Abstract
Several studies have reported a crucial role for cholesterol-enriched membrane lipid rafts and cell-associated heparan sulfate proteoglycans (HSPGs), a class of molecules that can localize in lipid rafts, in the entry of human immunodeficiency virus type 1 (HIV-1) into permissive cells. For the present study, we examined the role of these cell surface moieties in HIV-1 entry into primary human brain microvascular endothelial cells (BMVECs), which represent an important HIV-1 central nervous system-based cell reservoir and a portal for neuroinvasion. Cellular cholesterol was depleted by exposure to β-cyclodextrins and 3-hydroxy-3-methylglutaryl (HMG)-coenzyme A reductase inhibitors (statins), the loss of cholesterol was quantitated, and disruption of membrane rafts was verified by immunofluorescence. Nevertheless, these treatments did not affect binding of several strains of HIV-1 virions to BMVECs at 4°C or their infectivities at 37°C. In contrast, we confirmed that cholesterol depletion and raft disruption strongly inhibited HIV-1 binding and infection of Jurkat T cells. Enzymatic digestion of cell-associated HSPGs on human BMVECs dramatically inhibited HIV-1 infection, and our data from quantitative HIV-1 DNA PCR analysis strongly suggest that cell-associated chondroitin sulfate proteoglycans greatly facilitate infective entry of HIV-1 into human BMVECs. These findings, in combination with our earlier work showing that human BMVECs lack CD4, indicate that the molecular mechanisms for HIV-1 entry into BMVECs are fundamentally different from that of viral entry into T cells, in which lipid rafts, CD4, and probably HSPGs play important roles.
Human immunodeficiency virus type 1 (HIV-1) enters permissive cells by fusion of its envelope (Env) with the plasma membrane after binding to the CD4 receptor molecule and interaction with the chemokine coreceptors CXCR4 and CCR5, which determine the tropism of different HIV-1 isolates (6, 9, 10, 14, 66). The viral Env-mediated fusion is initiated by binding of the envelope gp120 to CD4. This event leads to subsequent conformational changes in gp120, resulting in engagement of the chemokine receptors with critical domains in gp120. The resultant switch of HIV-1 gp41 to a fusion-active condition enables the exposure of its fusion peptide domain (15, 16, 27).
Early after primary infection, HIV-1 also enters the central nervous system (CNS) (2, 52, 58). Despite extensive research on HIV-1 neuroinvasion, the mechanisms of initial entry into the CNS, and the precise causes of the AIDS dementia complex, which leads to neurological impairment in many HIV-1-seropositive patients, remain enigmatic. One of the hypotheses regarding how HIV-1 enters the CNS suggests direct infection of brain microvascular endothelial cells (BMVECs) as a major route of viral entry into the CNS, followed by replication of the virus in CNS-based cells, such as neurons, microglia, and astrocytes (39, 55). Since BMVECs represent the major cellular constituent of the blood-brain barrier (BBB), either HIV-1 can cross the barrier by transcellular migration or the infection may alter the tight junction property of BMVECs, creating a breach, which allows viral entry (4, 5). Another hypothesis suggests cell-associated HIV-1 entry into the CNS via CD4+ T cells and monocytes that traffic across the BBB, potentially transferring the infection to other CNS-based cells (5, 28, 31, 50, 53, 57). It has also been suggested that certain cytokines, HIV-1-specific proteins, and various cellular factors may also induce alterations in the BBB, creating a breach in the tight junctions of BMVECs. Consequently, this breach may assist the virus in gaining entry into CNS-based cells (12, 18, 32, 51).
In our laboratories, we have extensively studied HIV-1 neuroinvasion by means of a novel human in vitro BBB system composed of CNS-based cell systems (4, 40-42). In prior studies using well-characterized primary human BMVECs, we found that these cells lack the crucial CD4 molecule, indicating that mechanisms for HIV-1 entry and infection across the BBB must be CD4 independent. Interestingly, BMVECs highly express many chemokine receptors, including CXCR4, CCR5, APJ, and CCR3, plus C-type lectins DC-SIGN and L-SIGN, but blockage of these molecules individually does not inhibit binding of HIV-1 to BMVECs (41). Thus, viral attachment to primary BMVECs is mediated by an unidentified receptor(s) or by an uncharacterized cooperation among these various coreceptors.
Recent studies have reported a crucial role for cholesterol-rich plasma membrane rafts in the entry of HIV-1 into permissive cells, such as T-cell lines and primary human T lymphocytes (8, 13, 17, 23, 26, 29, 33, 34, 54, 56). Membrane rafts, also known as detergent-insoluble lipid microdomains, are specialized regions of the host cell membrane that are characterized by an unusually high content of cholesterol, glycophosphatidylinositol (GPI)-anchored proteins, and sphingolipids that serve several distinct functions, including crucial roles in signaling (3, 17, 21, 22, 34, 35, 49, 63). The presence of GPI-linked proteins in lipid rafts is controversial (3, 37, 49). It was recently demonstrated that the gp120 protein induces coclustering of CD4 and chemokine coreceptors into membrane regions enriched in GM1, a sphingolipid characteristic of membrane rafts, and that this process is prevented by cholesterol depletion of the plasma membrane (33, 34, 56, 64, 65). More importantly, cholesterol depletion inhibits the susceptibility of T-cell lines and primary human T lymphocytes to HIV-1 infection in vitro (29, 33, 54). A recent report by Viard et al. proposed that gp41-mediated membrane fusion may not require cholesterol per se. Instead, cholesterol depletion from cells with relatively low surface densities of coreceptors reduces the capacity of HIV-1 envelope proteins to engage these coreceptors, which is an essential trigger for fusion (69). Thus, membrane rafts on T cells are thought to facilitate the assembly of gp120 and gp41 with CD4 and chemokine coreceptors by concentrating these molecules within a restricted area on the host cell surface.
Along similar lines, recent work has implicated a role in HIV-1 entry for cell surface heparan sulfate proteoglycans (HSPGs), particularly syndecans (7, 11, 48, 61). Saphire et al. have shown that syndecans serve as attachment receptors for HIV-1 on macrophages (61). Bobardt et al. have recently demonstrated that syndecan functions as an in trans HIV-1 receptor and, when expressed in nonpermissive cells, becomes the major mediator for HIV-1 adsorption (7). Our laboratory (19) and others (68) have shown that syndecans concentrate into membrane rafts when they bind large, multivalent ligands. A recent study by Liu et al. suggested that HIV-1 penetrates BMVECs (isolated from discarded temporal lobe tissues from adults) by macropinocytosis and that this mechanism involves lipid rafts, mitogen-activated protein kinase signaling, and glycosaminoglycans, while CD4 and chemokine receptors play limited roles in this process (30).
In the present study, we examined the role of cholesterol-rich membrane rafts and cell-associated HSPGs and chondroitin sulfate proteoglycans (CSPGs) in HIV-1 entry into primary human BMVECs, which represent an important HIV-1 reservoir and a portal for neuroinvasion. We found that viral binding and infection of these cells are independent of the presence of membrane rafts, yet our data demonstrate that cell-associated CSPGs, but not HSPGs, greatly facilitate infective entry of HIV-1 into human BMVECs. These findings, in combination with our earlier work showing that BMVECs lack CD4, indicate that the molecular mechanisms for HIV-1 entry into BMVECs are fundamentally different from those for viral entry into T cells, in which rafts, CD4, and possibly cell-associated HSPGs and CSPGs play crucial roles.
MATERIALS AND METHODS
Reagents.
Anti-human von Willebrand factor antibody was purchased from Sigma (St. Louis, Mo.), anti-ZO-1 antibody was purchased from Zymed (South San Francisco, Calif.), anti-heparin/heparan sulfate (recognizes intact heparan sulfates) antibody was from Research Diagnostics, Inc. (Flanders, N.J.), and anti-chondroitin sulfate (recognizes intact chondroitin-6-sulfate and/or chondroitin-4-sulfates) antibody and the fluorescent conjugate (Alexa Fluor 488) of cholera toxin subunit B (CTB), which is a marker for ganglioside GM1-containing lipid rafts, were obtained from Molecular Probes Inc. (Eugene, Oreg.). For cholesterol depletion, we used randomly methylated β-cyclodextrin (BCD) (commercial name, TRMBP) and hydroxypropyl BCD (commercial name, THPBP) from Cyclodextrin Technologies Development, Inc. (High Springs, Fla.), and for inhibition of cholesterol synthesis, we used the 3-hydroxy-3-methylglutaryl (HMG)-coenzyme A (CoA) reductase inhibitors mevinolin (Sigma), atorvastatin, and pravastatin (Calbiochem, San Diego, Calif.). The lyases heparinase III (also known as heparitinase) and chondroitinase-ABC, which digest heparan and chondroitin sulfate moieties from cell surface proteoglycans, were obtained from Sigma.
Cell cultures.
Primary isolated human fetal BMVECs were obtained from Cell Systems Corp. (Kirkland, Wash.). The cells were initially seeded into 75-cm2 flasks in supplemented endothelial cell basal medium 2 (Biowhittaker, Walkersville, Md.) and were maintained and passaged in vitro under strict conditions established in our laboratory (37°C, 5% CO2, 100% humidity in human endothelial growth medium). For all experiments, BMVECS were seeded in 6-well tissue culture plates at a density of 0.8 × 106 to 1 × 106 cells/well (confluent). The purity of BMVECs was analyzed by immunofluorescent staining and microscopy with antibodies against von Willebrand factor and ZO-1 (a tight junction-associated protein characteristic of endothelium). Culture conditions for primary human BMVECs and immunofluorescent staining were optimized previously (39-41). Jurkat T cells (American Type Culture Collection, Manassas, Va.) served as a positive control in our studies and were maintained in complete medium consisting of RPMI 1640 (Cellgro; Mediatech, Inc., Herndon, Va.) supplemented with 2 mM l-glutamine, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 10% heat-inactivated fetal bovine serum (Gibco-BRL and Invitrogen).
Viral stocks.
The viral stocks used for our studies were the X4-tropic HIV-1 strain NL4-3, the R5-tropic strain YU2, and the dual-tropic strain 89.6. These were produced by transfection of 293T cells with the respective proviral DNA, as described previously (47). Briefly, 48 h after transfection of proviral DNA via the calcium phosphate coprecipitation method, supernatant was collected, filtered through a 0.45-μm-pore-size filter, quantified by HIV-1 p24 antigen enzyme-linked immunosorbent assay (NEN Life Science Products, Inc., Boston, Mass.), and stored at −70°C until further use. The viral stock of NL4-3 Env−Vpr− (pNL4-3.Luc.R−E−), an envelope- and Vpr-negative viral construct expressing luciferase, was kindly provided by Michael J. Root (Thomas Jefferson University, Philadelphia, Pa.).
Cholesterol depletion and inhibition of cholesterol synthesis.
Depletion of cellular cholesterol and disruption of plasma membrane rafts were achieved by preincubation of cells in serum-free medium at 37°C for 30 min, followed by two washes with medium. Cells were then treated with 10 mM concentrations of different cyclodextrins for 30 to 40 min at 37°C and were finally washed twice in serum-free medium before the addition of HIV-1-containing supernatants. The viability (trypan blue viability test) and morphology of cells were monitored very carefully in our assays, and treatment with cyclodextrins never exceeded a final concentration of 10 mM, as higher concentrations of these compounds proved to be toxic to the cells. For inhibition of cholesterol synthesis, cells were treated with 1 to 3 μM concentrations of different statins in serum-free medium at 37°C for 24 h, followed by two or three washes before the addition of HIV-1-containing supernatants (38). To avoid inadvertent replenishment of cellular cholesterol, all of these incubations and washes were performed in the absence of serum.
Cholesterol measurements.
Cellular cholesterol was measured with a sensitive cholesterol oxidase-based colorimetric assay (1) using the Cholesterol CII kit from Wako Inc. (Richmond, Va.) on propan-2-ol extracts of cells. The cholesterol content of cells was normalized to the total cellular protein, which was quantified by use of the SDS-Lowry kit from Sigma Diagnostics (36).
Treatment of cells with heparan and chondroitin sulfate-degrading enzymes (lyases).
The enzymatic removal of cell surface heparan and/or chondroitin sulfate moieties was performed as described previously (19, 61, 70). Briefly, because heparan-binding components of serum may interfere with heparitinase and/or chondroitinase digestion, the cells (0.8 × 106 to 1 × 106 primary human BMVECs) were placed into serum-free medium 2 h before the heparitinase and/or chondroitinase pretreatment and kept serum-free until the end of the experiment (19, 67). Cells were then incubated for 2 h at 37°C in the absence or presence of heparitinase and/or chondroitinase-ABC at a concentration of 5 U/ml. Subsequently, BMVECs were processed and analyzed by cell-free HIV-1 binding assay (at 4°C) and internalization and infectivity assays (at 37°C).
HIV-1 binding, internalization, and infectivity assays.
HIV-1 (NL4-3, YU2, and 89.6) attachment to cells was analyzed by cell-free virion binding assays at 4°C, as described previously (29, 33, 61). Briefly, 0.8 × 106 to 1.0 × 106 target cells in duplicate (untreated or pretreated with BCDs and/or statins or heparitinase and/or chondroitinase) were preincubated for 30 min at 4°C before exposure to virus. Viral supernatant (10 ng of p24 antigen equivalents of viral stock) was then added to target cells and incubated for 3 h (6 h for cells that were pretreated, or not, with lyases) at 4°C in a final volume of 1 ml of cold, serum-free medium. Unbound virus was removed by three washes, and then the cells were lysed in 1 ml of cold phosphate-buffered saline containing 2% NP-40 or 2% Triton X-100. To measure internalization, the same conditions were used, except that cells (untreated or pretreated with BCDs and statins) were incubated for 3 h (6 h for cells that were pretreated, or not, with lyases) directly with virus at 37°C, followed by assay of p24 antigen in cell lysates and supernatants as well as quantitative HIV-1 DNA PCR analysis for the gag gene from cell lysates. In addition, the replication of HIV-1 in cells that were untreated or pretreated with BCDs and statins was analyzed in cultures at 3 and 5 days postinfection. Cell lysates and supernatants were collected, and HIV-1 p24 antigen was quantitated by enzyme-linked immunosorbent assay (NEN Life Science Products, Inc.).
Immunostaining and immunofluorescence assays.
Immunofluorescence microscopy of primary isolated human BMVECs and Jurkat T cells for different markers was performed as described previously (40-42). Briefly, primary isolated human BMVECs were cultured in 2-well chamber slides for 48 h. Jurkat T cells were cultured in suspension and then transferred to 2-well chamber slides. The cells were fixed with 3.5% formaldehyde and incubated for 2 h at room temperature with primary antibodies (diluted 1:100) against their respective cell markers. Incubation of cells with secondary antibodies conjugated to Cy2 (green), obtained from Jackson ImmunoResearch Laboratories, Inc., or Alexa Fluor 488, obtained from Molecular Probes, Inc., was performed at room temperature for 1 h. The preparations were examined with an Olympus Research BX60 microscope.
Quantitative HIV-1 DNA analysis.
The cellular DNA was extracted as described previously (71, 72). The presence of HIV-1 proviral DNA was analyzed by utilizing a sensitive PCR and Southern blotting assay for HIV-1 gag sequences, using the primer-probe set SK38, SK39, and SK19. Southern blotting was utilized to visualize the specific bands and compared with a serially diluted standard curve to quantitate proviral DNA to 5 copies, with detection but not quantification between 1 and 5 copies, as described previously (71, 72). To strictly normalize the cell number, the human beta-globin gene was quantitated with a specific primer pair and a radiolabeled probe as described previously (71, 72). A PhosphorImager (Molecular Dynamics) was utilized for quantitation.
Statistical analysis.
Unless otherwise indicated, results are given as means ± standard errors (n = 2). Statistical comparisons were performed by two-tailed Student's t tests.
RESULTS
Characterization of primary human BMVEC preparations.
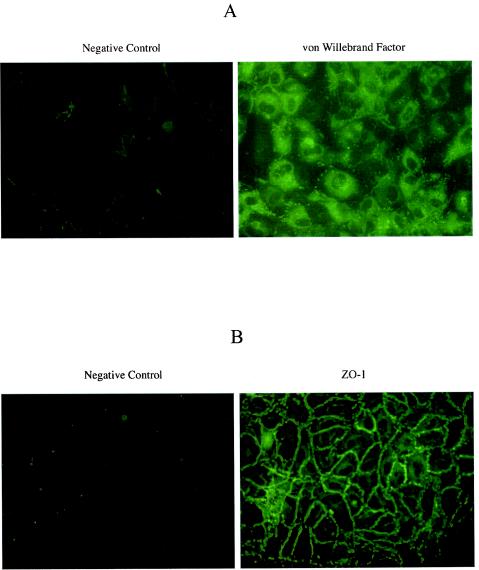
We began our studies by specifically characterizing our primary human BMVECs. Only cells from passage 3 or previous passages were used in our studies. The purity of primary isolated BMVECs was analyzed by immunofluorescent staining with antibody against von Willebrand factor (or factor VIII), which is synthesized by endothelial cells and stored in the Weibel-Palade granules. As shown in Fig. 1A, >97% of primary BMVECs stained positively for von Willebrand factor. Cells were also characterized and stained positively for ZO-1 marker, which is a major component of tight junctions in the paracellular pathway of endothelial cells (Fig. 1B).
FIG. 1.
Immunofluorescence microscopy of primary isolated human BMVECs for endothelial cell markers von Willebrand factor (A) and ZO-1 (B). Primary isolated human BMVECs were cultured on 2-well chamber slides for 48 h. The cells were fixed with 3.5% formaldehyde and incubated with primary antibodies against the respective cell markers. The negative control represents immunostaining of primary human BMVECS with secondary antibody. The figure is representative of 10 independent studies.
Plasma membrane rafts are not implicated in HIV-1 binding or viral entry into primary human BMVECs.
As a first step for investigating the role of lipid rafts and cellular cholesterol on HIV-1 entry into primary human BMVECs, we depleted cellular cholesterol and dispersed lipid rafts on cell membranes by means of BCDs and inhibited cholesterol synthesis by using HMG-CoA reductase inhibitors (statins). For our experiments, we utilized randomly methylated BCD (TRMBP) and hydroxypropyl BCD (THPBP), which are potent cholesterol-depleting agents, at final concentrations that did not exceed 10 mM. This was crucial for the viability of our cell systems, since treatment of BMVECs with cholesterol-depleting agents exceeding a 10 mM concentration proved to be highly toxic for the cells (not illustrated). For inhibition of cholesterol synthesis, we also utilized various potent HMG-CoA reductase inhibitors, such as mevinolin, atorvastatin, and pravastatin, at final concentrations not exceeding 3 μM.
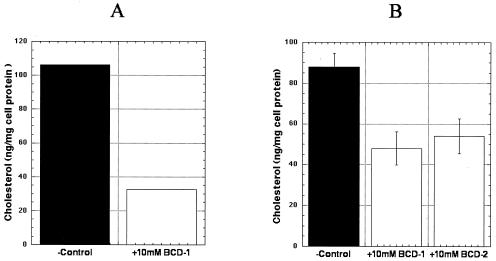
To verify that cells (primary human BMVECS and control Jurkat T cells) treated with different BCDs and statins were cholesterol depleted and that lipid rafts on the cell membranes were dispersed, we analyzed the cells by both staining with the fluorescent conjugate of CTB against ganglioside M1 (GM1), which is present in lipid rafts, and by cholesterol measurement using an enzymatic colorimetric assay. As shown in Fig. 2, treatment of primary human BMVECs and Jurkat T cells with BCDs resulted in over 50% depletion of total cellular cholesterol. Similar results were obtained with cells treated with statins for 24 h (data not shown).
FIG. 2.
Depletion of cellular cholesterol by BCDs in primary human BMVECs (A) and Jurkat T cells (B). Cellular cholesterol was measured by cholesterol oxidase-based colorimetric assay as described in Materials and Methods. The cholesterol content of cells was normalized to total cellular protein. Shown are the results of an experiment with primary human BMVECs and of two independent studies with the control Jurkat T cells. BCD-1, cells pretreated with 10 mM randomly methylated BCD (TRMBP); BCD-2, cells pretreated with 10 mM hydroxypropyl BCD (THPBP); −Control, untreated cells.
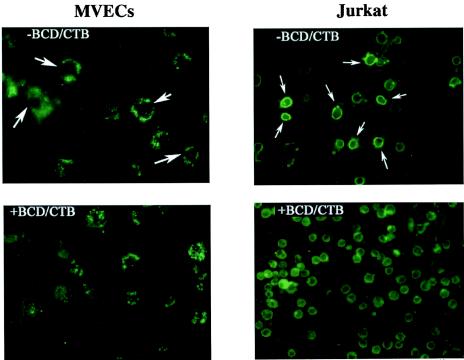
As shown in Fig. 3 (left panels), primary human BMVECs that were not treated with BCDs consistently demonstrated a distinct ring pattern when stained for GM1 with Alexa Fluor 488-CTB. In contrast, cells that were treated with BCDs exhibited granular staining over the entire cell, consistent with disruption of the normal GM1-raft structure. Similar results were obtained with Jurkat T cells, in which a distinct ring pattern in untreated cells was replaced by diffuse staining after BCD treatment (Fig. 3, right panels). Cells (BMVECs and Jurkat T cells) treated with 3 μM concentrations of the different statins for 24 h in serum-free medium and then stained for GM1 also showed a similar pattern of disruption of the normal ring pattern (data not illustrated).
FIG. 3.
Staining of primary human BMVECs and Jurkat T cells for GM1-lipid rafts. Cells were first fixed with 3.5% formaldehyde on 2-well chamber slides and then incubated with 2 μg of fluorescent conjugate (Alexa Fluor 488-CTB) per ml for 2 h at room temperature. The figure is representative of over four independent studies. −BCD/CTB, cells not treated with BCDs and stained with Alexa-CTB against GM1, demonstrating a distinct ring pattern (white arrows); +BCD/CTB, cells treated with BCDs and stained with Alexa-CTB against GM1.
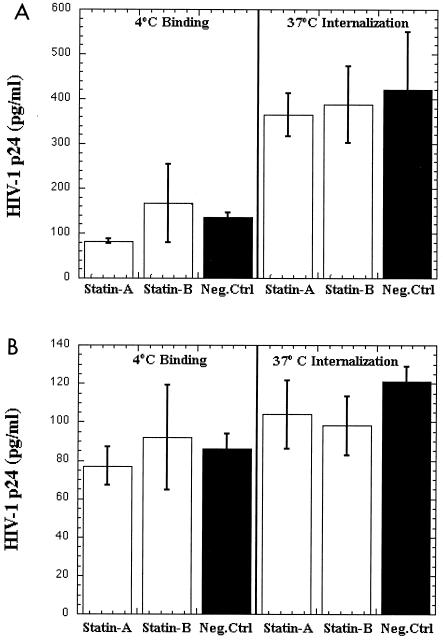
We further examined virus binding and internalization into primary human BMVECs (treated with various BCDs) by utilizing different HIV-1 strains (YU2, NL4-3, and 89.6, as well as the envelope-negative virus control, NL4-3 Env− Vpr−) in cell-free HIV-1 virion binding assays at 4°C and viral internalization assays at 37°C. As illustrated in Fig. 4, our data from the cell-free HIV-1 virion binding and internalization assays demonstrated that membrane cholesterol and lipid rafts were not implicated in HIV-1 binding and viral entry into primary human BMVECs. No statistically important differences (P > 0.5) were observed for binding (Fig. 4, left three columns) and internalization (Fig. 4, right three columns) of various HIV-1 strains (YU-2 [panel A] and NL4-3 [panel B]) between primary human BMVECs treated with cyclodextrins and untreated cells. Overall, the R5-tropic YU-2 strain demonstrated higher levels of binding and internalization properties in human BMVECs, as we were able to recover higher levels of YU-2 virus p24 antigen from cell lysates from both binding (4°C) and internalization (37°C) assays than for X4-tropic NL4-3 (compare panels A and B), which was consistent with our previous findings (44). Similar to the NL4-3 results were those obtained from binding and internalization assays of the dual-tropic HIV-1 strain 89.6 between BMVECs that were treated and/or untreated with various cyclodextrins (data not shown). Finally, as shown in Fig. 4C, testing of the Env-negative virus control (NL4-3 Env− Vpr−) in binding and internalization assays in BMVECs demonstrated that HIV-1 Env is clearly required for viral entry and replication, since only a very minimal background amount of NL4-3 Env− Vpr− p24 antigen from cell lysates was recovered (5 to 10 pg/ml).
FIG. 4.
Effects of BCDs on HIV-1 binding and entry into primary human BMVECs. (A) Effects of different BCD compounds on HIV-1 CCR-5-tropic YU-2 binding (4°C) and entry (37°C) into primary human BMVECs. (B) Effects of BCDs on HIV-1 X4-tropic NL4-3 binding (4°C) and entry (37°C) into BMVECs. (C) Effects of BCDs on HIV-1 NL4-3 Env− Vpr− binding (4°C) and entry (37°C) into BMVECs. The graphs represent the mean values of two independent experiments. BCD-1, cells pretreated with 10 mM randomly methylated BCD (TRMBP); BCD-2, cells pretreated with 10 mM hydroxypropyl BCD (THPBP); Neg.Ctrl, untreated BMVECs.
We also examined viral binding and internalization into primary human BMVECs treated with different HMG-CoA reductase inhibitors by utilizing different HIV-1 strains (YU-2, NL4-3, and 89.6) in cell-free HIV-1 virion binding assays at 4°C and viral internalization assays at 37°C. As shown in Fig. 5, similar to the effects with BCDs, no statistically important differences (P > 0.5) were observed in binding (Fig. 5, left three columns) and internalization (Fig. 5, right three columns) of various HIV-1 strains (YU-2 [panel A], NL4-3 [panel B], and 89.6 [not shown]) between primary human BMVECs treated with various statins and untreated cells. In addition, we analyzed the effects in supernatants collected at 3 and 5 days postinfection of cholesterol-depleting and -inhibiting agents on HIV-1 replication in human BMVECs that were untreated or treated with different BCDs and statins. As expected, no differences were observed in viral replication between BMVECs treated with cyclodextrins and/or statins and untreated cells (data not shown), suggesting that cellular cholesterol and lipid rafts are not important for viral attachment, entry, and replication into primary human BMVECs. Our results also confirmed findings in the literature for low-level HIV-1 replication in human BMVECs (29, 40).
FIG. 5.
Effects of statins on HIV-1 binding and entry into primary human BMVECs. (A) Effects of different statins on HIV-1 CCR-5-tropic YU-2 binding (4°C) and entry (37°C) into primary human BMVECs. (B) Effects of different statins on HIV-1 X4-tropic NL4-3 binding (4°C) and entry (37°C) into BMVECs. The graphs represent the mean values of two independent experiments. Statin-A, cells pretreated with 3 μM atorvastatin; Statin-B, cells pretreated with 3 μM pravastatin; Neg.Ctrl, untreated BMVECs.
Cellular cholesterol and lipid rafts are important in HIV-1 attachment and viral entry into T cells.
Recent studies by Hildreth and others have demonstrated the importance of lipid rafts and cellular cholesterol in HIV-1 attachment and entry into permissive cells, such as the T-cell lines Jurkat, PM1, and CEMX174, as well as primary cells (29, 33). To verify this finding and to assess T cells as positive controls for our primary human BMVEC studies, we also examined in our laboratory the role of cellular cholesterol and lipid rafts in HIV-1 binding and entry by utilizing the Jurkat T-cell line. For this purpose, we depleted cellular cholesterol and disrupted the lipid rafts in Jurkat cells treated with BCDs and/or statins (Fig. 2 and 3) and subsequently examined the ability of the CXCR4-tropic NL4-3 virus strain to bind, internalize, and replicate in these cells. As shown in Fig. 6, our data from the cell-free HIV-1 (NL4-3) binding assays, performed at 4°C (Fig. 6A), as well as from our studies on viral entry into Jurkat cells, performed at 37°C (Fig. 6B), confirmed that membrane cholesterol and lipid rafts are important in HIV-1 binding and virus internalization into T lymphocytes. Significant differences (P < 0.00005) were observed in binding and entry of HIV-1 NL4-3 between Jurkat cells treated with 10 mM hydroxypropyl BCD (THPBP) and/or 3 μM mevinolin and untreated cells. We also analyzed the effects in supernatants collected at 3 and 5 days postinfection of cholesterol-depleting and -inhibiting agents on HIV-1 replication in Jurkat T cells that were untreated or treated with BCDs and statins. Not surprisingly, significant differences were also observed in viral replication between Jurkat cells treated with cyclodextrins and/or statins and untreated cells (data not shown), confirming that cellular cholesterol and lipid rafts are important for viral attachment, entry, and replication into T lymphocytes.
FIG. 6.
Effects of BCDs and statins on HIV-1 binding and entry into Jurkat T cells. (A) Effects of hydroxypropyl BCD and/or mevinolin on HIV-1 NL4-3 binding (4°C) on Jurkat T cells. (B) Effects of hydroxypropyl BCD and/or mevinolin on HIV-1 NL4-3 entry (37°C) into Jurkat T cells. The graphs represent the mean values of two independent experiments. THPBP, 10 mM hydroxypropyl BCD; Neg.Ctrl, untreated Jurkat T cells.
Cell surface CSPGs facilitate HIV-1 internalization and replication into primary human BMVECs.
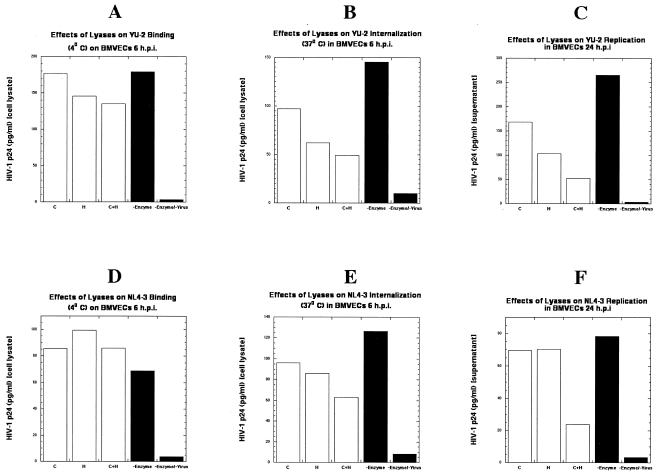
Certain cell surface HSPGs migrate into membrane rafts upon binding large, multivalent ligands, and several recent lines of evidence have implicated these HSPGs in HIV-1 binding and infection of T-cell lines, primary lymphocytes, and macrophages (7, 19, 61, 70). To examine this hypothesis, we employed primary human BMVECs in cell-free virion binding assays at 4°C and viral internalization assays at 37°C. The cells were first analyzed by immunostaining for cell surface HSPGs and CSPGs, and as shown in Fig. 7B and D, the cells were positive for these proteoglycans. To determine the role of cell surface glycosaminoglycans in HIV-1 attachment and entry, primary human BMVECs were either left untreated or pretreated with heparitinase, which removes cell surface heparan sulfate moieties, chondroitinase-ABC, which removes cell surface chondroitin sulfate moieties, or a combination of both lyases, following standard methodologies. Immunostaining of BMVECS pretreated with lyases confirmed the removal of cell surface sulfate moieties (Fig. 7C and E). We then tested the capacity of R5-tropic YU-2 and the X4-tropic HIV-1 strain NL4-3 to bind (at 4°C), internalize (at 37°C), and replicate in primary human BMVECs by analyzing the HIV-1 p24 antigen content in cell lysates and/or supernatants of BMVECs. As shown in Fig. 8A and D, no statistically significant effects (P > 0.5) of these predigestions were observed in the binding of either YU-2 or NL4-3 strains, after 6 h of incubation, on BMVECs. In contrast, as shown in Fig. 8B and E, significant differences were observed in YU-2 and NL4-3 entry into the cells at 6 h postinfection. These differences were more profound in BMVECs that were pretreated with a combination of both lyases (heparitinase plus chondroitinase-ABC). Similarly, as shown in Fig. 8C and F, significant effects of these predigestions, which were more evident in cells pretreated with both lyases, were observed in the replication of both YU-2 and NL4-3 strains in BMVECs at 24 h postinfection.
FIG. 7.
Immunofluorescence microscopy of primary isolated human BMVECs for heparan and chondroitin sulfate proteoglycans. (A) The negative control represents immunostaining of primary human BMVECs with secondary antibody. (B) Primary human BMVECs stained for cell surface heparan sulfate moieties. (C) Immunostaining of primary human BMVECs pretreated with 5 U of heparitinase confirms the removal of cell surface heparan sulfate moieties. (D) Primary human BMVECs stained for cell surface chondroitin sulfate moieties. (E) Immunostaining of primary human BMVECs pretreated with 5 U of chondroitinase-ABC confirms the removal of cell surface chondroitin sulfate moieties. The figure is representative of three independent studies.
FIG. 8.
Effects of lyases (heparitinase and chondroitinase) on HIV-1 binding, entry, and replication in primary human BMVECs. Effects of lyases on HIV-1 YU-2 binding (4°C) (A), internalization (37°C) (B), and replication (C) in BMVECs and HIV-1 NL4-3 binding (4°C) (D), internalization (37°C) (E), and replication (F) in BMVECs. Cells were treated according to standard procedures, as described in Materials and Methods. The graphs represent a single experiment (samples were analyzed in duplicate). C, BMVECs pretreated with 5 U of chondroitinase per ml; H, BMVECs pretreated with 5 U of heparitinase per ml; C+H, BMVECs pretreated with a combination of 5 U of chondroitinase per ml and 5 U of heparitinase per ml; −Enzyme, untreated BMVECs; −Enzyme/−Virus, untreated and uninfected BMVECs.
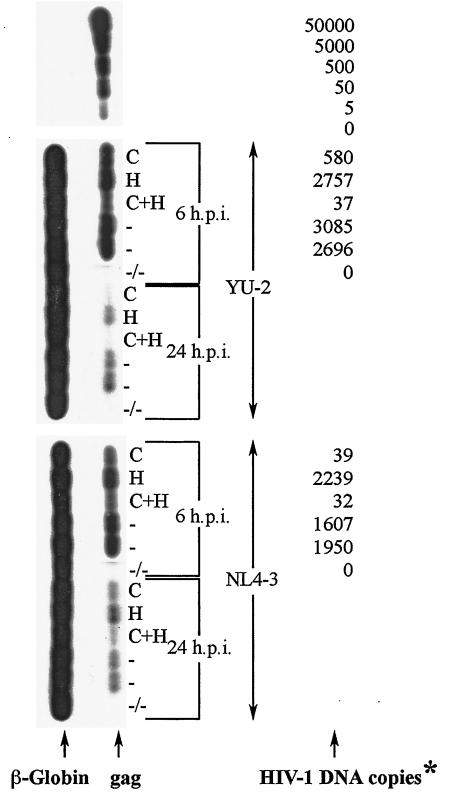
In order to further investigate the role of cell surface HSPGs and CSPGs in HIV-1 replication, we also quantitatively analyzed the proviral DNA (HIV-1 gag) in BMVECs that were pretreated (or not) with lyases and infected with the R5-tropic YU-2 and/or the X4-tropic HIV-1 strain NL4-3. As shown in Fig. 9, at 6 h postinfection, there was a dramatic decrease in the number of proviral DNA copies, for both YU-2 and NL4-3, in BMVECs that were pretreated with chondroitinase-ABC or a combination of both lyases compared to that in untreated cells. The same effects were also clearly evident at a later time point (24 h postinfection), while no differences were observed in the proviral copy number in cells that were pretreated with heparitinase versus untreated BMVECs. These findings strongly suggest that cell-associated CSPGs greatly facilitate entry and replication of HIV-1 in human BMVECs.
FIG. 9.
Detection of proviral DNA (YU-2 and NL4-3) at 6 and 24 h postinfection in primary human BMVECs pretreated with lyases or left untreated. Cells were treated according to standard procedures, as described in Materials and Methods. Cellular DNA was extracted from BMVECs and amplified by PCR with gag SK38 and SK39 as the primer pair. As a means of confirming that each sample contained similar quantities of cellular DNA before PCR, β-globin DNA (left) was also amplified in each sample, with PCO3 and PCO4 as the primer pair. The HIV-1 DNA standards (50,000 to 0 copies; top) were prepared from ACH-2 cells (one proviral copy per cell) and amplified by PCR at the same time as all the samples; values (right) for each sample are given as numbers of copies per 2.5 × 105 cells and correspond to the matching HIV-1 gag DNA bands (left). *, at 24 h postinfection, samples were analyzed by detection only, not by DNA-copy quantification (between 1 and 5 copies); h.p.i., hours postinfection; C, BMVECs pretreated with 5 U of chondroitinase per ml; H, BMVECs pretreated with 5 U of heparitinase per ml; C+H, BMVECs pretreated with a combination of 5 U of chondroitinase per ml and 5 U of heparitinase per ml; −, untreated BMVECs; −/−, untreated and uninfected BMVECs.
DISCUSSION
The precise molecular mechanisms involved in HIV-1 attachment and entry into the BBB-associated cell systems, as well as infection of the CNS, still remain relatively unclear. The BBB consists mainly of microvascular endothelial cells and astrocytic foot processes and separates the CNS from the periphery (25, 42, 50, 59). The main function of the BBB is to ensure a constant internal environment for proper synaptic transmission and supply of necessary nutrients for CNS-resident cells. This structure is constantly exposed to a variety of inflammatory cells as well as to infections in the body fluids. HIV-1 frequently infects the cellular elements of the CNS soon after primary seroconversion (2, 44-46, 50, 55). A number of independent studies have shown that microglia and monocytes/macrophages are the main cellular reservoirs for productive HIV-1 infection in the CNS (5, 28, 31, 55). A number of studies have also demonstrated limited (low-level) nonproductive replication of HIV-1, in vivo and in vitro, within BMVECs, astrocytes, and specific neuronal cells (4, 44-46). HIV-1 infection of the CNS induces neurodegenerative disorders in patients with AIDS (AIDS-related dementia), and the presence of virus in cerebrospinal fluid and CNS-based cells strongly suggests the need for further exploration of HIV-1 entry into the brain and the role of the BBB in the neuropathogenesis of HIV-1 infection (20, 62, 73).
Our recent data and a number of other laboratories' findings (30, 41) show that primary isolated human BMVECs, which represent the major cellular constituent of the BBB, are devoid of CD4, thus suggesting a CD4-independent HIV-1 entry and infection of these cells (41). More importantly, primary human BMVECs express various chemokine receptors (APJ, CCR3, CXCR4, CCR5, DC-SIGN, and L-SIGN), yet chemokines for cognate chemokine receptors individually do not block binding of HIV-1 to BMVECs. These findings suggest the possibility of some alternative receptors, such as cell surface proteoglycans, or an interaction of chemokine receptors with proteoglycan-like molecules.
A recent study of Liu et al. proposed that HIV-1 enters BMVECs by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway (30). In many recent studies, lipid rafts, localized in the plasma membranes of susceptible cells, as well as host membrane cholesterol, were proposed to play key roles in HIV-1 entry and viral budding (8, 13, 17, 23, 26, 29, 30, 33, 34, 43).
In our present studies, we employed a well-characterized CNS-associated system, early passages of primary human BMVECs, to explore the role of cellular cholesterol and lipid rafts in HIV-1 binding, entry, and replication into this major constituent of the BBB. As a positive control in our studies we included the Jurkat T-cell line. Recent reports have demonstrated that lipid rafts are involved in HIV-1 entry into this T-cell line (29). Our findings from HIV-1 binding and internalization assays performed on primary human BMVECs that were left untreated or treated with various cholesterol-depleting agents that disrupt lipid rafts strongly suggest a cholesterol- and lipid raft-independent mechanism of HIV-1 entry into primary human BMVECs. As a positive control, our data support previous findings for the important role of lipid rafts and cellular cholesterol in HIV-1 binding of and entry into T lymphocytes.
A possible explanation for the discrepancy of our data with the report of Liu et al. may be in the differences between the cell systems used. Liu et al. (30) used BMVECs isolated from discarded temporal lobe tissues obtained from temporal lobectomies from adults with epilepsy surgery, while in all our present and previous studies, we have utilized early passages of well-characterized primary human fetal BMVECs for the expression of von Willebrand factor as well as the endothelial tight-junction marker ZO-1. A recent study by Viard et al. has demonstrated that treatment of peripheral blood lymphocytes with the cholesterol-depleting agent methyl-β-cyclodextrin reduced their susceptibility to membrane fusion with cells expressing HIV-1 Env, which utilizes CXCR4 or CCR5. However, treatment of human osteosarcoma (HOS) cells expressing high levels of CD4 and coreceptors with cholesterol-depleting agents did not affect their susceptibility to HIV-1 Env-mediated membrane fusion (69). These findings indicate that although cholesterol is not required for HIV-1 Env-mediated membrane fusion per se, its depletion from cells with relatively low coreceptor densities reduces the capacity of HIV-1 Env to engage coreceptor clusters required to trigger fusion. In our case, primary human fetal BMVECs, which are shown to be CD4 negative but found to express significant levels of various chemokine receptors and possibly other unknown receptors for viral attachment, may not require lipid rafts and cholesterol for HIV-1 attachment and entry.
We also examined the possibility of cell surface glycosaminoglycan involvement in viral binding and internalization into primary human BMVECs. Recent studies by Saphire and others have proposed that cell surface proteoglycans (syndecans) may play a key role in virus attachment and entry (7, 61). Saphire et al. have shown that removal of cell surface heparan sulfate moieties by means of the heparan sulfate-degrading enzyme heparitinase totally inhibited the attachment of HIV-1 to monocyte-derived macrophages but did not influence HIV-1 attachment to activated CD4+ T lymphocytes. They suggest that CD4 alone is not sufficient to support the initial adsorption of HIV-1 to macrophages, which express low levels of CD4, and that HSPGs, especially syndecans that are expressed in abundance, serve as the main class of attachment receptors for HIV-1 on macrophages. Other studies have proposed that cell surface HSPGs facilitate HIV-1 entry into certain T-cell lines and that infection of primary lymphocytes by either monocyte-tropic or lymphocyte-tropic strains of HIV-1 is not substantially facilitated by heparan sulfate glycosaminoglycans (24). Our data for the first time clearly demonstrate that heparan sulfate, and especially chondroitin sulfate, moieties on the cell surfaces of primary human BMVECs are involved in HIV-1 infection and greatly facilitate viral entry and replication into these cells. Interestingly, our HIV-1 p24 antigen recovery data (Fig. 8A and D) did not reveal any lyase effect on viral binding to BMVECs at 4°C, which suggests that cell surface HSPGs or CSPGs are not required in the very earliest steps of viral attachment to primary human BMVECs. Our findings clearly demonstrate that cell-associated HSPGs and CSPGs are important for viral entry and HIV-1 replication in BMVECs, as demonstrated in Fig. 8B, C, E, and F. Quantitative proviral DNA analysis (Fig. 9), which is a more precise and accurate method of detection, clearly indicated that the chondroitin sulfate moieties on cell-associated CSPGs represent a major component in viral entry and replication. Currently, it is unclear how these moieties interact with HIV-1 and what exactly is their mode of action. A possibility is that CSPGs rescue HIV-1 on the cell surfaces of BMVECs from being endocytosed (or pinocytosed), as has recently been reported for other CSPG ligands (60, 67). CSPGs may also redirect the rescued virions to other pathways and may promote engagement and interaction of other HIV-1 coreceptor molecules on the host membrane for HIV-1 entry.
The molecular mechanisms of viral entry, infection, and spread in the CNS and its cellular components remain unclear. Further work is required in order to fully characterize the pathways of HIV-1 entry into the CNS, involving not only BMVECs but also other critical CNS cellular components, such as microglia, neurons, and astrocytes. Understanding the infection of the human brain by HIV-1 will be critical in targeting this potential viral reservoir site.
Acknowledgments
We thank Rita Victor and Brenda Gordon for excellent secretarial assistance and Mike Root for kindly providing the viral stock of NL4-3 Env− Vpr−.
This work was supported in part by U.S. Public Health Service grants NS41864, MH58526, and NS27405 to R.J.P. and HL58884 to K.J.W. and the Pfizer Atorvastatin Research Award (ARA) to M.M.
REFERENCES
- 1.Allain, C. C., L. S. Poon, C. S. Chan, W. Richmond, and P. C. Fu. 1974. Enzymatic determination of total serum cholesterol. Clin. Chem. 20:470-475. [PubMed] [Google Scholar]
- 2.An, S. F., M. Groves, F. Gray, and F. Scaravilli. 1999. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J. Neuropathol. Exp. Neurol. 58:1156-1162. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R. G., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296:1821-1825. [DOI] [PubMed] [Google Scholar]
- 4.Bagasra, O., E. Lavi, L. Bobroski, K. Khalili, J. P. Pestaner, R. Tawadros, and R. J. Pomerantz. 1996. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS 10:573-585. [DOI] [PubMed] [Google Scholar]
- 5.Bell, J. E., A. Busuttil, J. W. Ironside, S. Rebus, Y. K. Donaldson, P. Simmonds, and J. F. Peutherer. 1993. Human immunodeficiency virus and the brain: investigation of virus load and neuropathologic changes in pre-AIDS subjects. J. Infect. Dis. 168:818-824. [DOI] [PubMed] [Google Scholar]
- 6.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 7.Bobardt, M. D., A. C. Saphire, H. C. Hung, X. Yu, B. Van Der Schueren, Z. Zhang, G. David, and P. A. Gallay. 2003. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 18:27-39. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, S. M., S. M. Crowe, and J. Mak. 2001. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J. Clin. Virol. 22:217-227. [DOI] [PubMed] [Google Scholar]
- 9.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 10.Dalgleish, A. G., P. C. Beverley, P. R. Clapham, D. H. Crawford, M. F. Greaves, and R. A. Weiss. 1985. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763-767. [DOI] [PubMed] [Google Scholar]
- 11.Del Real, G., S. Jimenez-Baranda, R. A. Lacalle, E. Mira, P. Lucas, C. Gomez-Mouton, A. C. Carrera, C. Martinez-A, and S. Manes. 2002. Blocking of HIV-1 infection by targeting CD4 to nonraft membrane domains. J. Exp. Med. 196:293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Parseval, A., and J. H. Elder. 2001. Binding of recombinant feline immunodeficiency virus surface glycoprotein to feline cells: role of CXCR4, cell-surface heparans, and an unidentified non-CXCR4 receptor. J. Virol. 75:4528-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vries, H. E., M. C. Blom-Roosemalen, M. Van Oosten, A. G. De Boer, T. J. Van Berkel, D. D. Breimer, and J. Kuiper. 1996. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J. Neuroimmunol. 64:37-43. [DOI] [PubMed] [Google Scholar]
- 14.Dimitrov, D. S. 2000. Cell biology of virus entry. Cell 101:697-702. [DOI] [PubMed] [Google Scholar]
- 15.Dimitrov, D. S., D. Norwood, T. S. Stantchev, Y. Feng, X. Xiao, and C. C. Broder. 1999. A mechanism of resistance to HIV-1 entry: inefficient interactions of CXCR4 with CD4 and gp120 in macrophages. Virology 259:1-6. [DOI] [PubMed] [Google Scholar]
- 16.Doms, R. W. 2000. Beyond receptor expression: the influence of receptor conformation, density, and affinity in HIV-1 infection. Virology 276:229-237. [DOI] [PubMed] [Google Scholar]
- 17.Fantini, J., M. Maresca, D. Hammache, N. Yahi, and O. Delezay. 2000. Glycosphingolipid (GSL) microdomains as attachment platforms for host pathogens and their toxins on intestinal epithelial cells: activation of signal transduction pathways and perturbations of intestinal absorption and secretion. Glycoconj. J. 17:173-179. [DOI] [PubMed] [Google Scholar]
- 18.Fiala, M., D. J. Looney, M. Stins, D. D. Way, L. Zhang, X. Gan, F. Chiappelli, E. S. Schweitzer, P. Shapshak, M. Weinand, M. C. Graves, M. Witte, and K. S. Kim. 1997. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol. Med. 3:553-564. [PMC free article] [PubMed] [Google Scholar]
- 19.Fuki, I. V., M. E. Meyer, and K. J. Williams. 2000. Transmembrane and cytoplasmic domains of syndecan mediate a multi-step endocytic pathway involving detergent-insoluble membrane rafts. Biochem. J. 351:607-612. [PMC free article] [PubMed] [Google Scholar]
- 20.Gabuzda, D. H. 1990. Neurologic disorders associated with HIV infections. J. Am. Acad. Dermatol. 22:1232-1236. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Mouton, C., J. L. Abad, E. Mira, R. A. Lacalle, E. Gallardo, S. Jimenez-Baranda, I. Illa, A. Bernad, S. Manes, and C. Martinez-A. 2001. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc. Natl. Acad. Sci. USA 98:9642-9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammache, D., N. Yahi, M. Maresca, G. Pieroni, and J. Fantini. 1999. Human erythrocyte glycosphingolipids as alternative cofactors for human immunodeficiency virus type 1 (HIV-1) entry: evidence for CD4-induced interactions between HIV-1 gp120 and reconstituted membrane microdomains of glycosphingolipids (Gb3 and GM3). J. Virol. 73:5244-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hug, P., H. M. Lin, T. Korte, X. Xiao, D. S. Dimitrov, J. M. Wang, A. Puri, and R. Blumenthal. 2000. Glycosphingolipids promote entry of a broad range of human immunodeficiency virus type 1 isolates into cell lines expressing CD4, CXCR4, and/or CCR5. J. Virol. 74:6377-6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim, J., P. Griffin, D. R. Coombe, C. C. Rider, and W. James. 1999. Cell-surface heparan sulfate facilitates human immunodeficiency virus type 1 entry into some cell lines but not primary lymphocytes. Virus Res. 60:159-169. [DOI] [PubMed] [Google Scholar]
- 25.Joseph, J., F. D. Lublin, and R. L. Knobler. 1997. Modulation of T cell-endothelial adhesion by astrocyte conditioned medium. Glia 21:408-412. [PubMed] [Google Scholar]
- 26.Kozak, S. L., J. M. Heard, and D. Kabat. 2002. Segregation of CD4 and CXCR4 into distinct lipid microdomains in T lymphocytes suggests a mechanism for membrane destabilization by human immunodeficiency virus. J. Virol. 76:1802-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhmann, S. E., E. J. Platt, S. L. Kozak, and D. Kabat. 2000. Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J. Virol. 74:7005-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kure, K., W. D. Lyman, K. M. Weidenheim, and D. W. Dickson. 1990. Cellular localization of an HIV-1 antigen in subacute AIDS encephalitis using an improved double-labeling immunohistochemical method. Am. J. Pathol. 136:1085-1092. [PMC free article] [PubMed] [Google Scholar]
- 29.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retrovir. 17:1009-1019. [DOI] [PubMed] [Google Scholar]
- 30.Liu, N. Q., A. S. Lossinsky, W. Popik, X. Li, C. Gujuluva, B. Kriederman, J. Roberts, T. Pushkarsky, M. Bukrinsky, M. Witte, M. Weinand, and M. Fiala. 2002. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J. Virol. 76:6689-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, Y., X. P. Tang, J. C. McArthur, J. Scott, and S. Gartner. 2000. Analysis of human immunodeficiency virus type 1 gp160 sequences from a patient with HIV dementia: evidence for monocyte trafficking into brain. J. Neurovirol. 6(Suppl. 1):S70-S81. [PubMed] [Google Scholar]
- 32.Lossinsky, A. S., K. F. Buttle, R. Pluta, M. J. Mossakowski, and H. M. Wisniewski. 1999. Immunoultrastructural expression of intercellular adhesion molecule-1 in endothelial cell vesiculotubular structures and vesiculovacuolar organelles in blood-brain barrier development and injury. Cell Tissue Res. 295:77-88. [DOI] [PubMed] [Google Scholar]
- 33.Manes, S., G. Del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and C. Martinez-A. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manes, S., R. A. Lacalle, C. Gomez-Mouton, G. Del Real, E. Mira, and C. Martinez-A. 2001. Membrane raft microdomains in chemokine receptor function. Semin. Immunol. 13:147-157. [DOI] [PubMed] [Google Scholar]
- 35.Manes, S., E. Mira, C. Gomez-Mouton, R. A. Lacalle, P. Keller, J. P. Labrador, and C. Martinez-A. 1999. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 18:6211-6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markwell, M. A., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87:206-210. [DOI] [PubMed] [Google Scholar]
- 37.Mayor, S., S. Sabharanjak, and F. R. Maxfield. 1998. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 17:4626-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maziere, J. C., J. C. Landureau, P. Giral, M. Auclair, L. Fall, A. Lachgar, A. Achour, and D. Zagury. 1994. Lovastatin inhibits HIV-1 expression in H9 human T lymphocytes cultured in cholesterol-poor medium. Biomed. Pharmacother. 48:63-67. [DOI] [PubMed] [Google Scholar]
- 39.Moses, A. V., F. E. Bloom, C. D. Pauza, and J. A. Nelson. 1993. Human immunodeficiency virus infection of human brain capillary endothelial cells occurs via a CD4/galactosylceramide-independent mechanism. Proc. Natl. Acad. Sci. USA 90:10474-10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukhtar, M., H. Duke, M. BouHamdan, and R. J. Pomerantz. 2000. Anti-human immunodeficiency virus type 1 gene therapy in human central nervous system-based cells: an initial approach against a potential viral reservoir. Hum. Gene Ther. 11:347-359. [DOI] [PubMed] [Google Scholar]
- 41.Mukhtar, M., S. Harley, P. Chen, M. BouHamdan, C. Patel, E. Acheampong, and R. J. Pomerantz. 2002. Primary isolated human brain microvascular endothelial cells express diverse HIV/SIV-associated chemokine coreceptors and DC-SIGN and L-SIGN. Virology 297:78-88. [DOI] [PubMed] [Google Scholar]
- 42.Mukhtar, M., and R. J. Pomerantz. 2000. Development of an in vitro blood-brain barrier model to study molecular neuropathogenesis and neurovirologic disorders induced by human immunodeficiency virus type 1 infection. J. Hum. Virol. 3:324-334. [PubMed] [Google Scholar]
- 43.Nguyen, D. H., and D. Taub. 2002. CXCR4 function requires membrane cholesterol: implications for HIV infection. J. Immunol. 168:4121-4126. [DOI] [PubMed] [Google Scholar]
- 44.Nottet, H. S., Y. Persidsky, V. G. Sasseville, A. N. Nukuna, P. Bock, Q. H. Zhai, L. R. Sharer, R. D. McComb, S. Swindells, C. Soderland, and H. E. Gendelman. 1996. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J. Immunol. 156:1284-1295. [PubMed] [Google Scholar]
- 45.Nuovo, G. J., and M. L. Alfieri. 1996. AIDS dementia is associated with massive, activated HIV-1 infection and concomitant expression of several cytokines. Mol. Med. 2:358-366. [PMC free article] [PubMed] [Google Scholar]
- 46.Nuovo, G. J., F. Gallery, P. MacConnell, and A. Braun. 1994. In situ detection of polymerase chain reaction-amplified HIV-1 nucleic acids and tumor necrosis factor-alpha RNA in the central nervous system. Am. J. Pathol. 144:659-666. [PMC free article] [PubMed] [Google Scholar]
- 47.Ohagen, A., S. Ghosh, J. He, K. Huang, Y. Chen, M. Yuan, R. Osathanondh, S. Gartner, B. Shi, G. Shaw, and D. Gabuzda. 1999. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J. Virol. 73:897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohshiro, Y., T. Murakami, K. Matsuda, K. Nishioka, K. Yoshida, and N. Yamamoto. 1996. Role of cell surface glycosaminoglycans of human T cells in human immunodeficiency virus type-1 (HIV-1) infection. Microbiol. Immunol. 40:827-835. [DOI] [PubMed] [Google Scholar]
- 49.Parolini, I., S. Topa, M. Sorice, A. Pace, P. Ceddia, E. Montesoro, A. Pavan, M. P. Lisanti, C. Peschle, and M. Sargiacomo. 1999. Phorbol ester-induced disruption of the CD4-Lck complex occurs within a detergent-resistant microdomain of the plasma membrane. Involvement of the translocation of activated protein kinase C isoforms. J. Biol. Chem. 274:14176-14187. [DOI] [PubMed] [Google Scholar]
- 50.Persidsky, Y. 1999. Model systems for studies of leukocyte migration across the blood-brain barrier. J. Neurovirol. 5:579-590. [DOI] [PubMed] [Google Scholar]
- 51.Persidsky, Y., A. Ghorpade, J. Rasmussen, J. Limoges, X. J. Liu, M. Stins, M. Fiala, D. Way, K. S. Kim, M. H. Witte, M. Weinand, L. Carhart, and H. E. Gendelman. 1999. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am. J. Pathol. 155:1599-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Persidsky, Y., H. S. Nottet, V. G. Sasseville, L. G. Epstein, and H. E. Gendelman. 1995. The development of animal model systems for HIV-1 encephalitis and its associated dementia. J. Neurovirol. 1:229-243. [DOI] [PubMed] [Google Scholar]
- 53.Persidsky, Y., M. Stins, D. Way, M. H. Witte, M. Weinand, K. S. Kim, P. Bock, H. E. Gendelman, and M. Fiala. 1997. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J. Immunol. 158:3499-3510. [PubMed] [Google Scholar]
- 54.Pierini, L. M., and F. R. Maxfield. 2001. Flotillas of lipid rafts fore and aft. Proc. Natl. Acad. Sci. USA 98:9471-9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poland, S. D., G. P. Rice, and G. A. Dekaban. 1995. HIV-1 infection of human brain-derived microvascular endothelial cells in vitro. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:437-445. [DOI] [PubMed] [Google Scholar]
- 56.Popik, W., T. M. Alce, and W. C. Au. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells. J. Virol. 76:4709-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pumarola-Sune, T., B. A. Navia, C. Cordon-Cardo, E. S. Cho, and R. W. Price. 1987. HIV antigen in the brains of patients with the AIDS dementia complex. Ann. Neurol. 21:490-496. [DOI] [PubMed] [Google Scholar]
- 58.Resnick, L., J. R. Berger, P. Shapshak, and W. W. Tourtellotte. 1988. Early penetration of the blood-brain-barrier by HIV. Neurology 38:9-14. [DOI] [PubMed] [Google Scholar]
- 59.Rubin, L. L., and J. M. Staddon. 1999. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 22:11-28. [DOI] [PubMed] [Google Scholar]
- 60.Sachais, B. S., A. Kuo, T. Nassar, J. Morgan, K. Kariko, K. J. Williams, M. Feldman, M. Aviram, N. Shah, L. Jarett, M. Poncz, D. B. Cines, and A. A. Higazi. 2002. Platelet factor 4 binds to low-density lipoprotein receptors and disrupts the endocytic machinery, resulting in retention of low-density lipoprotein on the cell surface. Blood 99:3613-3622. [DOI] [PubMed] [Google Scholar]
- 61.Saphire, A. C., M. D. Bobardt, Z. Zhang, G. David, and P. A. Gallay. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 75:9187-9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi, B., U. De Girolami, J. He, S. Wang, A. Lorenzo, J. Busciglio, and D. Gabuzda. 1996. Apoptosis induced by HIV-1 infection of the central nervous system. J. Clin. Investig. 98:1979-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 64.Sorice, M., T. Garofalo, R. Misasi, A. Longo, V. Mattei, P. Sale, V. Dolo, R. Gradini, and A. Pavan. 2001. Evidence for cell surface association between CXCR4 and ganglioside GM3 after gp120 binding in SupT1 lymphoblastoid cells. FEBS Lett. 506:55-60. [DOI] [PubMed] [Google Scholar]
- 65.Sorice, M., I. Parolini, T. Sansolini, T. Garofalo, V. Dolo, M. Sargiacomo, T. Tai, C. Peschle, M. R. Torrisi, and A. Pavan. 1997. Evidence for the existence of ganglioside-enriched plasma membrane domains in human peripheral lymphocytes. J. Lipid Res. 38:969-980. [PubMed] [Google Scholar]
- 66.Stantchev, T. S., and C. C. Broder. 2001. Human immunodeficiency virus type-1 and chemokines: beyond competition for common cellular receptors. Cytokine Growth Factor Rev. 12:219-243. [DOI] [PubMed] [Google Scholar]
- 67.Tabas, I., Y. Li, R. W. Brocia, S. W. Xu, T. L. Swenson, and K. J. Williams. 1993. Lipoprotein lipase and sphingomyelinase synergistically enhance the association of atherogenic lipoproteins with smooth muscle cells and extracellular matrix. A possible mechanism for low density lipoprotein and lipoprotein(a) retention and macrophage foam cell formation. J. Biol. Chem. 268:20419-20432. [PubMed] [Google Scholar]
- 68.Tkachenko, E., and M. Simons. 2002. Clustering induces redistribution of syndecan-4 core protein into raft membrane domains. J. Biol. Chem. 277:19946-19951. [DOI] [PubMed] [Google Scholar]
- 69.Viard, M., I. Parolini, M. Sargiacomo, K. Fecchi, C. Ramoni, S. Ablan, F. W. Ruscetti, J. M. Wang, and R. Blumenthal. 2002. Role of cholesterol in human immunodeficiency virus type 1 envelope protein-mediated fusion with host cells. J. Virol. 76:11584-11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wyma, D. J., A. Kotov, and C. Aiken. 2000. Evidence for a stable interaction of gp41 with Pr55Gag in immature human immunodeficiency virus type 1 particles. J. Virol. 74:9381-9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang, H., O. Bagasra, M. Niikura, B. J. Pouiesz, and R. J. Pomerantz. 1994. Intravirion reverse transcripts in the peripheral blood plasma of human immunodeficiency virus type 1-infected individuals. J. Virol. 68:7591-7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang, H., G. Dornadula, M. Beumont, L. Livornese, Jr., B. Van Uitert, K. Henning, and R. J. Pomerantz. 1998. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N. Engl. J. Med. 339:1803-1809. [DOI] [PubMed] [Google Scholar]
- 73.Zink, W. E., J. Zheng, Y. Persidsky, L. Poluektova, and H. E. Gendelman. 1999. The neuropathogenesis of HIV-1 infection. FEMS Immunol. Med. Microbiol. 26:233-241. [DOI] [PubMed] [Google Scholar]