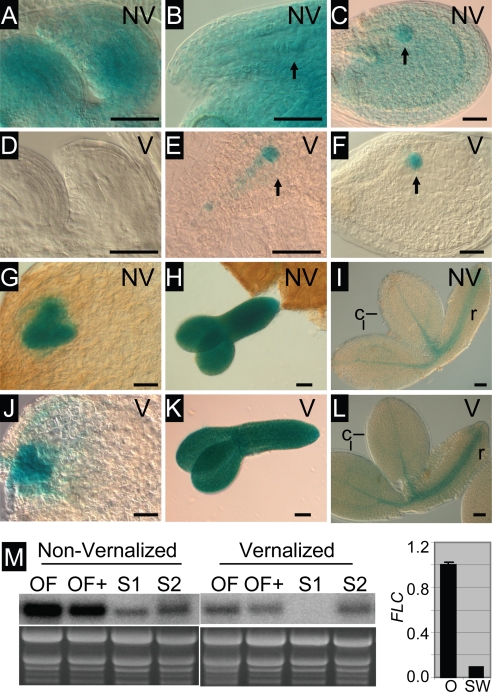
In contrast to the resetting of FLC:GUS that occurs during the development of the male reproductive structures, there was no detectable expression of either FLC-C24:GUS or FLC-Col:GUS during female meiosis, after formation of the functional megaspore, or in the mature female gametophyte of ovules on vernalized plants (Figs. 2B, E, G, and J and 3D). Nonvernalized ovules expressed FLC:GUS strongly in the integuments (Fig. 2 A and D).
Fig. 2.
FLC:GUS is not expressed during female gametogenesis, and the paternally derived gene is expressed in the single-celled zygotes from vernalized plants. (A and D) Youngest open pollinated flower of nonvernalized Ler FRI + FLC-Col:GUS. (B and E) Youngest open pollinated flower of vernalized Ler FRI + FLC-Col:GUS. (C and F) Second youngest open pollinated flower of vernalized Ler FRI + FLC-Col:GUS. (A–C) GUS-stained viewed with DIC optics. (D–F) Cleared ovules for stage comparison. (G and J) Sections of C24 + FLC-C24:GUS through ovule primordia around the time of meiosis (G) and at the functional megaspore stage (J) viewed under dark-field conditions. (H and K) Ovules resulting from a cross between male Ler FRI + FLC-Col:GUS vernalized and female Ler FRI-Sf2 vernalized. (I and L) Ovules resulting from a cross between female Ler FRI + FLC-Col:GUS vernalized and male Ler FRI-Sf2 vernalized. (H and I) Single-celled zygote stage ovules (1 day after pollination). (K and L) Early-globular stage embryos (3 days after pollination). Black arrows, endosperm nuclei; red arrows, zygotic nuclei. (Scale bars: 50 μm.)
Fig. 3.
FLC-C24:GUS is expressed throughout embryo development. (A–L) GUS-stained ovules and embryos from nonvernalized (NV) or vernalized (V) C24 + FLC-C24:GUS plants. (A and D) Unfertilized ovules. (B and E) Ovules with single-cell pro-embryo and suspensor indicated by arrow. (C and F) Ovules with globular embryo indicated by arrow. (G and J) Ovules with heart-stage embryo. (H and K) Torpedo-stage embryos. (I and L) Late-stage (bent cotyledon) embryos. (M Left) RNA gel blot showing FLC expression in flowers and siliques from nonvernalized and vernalized C24 plants. OF, open flowers; OF+, open flowers with up to 5-mm siliques (containing pro-embryos up to the four-cell stage); S1, siliques between 5 and 10 mm in length (containing embryos from four-cell to globular stage); S2, siliques >10 mm (containing embryos larger than globular stage). The ethidium bromide-stained gel is shown as a loading control. (M Right) Quantitative RT-PCR comparing FLC expression in ovules (O) and silique walls (SW) from ≈10-mm siliques from vernalized plants.
Approximately 24 h after pollination, weak FLC-Col:GUS activity was detected in the single-celled zygote formed by the fusion of the egg cell with a male gamete (Fig. 2 C and F). There was no GUS activity in the endosperm, the product of fertilization of the diploid central cell by a second male gamete, or in the integuments. Expression in the single-celled zygote was rarely detectable in the C24 + FLC-C24:GUS line, which had an overall lower level of expression than the Ler FRI + FLC-Col:GUS line.
The Timing of Expression Is Different for Maternal and Paternal FLC:GUS Genes.
We investigated whether both the male- and female-derived FLC-Col:GUS genes were expressed in the zygote. When the vernalized FLC-Col:GUS gene copy was contributed by the male, activity was observed in single-cell zygotes (Fig. 2H) at the same stage as in selfed vernalized plants (Fig. 2C). The frequency of detectable GUS activity of the male-derived FLC:GUS gene was lower (10–20%) than that of zygotes generated by self-pollination of a homozygous FLC:GUS plant (50%). The reason for this difference is not known. Expression of the male-derived FLC:GUS gene continued in early-globular and subsequent-stage embryos (Fig. 2K). When the vernalized FLC-Col:GUS gene copy was contributed by the female, no expression was seen in the zygote and the earliest expression was around the early-globular stage (Fig. 2 I and L).
Endogenous FLC Expression Affects Early Embryo Expression of FLC:GUS.
When pollen containing FLC-C24:GUS from either a nonvernalized flc-null (flc-20) or FLC wild-type (C24) plant was used to fertilize nonvernalized flc-20 or C24 plants, GUS activity was reduced when both parents contributed wild-type FLC alleles compared with when either parent, or both, contributed an flc-20 allele (Table 1). This suggests that endogenous FLC expression, from either the maternally or paternally derived gene copy, directly or indirectly results in decreased expression of the male-derived FLC-C24:GUS gene in early-globular embryos developing in ovules of nonvernalized plants. This effect is short-lived, because by the late-globular stage the male-derived FLC(C24):GUS embryonic expression was similar regardless of the FLC genotype (data not shown).
Table 1.
Paternally derived FLC-C24:GUS activity in early-globular embryos is reduced by endogenous FLC expression
| Female parent | Male parent* |
Embryo FLC genotype |
No. of embryos† (%) |
||
|---|---|---|---|---|---|
| N | S | Total | |||
| C24 NV | C24 NV | FLC/FLC | 173 (86.1) | 28 (13.9) | 201 |
| flc-20 NV | C24 NV | flc/FLC | 84 (59.2) | 58 (40.8) | 142 |
| C24 NV | flc-20 NV | FLC/flc | 27 (57.4) | 20 (42.6) | 47 |
| flc-20 NV | flc-20 NV | flc/flc | 37 (55.2) | 30 (44.8) | 67 |
| C24 V | C24 V | FLC(reset)/FLC(reset) | 90 (62.5) | 54 (37.5) | 144 |
*Also carries FLC-C24:GUS.
†N, no detectable staining; S, detectable staining. Similar results were obtained in independent experiments.
Early-globular embryos from vernalized C24 plants had increased expression of the male-derived FLC-C24:GUS transgene compared with embryos from nonvernalized C24 (Table 1). This observation suggests that early embryos developing on vernalized plants have a reduced level of endogenous FLC expression compared with embryos developing on a nonvernalized plant, consistent with the maternally derived FLC:GUS gene being first expressed around the early-globular stage (Fig. 2 I and L).
FLC:GUS Is Expressed Throughout Embryogenesis.
In nonvernalized flowers FLC-C24:GUS activity was present in the ovule integuments before and after pollination and in the pro-embryo and globular-stage embryo 2–3 days after pollination (Fig. 3 A–C). Around the late-globular to heart stage of embryo development only embryo expression and not integument expression is evident (Fig. 3G). At early to mid stages of embryo development GUS activity was present throughout the embryo (Fig. 3 C, G, and H). In older embryos, GUS activity was strongest in the provascular tissue of both the embryonic root and cotyledon (Fig. 3I).
In fertilized vernalized ovules, FLC-C24:GUS activity was present in the single-celled embryo and attached suspensor and in globular embryos, but not in the endosperm or in the integuments (Fig. 3 E and F). At the heart stage of embryo development and subsequently, GUS activity was identical in nonvernalized and vernalized ovules (Fig. 3 G–L).
FLC(C24):GUS activity is consistent with the expression of the endogenous FLC gene (Fig. 3M). In flowers containing ovules at the zygote stage, at the one- to four-cell pro-embryo stage, and in siliques containing ovules at the four-cell to globular embryo stage, there is higher expression in the nonvernalized compared with the vernalized samples, consistent with repression of maternal expression in the vernalized samples. In siliques containing larger embryos, expression of the nonvernalized and vernalized samples was similar, consistent with FLC expression having been reset by this stage. Expression in vernalized siliques is largely limited to ovules (Fig. 3M).
FLC Expression During Late Embryogenesis Is Required for Late Flowering.
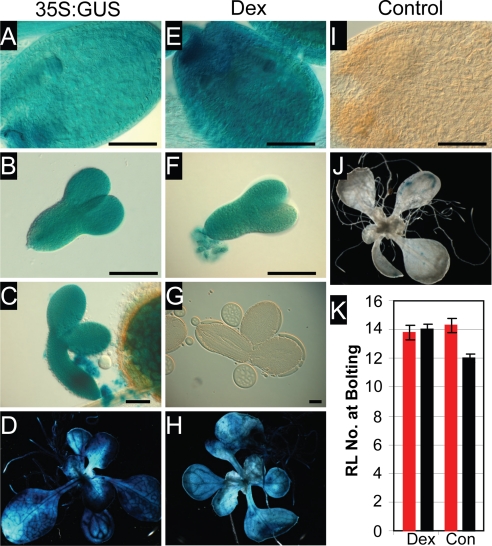
To address the role of FLC expression during embryo development, we used a bipartite dexamethasone inducible system (13). In this line (LhGR/pOp-FLC/GUS in Ler) both the FLC cDNA and GUS gene are induced by application of dexamethasone, with expression under the control of the 35S promoter that drives the activator construct (35S:LhGR). The level of FLC protein expression in induced vegetative plants was similar to that of 35S:FLC vegetative plants (data not shown). In the absence of dexamethasone no induction of either FLC or GUS occurred (Fig. 4 I and J and data not shown). Seeds were harvested from plants grown either with or without dexamethasone during flower and seed formation and were subsequently grown either with or without dexamethasone treatment from imbibition. All dexamethasone-treated and control plants flowered at around the same time as wild-type Ler (Fig. 4K). Ler + 35S:FLC plants are late-flowering (4), suggesting that dexamethasone did not induce FLC in the same tissues as 35S:FLC. Dexamethasone induced both GUS and FLC expression during the vegetative phase of growth (Fig. 4H and data not shown), and GUS expression was detectable during early to mid embryogenesis (Fig. 4 E and F). After the torpedo stage of embryo development, dexamethasone appeared not to penetrate the developing seed coat because embryos did not express GUS (Fig. 4G). 35S:GUS (as a marker for 35S:FLC), in contrast, was expressed throughout the vegetative phase as well as throughout embryogenesis (Fig. 4 A–D). Expression of FLC during early to mid embryogenesis and during vegetative growth was not sufficient to confer late flowering in the dexamethasone-induced line, suggesting that expression of FLC during late embryogenesis, as well as during vegetative growth, is required for the plant to be late-flowering.
Fig. 4.
FLC expression in late embryogenesis is required for delayed flowering. (A–D) 35S:GUS as a marker for 35S:FLC. (E–H) Dexamethasone-treated LhGR/pOp-FLC/GUS in Ler. (I and J) Control-treated LhGR/pOp-FLC/GUS in Ler. (Scale bars: 100 μm.) (K) Flowering time measurements of LhGR/pOp-FLC/GUS in Ler, either dexamethasone-treated (Dex) or control-treated (Con). Seeds for the flowering time experiment came from plants that were either dexamethasone-treated (red bars) or control-treated (black bars) throughout vegetative growth, flowering, and seed development. Error bars indicate the standard error.
We used a related bipartite expression system (13) in which the reporter construct, containing both the FLC cDNA and GUS gene (pOp-FLC/GUS), was activated by a range of promoters, with differing tissue specificity, used in the activator constructs (promoter:LhG4). F1 progeny resulting from crossing of the reporter line and the activator lines all displayed a delay in flowering (Fig. 5A). CLAVATA (CLV):LhG4 × pOp-FLC/GUS strongly delayed flowering and directed expression in embryos from heart stage onwards, including in the provascular tissue of the cotyledons, as well as strong expression in the vegetative plant (Fig. 5 A and E–G). SHOOTMERISTEMLESS (STM):LhG4 × LhGh/pOp-FLC/GUS, in contrast, although expressed strongly at the heart stage, had weak expression in the shoot apical meristem region of late-stage embryos and in vegetative plants and had a weak effect on flowering (Fig. 5 A and N–P). SUCROSE TRANSPORTER2 (SUC2):LhG4 × LhGh/pOp-FLC/GUS, which conferred a substantial delay on flowering, was not expressed in early-stage ovules but was expressed in late-stage embryos including in the provascular tissue of the root and cotyledon and in the phloem of vegetative plants (Fig. 5 A and K–M). AINTEGUMENTA (ANT):LhG4 × LhGh/pOp-FLC/GUS and CUP-SHAPED COTYLEDONS2 (CUC2):LhG4 x LhGh/pOp-FLC/GUS were both expressed in heart-stage embryos and also in the provascular tissue of late-stage embryos, as well as in the vegetative plant, and both conferred a moderate delay on flowering (Fig. 5 A–D and H–J). FLC-C24:GUS was expressed in the provascular cells of late-stage embryos (Fig. 3 I and L). FLC has been shown to function in the leaves of vegetative plants to repress FT expression in the companion cells of the phloem (3). FT is also expressed in late-stage developing seeds (14). The correlation between expression of FLC in the provascular cells of late-stage embryos under the control of a range of promoters and the ability to delay flowering suggests that FLC activity may be required to repress FT expression in the provascular cells in late-stage embryos, thereby preventing precocious flowering.
Fig. 5.
FLC expression under the control of heterologous promoters results in delayed flowering. (A) Flowering time measurement of F1 plants of promoter:LhG4 × LhGh/pOp-FLC/GUS in Ler (red bars) and the corresponding promoter:LhG4 line in Ler (black bars), with the promoter indicated on the x axis. The standard errors are indicated. (B, E, H, K, and N) Ovules containing heart-stage embryos. (C, F, I, L, and O) Dissected bent-cotyledon-stage embryos. (D, G, J, M, and P) Ten-day-old vegetative plants. The promoter driving FLC and GUS expression is indicated at the top. (Scale bars: 100 μm.)
Discussion
The resetting of vernalization-repressed FLC in the next sexual generation ensures the need for vernalization in each generation to promote flowering. This study determines the timing of the first FLC:GUS expression in the progeny generation. We have used a functionally active region of the FLC gene (SI Fig. 6) (15) linked to the GUS reporter gene to follow resetting of FLC. This construct appears to have the same pattern of activity and vernalization response as the endogenous gene, but by following the activity of the GUS enzyme we are limited to observations on translational expression as opposed to transcriptional expression. The mitotic transmission of the repressed FLC state in the vernalized generation is associated with changes in FLC chromatin structure, including repressive histone modifications. Resetting presumably involves removal of these repressive histone modifications; however, this may not be sufficient for expression if required transcription factors are not present. Our analysis describes the timing of FLC:GUS expression but does not exclude the possibility of earlier events that result in an expression-competent FLC gene state.
In anthers of vernalized plants, FLC:GUS expression occurred in PMC and in somatic tissues of the anther, including the tapetum. The expression in PMC commenced before meiosis or in the early stages of meiosis and continued at a low level in the meiotic cells and in haploid microspores but ceased before pollen maturation. Although expression is observed around the time of meiosis, expression is not restricted to cells that undergo meiosis, indicating that meiosis is not required for resetting. FLC:GUS expression in the tapetum is initially low but increases markedly after release of the haploid microspores from the tetrads. During this stage the tapetal cells become binucleate and secrete materials for the formation of the pollen coat (16). FLC:GUS expression in the tapetum ceased as the tapetum degenerated before maturity of the anther. The observation of tapetum expression is consistent with the report of Zhang et al. (17) that FLC is high in nonvernalized wild-type young anthers and that expression is significantly reduced in mutant anthers that lack tapetal tissue. The function of FLC in these cells is not known. Plants without a functional FLC gene are fully fertile and have normal anther development. This may indicate that FLC function, if normally required, can be replaced by the action of other genes, perhaps the FLC-like MAF genes (18, 19).
Expression of FLC:GUS in a range of tissue types of the anther argues against resetting occurring in a common progenitor cell. The sporogenous cells and three cell layers of the anther wall, the tapetum, the middle layer, and the endothecium, have a common cellular origin, with a single archaesporial cell in the L2 layer of the stamen primordium giving rise to all four cell types. However, the other tissue types in which FLC:GUS is reset, the epidermis and the connective tissue, originate from L1 and L3, respectively (16).
The loss of FLC:GUS expression in tricellular pollen may be related to a general reduction in transcriptional activity as the chromatin of the gametes becomes compacted (20). Histone H3 variants and other histone variants have been reported in the two products of the first pollen mitosis, the generative cell and vegetative cell (21, 22), and it is possible that these variants contribute to loss of FLC:GUS expression. Although the male gametes do not express FLC:GUS, expression is detected in the single-cell zygote. FLC:GUS activity occurs around the time that has been reported for the DNA replication-independent removal of the male gamete-specific histone H3 variant (23). FLC:GUS expression does not occur in the syncytial endosperm, indicating that the second male gamete that fertilizes the central cell, although identical to the sperm cell fertilizing the egg cell, does not express FLC:GUS, perhaps because of the absence of required transcriptional activators.
In contrast to FLC:GUS expression during male gametophyte development and expression of the paternally derived gene in the zygote, we did not observe FLC:GUS expression during female gametogenesis, nor did we observe expression of the female-derived gene in the zygote. The first detectable expression occurred several cell divisions later in the early multicellular embryo. The differential timing of expression shows that there must be differences in the FLC chromatin of the maternal and paternal gametes. The delay in expression of the female-derived gene may indicate that the chromatin is still in the vernalization-repressed state at the zygote stage and that the modified histones are passively diluted out during the zygotic and early-embryo mitotic divisions. Passive loss of histone modifications could occur because of loss of activity of genes such as VRN2, which is required for the repressive histone modifications at FLC in response to vernalization (6, 8).
We have uncovered a regulatory activity whereby reduced endogenous FLC expression in early-globular embryos directly or indirectly results in an increased expression of the paternally derived FLC:GUS transgene. Consistent with the vernalized maternally inherited FLC gene not being active until the early-globular stage, there was higher expression of the male FLC:GUS gene in embryos on vernalized plants compared with nonvernalized plants.
This difference in FLC expression in embryos of vernalized and nonvernalized plants has no effect on flowering time (data not shown), indicating that early embryo FLC is not essential for flowering time control. FLC is expressed throughout embryo development; however, it is not essential for embryo development as flc-null mutant embryos develop normally. Data from the inducible construct suggest that FLC expression during late embryogenesis is required for delayed flowering of the adult plant. In the late-stage embryo FLC:GUS expression is localized to the embryonic provascular cells. Constructs that direct FLC expression in the embryonic provascular cells are able to confer a delay in flowering, whereas a construct with expression limited to the shoot meristem zone causes a reduced delay in flowering. FLC expression may be required to repress FT, thereby preventing movement of FT protein to the apex and avoiding precocious flowering.
Materials and Methods
Plant Lines and Constructs.
Plants were grown as described (24), except that a 56-day cold treatment was used. FLC-C24:GUS construct in C24 ecotype contains the C24 genomic FLC sequence with GUS replacing the stop codon (SI Fig. 6). Preliminary experiments were conducted with three independent lines, with all three lines giving similar results. The data presented derive from one line. This line was crossed into the flc-20 mutant (15) and homozygous F2 plants selected by flowering time and PCR testing. One line of Ler FRI + FLC-Col:GUS was used for the data presented. FLC-Col:GUS contains FLC-Col fused to the GUS gene at an NheI site in exon 6 (8). The FLC-Col has been characterized genetically as having higher activity than the C24 allele (25). Ler FRI was generated by introducing a genomic clone of FRI-H51 into Ler. Ler FRI-Sf2 was obtained from R. Amasino (University of Wisconsin, Madison, WI). For dexamethasone induction of FLC and misexpression of FLC, the amplified FLC-1/FLC-2 coding region (for primers see SI Table 2) was cloned into pVTOP (13) and transformed into Ler to generate pOp-FLC/GUS. Homozygous T3 lines were supertransformed with pBIN 35S:LhGR-N (13). Lines homozygous for both constructs (LhGR/pOp-FLC/GUS) were selected. Dexamethasone (10 μM; Sigma–Aldrich) in 0.1% ethanol was used in MS agar for induction of imbibed seed and seedlings. Flowering plants in soil were dipped in 10 μM dexamethasone, 0.1% ethanol, and 0.03% silwet-L77 (Lehle Seeds). Control plants were treated with 0.1% ethanol. Coinduction of both FLC and GUS was confirmed by immunodetection of FLC and histochemical analysis for GUS (26). Misexpression activator lines in Ler were obtained from J. Bowman and P. Brewer (Monash University, Melbourne, Australia). pANT and pCLV1 are described by Schoof et al. (27). pCUC2 contains 3.48 kb, pSUC2 contains 3.9 kb, and pSTM contains 7.2 kb upstream of the ATG. pOp-FLC/GUS was used as the reporter line.
Histochemical GUS Imaging.
GUS staining was carried out as described by Sheldon et al. (28). After staining, dissected embryos and seedlings were cleared in ethanol and ovules were cleared in 8:1:3 chloral hydrate:glycerol:water or 20% lactic acid/20% glycerol. Plants were photographed by using a Leitz M8 dissecting microscope with a Colorview Soft Imaging System camera (Leitz). Embryos and ovules were imaged by using DIC optics on a Leica DMR upright microscope with DC500 camera (Leica). After GUS staining, buds were fixed in 3% glutaraldehyde, dehydrated through an ethanol series, and embedded in LR White resin. One-micrometer transverse sections were cut and imaged under dark-field conditions.
mRNA Expression Analysis.
RNA extraction and gel blot analysis were as described (24). Real-time RT-PCR was carried out as described (7), using primers shown in SI Table 2. Expression data were normalized against expression of the FDH.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Bjorg Sherman, Aneta Ivanova, and Janice Norman for excellent assistance; Graham Scofield for microscopy advice; Dr. Mark Talbot for sectioning; and Drs. Jean Finnegan, Ben Trevaskis, and Takashi Okada for comments on the manuscript; Drs. J. Bowman and P. Brewer for the misexpression activator constructs; and Dr. I. Small (University of Western Australia, Perth, Australia) for pVTOP and pBIN 35S:LhGR-N.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711453105/DC1.
References
- 1.Hepworth SR, Valverde F, Ravenscroft D, Mouradov A, Coupland G. Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J. 2002;21:4327–4337. doi: 10.1093/emboj/cdf432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helliwell CA, Wood CC, Robertson M, Peacock WJ, Dennis ES. The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 2006;46:183–192. doi: 10.1111/j.1365-313X.2006.02686.x. [DOI] [PubMed] [Google Scholar]
- 3.Searle I, et al. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signalling in Arabidopsis. Genes Dev. 2006;20:898–912. doi: 10.1101/gad.373506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheldon CC, et al. The FLF MADS box gene: A repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell. 1999;11:445–458. doi: 10.1105/tpc.11.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung S, Amasino RM. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature. 2004;427:159–163. doi: 10.1038/nature02195. [DOI] [PubMed] [Google Scholar]
- 7.Wood CC, et al. The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc Natl Acad Sci USA. 2006;103:14631–14636. doi: 10.1073/pnas.0606385103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bastow R, et al. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature. 2004;427:164–167. doi: 10.1038/nature02269. [DOI] [PubMed] [Google Scholar]
- 9.Schubert D, et al. Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. EMBO J. 2006;25:4638–4649. doi: 10.1038/sj.emboj.7601311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheldon CC, Rouse DT, Finnegan EJ, Peacock WJ, Dennis ES. The molecular basis of vernalization: The central role of FLOWERING LOCUS C (FLC). Proc Natl Acad Sci USA. 2000;97:3753–3758. doi: 10.1073/pnas.060023597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smyth DR, Bowman JL, Meyerowitz EM. Early flower development in Arabidopsis. Plant Cell. 1990;2:755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders PM, et al. Anther development defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod. 1999;11:297–322. [Google Scholar]
- 13.Craft J, et al. New pOp/LhG4 vectors for stringent glucocorticoid-dependent transgene expression in Arabidopsis. Plant J. 2005;41:899–918. doi: 10.1111/j.1365-313X.2005.02342.x. [DOI] [PubMed] [Google Scholar]
- 14.Schmid M, et al. A gene expression map of Arabidopsis thaliana development. Nat Genet. 2005;37:501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- 15.Finnegan EJ, Sheldon CC, Jardinaud F, Peacock WJ, Dennis ES. A cluster of Arabidopsis genes with a coordinate response to an environmental stimulus. Curr Biol. 2004;14:911–916. doi: 10.1016/j.cub.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 16.Scott RJ, Spielman M, Dickinson HG. Stamen structure and function. Plant Cell. 2004;16:S46–S60. doi: 10.1105/tpc.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, et al. Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development. 2006;133:3085–3095. doi: 10.1242/dev.02463. [DOI] [PubMed] [Google Scholar]
- 18.Ratcliffe OJ, Nadzan GC, Reuber TL, Reichmann JL. Regulation of flowering in Arabidopsis by an FLC homologue. Plant Physiol. 2001;126:122–132. doi: 10.1104/pp.126.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratcliffe OJ, Kumimoto RW, Wong BJ, Reichmann JL. Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. Plant Cell. 2003;15:1159–1169. doi: 10.1105/tpc.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman WE. Expression of the cell cycle in sperm of Arabidopsis: Implications for understanding patterns of gametogenesis and fertilization in plants and other eukaryotes. Development. 1999;126:1065–1075. doi: 10.1242/dev.126.5.1065. [DOI] [PubMed] [Google Scholar]
- 21.Okada T, Endo M, Singh MB, Bhalla PL. Analysis of the histone H3 gene family in Arabidopsis and identification of the male-gamete-specific variant AtMGH3. Plant J. 2005;44:557–568. doi: 10.1111/j.1365-313X.2005.02554.x. [DOI] [PubMed] [Google Scholar]
- 22.Sano Y, Tanaka I. Detection of differentially expressed variant histone H3.3 in the vegetative nucleus of lily pollen. Sex Plant Reprod. 2007;20:27–33. [Google Scholar]
- 23.Ingouff M, Hamamura Y, Gourgues M, Higashiyama T, Berger F. Distinct dynamics of HISTONE3 variants between the two fertilization products in plants. Curr Biol. 2007;17:1032–1037. doi: 10.1016/j.cub.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Sheldon CC, Finnegan EJ, Dennis ES, Peacock WJ. Quantitative effects of vernalization on FLC and SOC1 expression. Plant J. 2006;45:871–883. doi: 10.1111/j.1365-313X.2006.02652.x. [DOI] [PubMed] [Google Scholar]
- 25.Sanda S, Amasino R. Genetic and physiological analysis of flowering time in the C24 line of Arabidopsis thaliana. Weeds World. 1995;2:2–8. [Google Scholar]
- 26.Hills MJ. Canberra, Australia: Australian Natl Univ; 2005. PhD thesis. [Google Scholar]
- 27.Schoof H, et al. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell. 2000;100:635–644. doi: 10.1016/s0092-8674(00)80700-x. [DOI] [PubMed] [Google Scholar]
- 28.Sheldon CC, Conn AB, Dennis ES, Peacock WJ. Different regulatory regions are required for the vernalization-induced repression of FLOWERING LOCUS C and for the epigenetic maintenance of repression. Plant Cell. 2002;14:2527–2537. doi: 10.1105/tpc.004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.