Abstract
Background:
Sleep-disordered breathing (SDB) is a treatable but markedly under-diagnosed condition of frequent breathing pauses during sleep. SDB is linked to incident cardiovascular disease, stroke, and other morbidity. However, the risk of mortality with untreated SDB, determined by polysomnography screening, in the general population has not been established.
Methods:
An 18-year mortality follow-up was conducted on the population-based Wisconsin Sleep Cohort sample (n = 1522), assessed at baseline for SDB with polysomnography, the clinical diagnostic standard. SDB was described by the number of apnea and hypopnea episodes/hour of sleep; cutpoints at 5, 15 and 30 identified mild, moderate, and severe SDB, respectively. Cox proportional hazards regression was used to estimate all-cause and cardiovascular mortality risks, adjusted for potential confounding factors, associated with SDB severity levels.
Results:
All-cause mortality risk, adjusted for age, sex, BMI, and other factors was significantly increased with SDB severity. The adjusted hazard ratio (HR, 95% CI) for all-cause mortality with severe versus no SDB was 3.0 (1.4,6.3). After excluding persons who had used CPAP treatment (n = 126), the adjusted HR (95% CI) for all-cause mortality with severe versus no SDB was 3.8 (1.6,9.0); the adjusted HR (95% CI) for cardiovascular mortality was 5.2 (1.4,19.2). Results were unchanged after accounting for daytime sleepiness.
Conclusions:
Our findings of a significant, high mortality risk with untreated SDB, independent of age, sex, and BMI underscore the need for heightened clinical recognition and treatment of SDB, indicated by frequent episodes of apnea and hypopnea, irrespective of symptoms of sleepiness.
Citation:
Young T; Finn L; Peppard PE; Szklo-Coxe M; Austin D; Nieto FJ; Stubbs R; Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the wisconsin sleep cohort. SLEEP 2008;31(8):1071-1078.
Keywords: Sleep-disordered breathing, sleep apnea, all-cause mortality, cardiovascular mortality, cohort
UNDERSTANDING THE HEALTH BURDEN OF SLEEP DISORDERED BREATHING (SDB), A HIGHLY PREVALENT CONDITION OF FREQUENT, INTERMITTENT breathing pauses during sleep, is critical.1–4 The prevalence of moderate or severe SDB is estimated to be at least 6% for US adults; moreover, 17% of adults are estimated to have mild or worse SDB.5 Prevalence will undoubtedly rise, because obesity, a causal factor for SDB, is increasing dramatically in adults and children.5 Despite the availability of effective treatment for SDB, and evidence that SDB contributes to significant morbidity, at least 75% of cases of severe SDB remain undiagnosed.4,6
Cost-effective screening and treatment recommendations to decrease the burden of SDB depend on the overall magnitude of adverse outcomes, including mortality, attributable to untreated SDB. Although numerous prospective7–10 and cross-sectional studies11–16 have linked SDB to hypertension, cardiovascular disease, depression, motor vehicle crashes, cognitive impairment, and diminished quality of life, definitive evidence on the role of SDB in increased mortality, a major component of the total burden of disease, is lacking.
Most previous studies of SDB and mortality have been based on clinic patient samples.17–23 Although many studies of sleep clinic patients report an increased mortality risk associated with SDB, interpretation is limited due to biases in referral of patients for evaluation. Of particular concern, referral for SDB evaluation is more likely to occur in patients under treatment for other serious morbidity. Although this type of bias would lead to overestimation of a mortality risk with SDB, other clinic-related biases, including lack of appropriate comparison groups, may lead to underestimation of risk. Mortality estimates from large population samples with SDB determined by polysomnography have not been reported. In the few mortality studies of older adults with objectively measured SDB, results are inconsistent.24–26
We hypothesized that SDB contributes to all-cause mortality. SDB could directly contribute to mortality through a possible causal role in hypertension, coronary heart disease or stroke. Similarly, the behavioral morbidity and daytime impairment of SDB may increase mortality by contributing to fatal injuries including suicide. Nightly exposure to the acute effects of apnea and hypopnea, including hypoxia, increased sympathetic drive, hemodynamic stress, including large swings in blood pressure and heart rate, and increased negative intrathoracic pressure may contribute indirectly to mortality by exacerbating a wide range of comorbid conditions.1 Furthermore, the daytime impairment associated with SDB may decrease motivation to maintain a healthy life style and may diminish compliance with therapy for comorbid conditions. Consequently, we sought to estimate the all-cause mortality associated with SDB, with 18-year follow-up of 1522 adults assessed for SDB with in-laboratory polysomnography at baseline. In addition, we investigated the risk of cardiovascular and cerebrovascular mortality with SDB and explored use of continuous positive air pressure therapy (CPAP), the most common treatment for SDB, as a modifier of mortality risk.
METHODS
Sample
The Wisconsin Sleep Cohort Study, established in 1988, is a prospective population-based study of the natural history of SDB. The study has been reviewed and approved by the University of Wisconsin Institutional Review Board. The sample is based on a two-stage weighted sampling procedure, previously described.27 In brief, a random sample of men and women, ages 30–60, was recruited from a sampling frame of payroll records of several Wisconsin state agencies, with a complete range of job titles from unskilled to professional, for a survey of sleep characteristics and other factors.
Of 2940 survey participants invited to participate in the overnight polysomnography protocol, 1546 agreed (53%). Potential participation bias was evaluated with data on the entire sampling frame and survey respondents.8,27,28 Participants, compared with nonparticipants, had a slightly healthier profile, including less hypertension. Consistent with a healthy volunteer bias, mortality follow-up (to March 2008) of the entire sampling frame showed a lower death rate among the cohort participants, compared to non-participants. After exclusion of two persons who died within one year of the baseline study and 22 with inadequate polysomnography data, the sample comprised 1522 persons.
It is important to clarify that the Wisconsin Sleep Cohort is an observational study of a random sample of community adults.27 Thus, cohort participants are not patients referred for a clinical sleep diagnostic evaluation. Participants are not given diagnoses and are not treated or referred to a specific clinic for treatment. A passive method of informing participants of their polysomnography findings is used. Participants are given a summary report of their sleep study, with the number of apneas and hypopneas detected during their study and other details about their sleep duration and sleep architecture. The report is also sent to the participant’s health care provider. The report stresses that this is a research study with the aim of determining how SDB is related to adverse health outcomes and thus test results cannot be considered a diagnosis, and participants should contact their doctor if they have unsatisfactory sleep regardless of their study report. However, participants with an AHI of 15 or greater are advised that frequent breathing pauses are linked to health problems and they are advised to talk to their health care provider about their sleep report.
Data Collection
Baseline Assessment
The overnight protocol, described previously,27 was conducted in home-like bedrooms at the University of Wisconsin General Clinical Research Center. Upon arrival participants were given an orientation and informed written consent was obtained. After completion of the evening protocol, participants were encouraged to follow their usual sleep and wake times. Data for this investigation included responses to queries on life style, sleep habits, medical history, measures of weight (kg), height (cm), and girths of neck, waist, and hips (cm),29 multiple measures of blood pressure while seated, and polysomnographic assessment of SDB.
Eighteen-channel polysomnography (Grass model 78; Quincy, MA) recorded sleep state, using electroencephalography, electro-oculography, and electromyography; breathing, using respiratory inductance plethysmography (Respitrace; Ambulatory Monitoring, Ardsley, NY), nasal and oral airflow (ProTec thermocouples; Hendersonville, TN and Validyne Engineering Corp pressure transducer, Northridge, CA) and oxyhemoglobin saturation, using pulse oximetry (Ohmeda Biox 3740; Englewood, CO). Each 30-second epoch of the polysomnographic recordings was scored for sleep stage and apnea and hypopnea events by trained technicians and reviewed using standard criteria.30,31 Apnea was defined as cessation of airflow for ≥ 10 seconds and hypopnea was defined as a discernible reduction in breathing (sum of chest and abdominal excursions) with a reduction in oxyhemoglobin saturation of ≥ 4%. The summary parameter for SDB was mean number of apnea and hypopnea events/hour of sleep (apnea-hypopnea index, AHI).
Mortality Follow-up
Deaths in the cohort occurring up to March 1, 2008 were identified by matching social security numbers with 2 death record sources: the US Social Security Death Index (SSDI) and the Wisconsin State Bureau of Health Information and Policy, Vital Records Section. Matches on social security number were verified with participants’ age and sex. All deaths in Wisconsin (WI) were identified in the SSDI; in addition, 4 deaths occurring outside of WI were identified by the SSDI. Date of death was available on all decedents, and underlying and contributory causes of deaths were available from WI Vital Statistics. WI Vital statistics supplied a file with data on each individual death, including the ICD-9 codes and corresponding description as abstracted from individual death certificates. A sample of 30 death certificates was compared with abstracted records for accuracy, with 100% agreement. Cause of death was lacking for 3 of the 4 out of state deaths and 2 of the WI deaths.
Data Analysis
Variables
Sleep Disordered Breathing Status
Widely-used cutpoints, chosen a priori, were used to create the following SDB severity categories: No SDB (AHI < 5); Mild (AHI ≥ 5 < 15); Moderate (AHI ≥ 15 < 30); and Severe (AHI ≥ 30).
Mortality Status
Based on deaths recorded to March 2008, vital status was coded as alive or dead. Underlying and contributing causes categorized with ICD-9 coding, were used to create the following cause-specific death categories:
1) Cardiovascular and stroke, including acute myocardial infarction, acute ischemic heart disease, heart failure with underlying chronic ischemic heart disease, stroke, atrial fibrillation and flutter, sudden cardiac arrest, cardiac dysrhythmias with underlying chronic ischemic heart disease, cardiomyopathy, and pulmonary hypertension;
2) Fatal events, including motor vehicle and motorcycle accidents, falls, injuries, and suicide;
3) Cancer, including cancer of the lung, bladder, breast, brain, and leukemia;
4) Other.
Covariables
Variables were created for: alcohol use (number of drinks/week), cigarette smoking (current or not), educational level (6 categories of school attainment), cardiovascular disease, stroke, diabetes (based on self-report of physician-diagnosis), hypertension (based on average of 3 measures of BP: systolic BP ≥ 140 mm Hg or diastolic ≥ 90 mm Hg or use of antihypertensive medication), self-rated general health (excellent, very good, good, fair, poor), and CPAP use for SDB (based on “usual use” at least 4 nights/week, reported at baseline or any follow-up visit). Variables for excessive daytime sleepiness included having excessive daytime sleepiness that interferes with daily living (yes, no), usual frequency (5 semi-quantitative categories) of excessive daytime sleepiness, and of feeling unrested, regardless of sleep duration. BMI (weight in kilograms divided by height in meters squared), neck girth, waist-hip ratio, total cholesterol, sleep duration (min/night), and age (y) at baseline were continuous variables.
Statistical Analysis
SAS software (SAS Institute, Version 9.1.3) and S plus (version 8) were used. Person-years were accumulated from baseline study to date of death or March 1, 2008. We computed all-cause and cardiovascular mortality rates (deaths/1000 person-years) and 95% confidence intervals (CI) by SDB categories at baseline. Kaplan-Meier techniques were used to compare survival by SDB categories, with log-rank test to assess differences.32
Cox proportional hazards regression was used to estimate adjusted hazard ratios and 95% CIs using SAS PHREG software. Trends were examined by testing the significance of SDB categories as a linear term. With multiple variable models, we examined sex, linear and quadratic terms for age and BMI, waist-hip ratio, neck girth, smoking, alcohol use, educational status, general heath status, total cholesterol, and sleep duration as potential confounding factors. Diagnosed disorders were explored as possible mediating factors. Interactions of SDB with age, sex, and excessive daytime sleepiness in predicting mortality were examined. Analyses were repeated after excluding participants who reported use of CPAP. Diagnostic and residual plots were examined to test the proportional hazards assumptions. In addition, mortality rates and hazard ratios were also estimated after accounting for the sample weighting. The estimates from the weighted and unweighted analyses did not differ.
RESULTS
Baseline characteristics of the sample and causes of death are shown in Table 1. The mean observation period of the cohort of 1522 men and women was 13.8 years (range, 1.5–18.7), for a total of 20,963 person-years. The average age at death was 61.1 years (range, 34–75). Cardiovascular mortality accounted for 26% of all deaths among persons without SDB at baseline and 42% of all deaths in persons with severe SDB at baseline.
Table 1.
Description of Sample (n = 1522) by Sleep-Disordered Breathing Category
| Baseline characteristics | Total n = 1522 |
Apnea Hypopnea Index Category |
|||
|---|---|---|---|---|---|
| None < 5 n = 1157 |
Mild 5 – < 15 n = 220 |
Moderate 15 – < 30 n = 82 |
Severe ≥ 30 (range, 30–97) n = 63 |
||
| Continuous variables, mean (SD) | |||||
| Age, y | 48 (8) | 47 (8) | 50 (8) | 50 (8) | 50 (9) |
| Body mass index, median kg/m2 | 28.6 | 27.6 | 31.5 | 33.3 | 37.2 |
| Alcoholic drinks/week | 2 (6) | 2 (6) | 2 (6) | 2 (4) | 3 (9) |
| Binary variables, n (%) | |||||
| Current smoker | 274 (18) | 214 (19) | 36 (16) | 12 (15) | 12 (19) |
| Sex, male | 839 (55) | 589 (51) | 142 (65) | 59 (72) | 49 (78) |
| Cardiovascular disease* | 54 (4) | 30 (3) | 11 (5) | 8 (10) | 5 (8) |
| Hypertension* | 495 (33) | 312 (27) | 95 (43) | 46 (56) | 42 (69) |
| Diabetes* | 50 (3) | 27 (2) | 11 (5) | 5 (6) | 7 (11) |
| Stroke* | 8 (0.5) | 4 (0.4) | 0 (0) | 0 (0) | 4 (6) |
| Excessive daytime sleepiness | 371 (25) | 266 (23) | 54 (25) | 81 (35) | 23 (37) |
| Education beyond high school | 1124 (74) | 876 (76) | 149 (69) | 57 (70) | 42 (68) |
| Very good or excellent health** | 1001 (69) | 808 (74) | 130 (61) | 37 (48) | 26 (43) |
| Deaths | |||||
| Cause-specific, n (% of total deaths in AHI category) | |||||
| Cardiovascular disease and Stroke | 25 (31) | 12 (26) | 6 (38) | 2 (33) | 5 (42) |
| Cancer | 37 (46) | 22 (48) | 6 (38) | 4 (67) | 5 (42) |
| Injury, suicide | 7 (9) | 4 (9) | 2 (12.5) | 0 (0) | 1 (8) |
| Other | 6 (8) | 4 (9) | 2 (12.5) | 0 (0) | 0 (0) |
| Unknown | 5 (6) | 4 (9) | 0 (0) | 0 (0) | 1 (8) |
| Total deaths, n (%) | 80 (100) | 46 (100) | 16 (100) | 6 (100) | 12 (100) |
physician-diagnosed condition, self reported at baseline protocol
Self-rated health evaluation
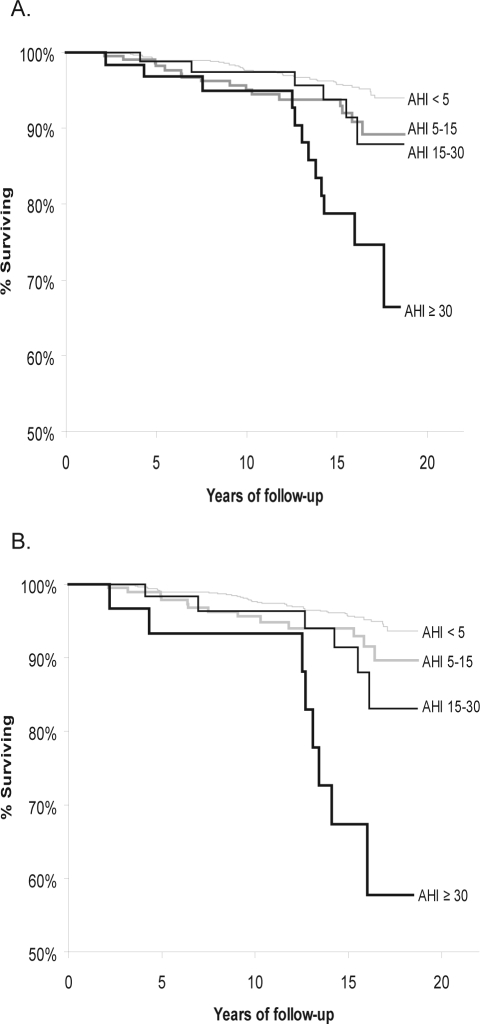
Kaplan-Meier survival curves (Figure 1) illustrate the decrease in survival with increasing SDB category in the total sample of 1522 participants (Panel A) and in the sample after excluding participants (n = 126) who had reported CPAP use (Panel B). Survival was significantly lower with increasing SDB severity (P < 0.0001). The decrease in survival with SDB not treated with CPAP was markedly greater.
Figure 1.
Kaplan-Meier estimates of survival probability according to sleep-disordered breathing severity for A) Total sample and B) Sample excluding 126 CPAP treated participants.
A. Kaplan-Meier estimates of the probability of survival (shown with y-axis truncated at 50% survival) according to sleep disordered breathing severity: none (AHI < 5), mild (AHI > 5, < 15), moderate (AHI > 15, < 30) and severe (AHI > 30), total sample (n = 1522); long-rank test for differences in survival by SDB category: P < 0.00001; AHI is mean number of apnea and hypopnea episodes/hr of sleep.
B. Kaplan-Meier estimates of the probability of survival (shown with y-axis truncated at 50% survival) according to untreated* sleep-disordered breathing category (none (AHI < 5), mild (AHI > 5, < 15), moderate (AHI > 15, < 30) and severe (AHI > 30), *participants who had used CPAP were excluded (n = 1396); long-rank test for differences in survival by SDB category: P < 0.00001; AHI is mean number of apnea and hypopnea episodes/hr of sleep.
The all-cause mortality rate /1000 person-years (Table 2) for those with either mild or moderate SDB (AHI 5–15 and 15–30) at baseline were nearly double the rate among those without SDB (AHI < 5). The all-cause death rate was highest for severe SDB, at 14.6/1000 person-years. There was a clear trend of increased cardiovascular mortality with increasing severity of baseline SDB.
Table 2.
All-Cause and Cause-Specific Mortality Rates Per 1000 Person-Years
| Baseline AHI category | n | All-cause mortality |
Cardiovascular mortality |
||
|---|---|---|---|---|---|
| Deaths n (%) | Rate per 1000 person-years (95% CI) | Deaths n (%) | Rate per 1000 person-years (95% CI) | ||
| None: 0 – < 5 | 1157 | 46 (4.0) | 2.85 (2.09, 3.80) | 12 (1.0) | 0.74 (0.38, 13.0) |
| Mild: 5 – < 15 | 220 | 16 (7.3) | 5.54 (3.16, 8.99) | 6 (2.7) | 2.08 (0.76, 4.52) |
| Moderate: 15 – < 30 | 82 | 6 (7.3) | 5.42 (1.99, 11.8) | 2 (2.4) | 1.81 (0.22, 6.53) |
| Severe: ≥ 30 | 63 | 12 (19.1) | 14.6 (7.52, 25.4) | 5 (7.9) | 6.07 (1.97, 14.1 ) |
Hazard ratios for all-cause mortality, adjusted for sex, and linear and quadratic terms for age and BMI, were significantly increased with SDB severity (P-trend = 0.008) (Table 3). The adjusted hazard ratio (95% CI) for all-cause mortality for severe versus no SDB at baseline was 3.0 (1.4, 6.3). These hazard ratios were not decreased after further adjustment for smoking, alcohol use, general health status, educational status, neck girth, waist-hip ratio, sleep duration, and total cholesterol; the fully adjusted hazard ratio (95% CI) for mortality with severe vs no SDB was 5.9 (2.6, 13.3). We did not consider this model robust, for several reasons, including multicollinearity and potential model instability due to outliers and influential points (of great concern with a small number of outcomes). With 80 deaths as the outcome, we chose the parsimonious model with adjustment for BMI, BMI-squared (BMI2), age, age-squared (age2), and sex as the basic model for other analyses. However, we describe the above expanded model to show that the hazard ratio for mortality with SDB in the basic model is not overestimated by failure to include common risk factors for mortality. In addition, mortality risk in our data was increased by the hallmark risk factors for mortality, including age, smoking, CVD risk factors, and general health status, increasing confidence in generalizability.
Table 3.
Mortality Risk with Sleep-Disordered Breathing (n = 1522): Adjusted Hazard Ratios*
| Baseline AHI category | All-cause mortality Hazard Ratio (95% CI) | Cardiovascular mortality Hazard Ratio (95% CI) |
|---|---|---|
| None: 0 – < 5 | Reference | Reference |
| Mild: 5 – < 15 | 1.6 (0.9, 2.8) | 1.8 (0.7, 4.9) |
| Moderate: 15 – < 30 | 1.4 (0.6, 3.3) | 1.2 (0.3, 5.8) |
| Severe: ≥ 30 | 3.0 (1.4, 6.3) | 2.9 (0.8, 10.0) |
| P trend = 0.008 | P trend = 0.12 |
adjusted for age, age2, sex, body mass index, and body mass index2
AHI denotes number of apnea and hypopnea events per hour of sleep, CI denotes confidence interval
We explored the potential intermediary effects of chronic disorders as pathways from SDB to death, by examining the effect on the hazard ratio for all-cause mortality with SDB by adding variables for hypertension and physician-diagnosed CVD, stroke, and diabetes to the regression model simultaneously and singly. As seen in Table 4, the all-cause mortality hazard ratio with severe SDB changed from 3.0 to 2.7, (P = 0.01), indicating the net mortality risk with severe SDB explained by chronic disorders was less than might be expected. Adjusted hazard ratios for mortality associated with mild or moderate SDB were negligible. After single addition of the intermediary variables to examine the effect of each comorbid condition, adjusted hazard ratios for mortality with severe versus no SDB were: 3.1 (P = 0.003) after adding cardiovascular disease; 2.9 (P < 0.001) after adding stroke; 2.7 (P = 0.01) after adding hypertension; and 2.4 (P = 0.03) after adding diabetes. In addition to interest in how the SDB-mortality risk is explained by overt, diagnosed disorders, it is possible that the comorbidity is a marker for unmeasured confounding factors, or that stroke or CVD cause SDB. To address these potentially ambiguous cause-effect relationships, 8 participants reporting a stroke prior to baseline evaluation and 43 reporting CVD were excluded from the regression analyses (Table 5). Hazard ratios for mortality according to SDB status were not affected by this exclusion.
Table 4.
Mortality Risk with Sleep-Disordered Breathing (n = 1522): Influence of Comorbidity
| Baseline AHI category | Hazard ratio* for all-cause mortality, accounting for comorbidity Hazard Ratio (95% CI) |
|---|---|
| None: 0 – < 5 | Reference |
| Mild: 5 – < 15 | 1.5 (0.8, 2.8) |
| Moderate: 15 – < 30 | 1.3 (0.5, 3.2) |
| Severe: ≥ 30 | 2.7 (1.3, 5.7) |
| P-trend = 0.01 |
model includes variables for age, age2, sex, body mass index, body mass index2, hypertension (BP ≥ 140 systolic or ≥ 90 diastolic or use of antihypertensive medication), self-reported diagnosis of diabetes, coronary artery disease, cardiovascular disease, heart failure, myocardial infarction, cardiac surgery, and stroke.
AHI denotes number of apnea and hypopnea events per hour of sleep, CI denotes confidence interval
Table 5.
Risk* of All-Cause Mortality with Sleep-Disordered Breathing Among Participants Free of Diagnosed Stroke and Cardiovascular Disease at Baseline (n = 1471)**
| Baseline AHI category | Hazard Ratio (95% CI) |
|---|---|
| None: 0 – < 5 | Reference |
| Mild: 5 – < 15 | 1.5 (0.8, 2.8) |
| Moderate: 15 – < 30 | 1.3 (0.5, 3.5) |
| Severe: ≥ 30 | 3.2 (1.4, 7.1) |
| P trend = 0.01 |
Hazard ratios adjusted for age, age2, sex, body mass index, and body mass index2
Participants with self-reported diagnosis of stroke (n = 8) and CVD (n = 43) excluded from sample
AHI denotes number of apnea and hypopnea events per hour of sleep, CI denotes confidence interval
We found no statistically significant interactions between SDB and age, sex, or excessive daytime sleepiness with regard to mortality risk (all P > 0.1). Because the lack of interaction of SDB and sleepiness with regard to mortality risk has implications regarding treatment of SDB patients who are not sleepy, we compared the adjusted hazard ratios for morbidity with SDB after stratification on several sleepiness variables defined by frequency of “feeling excessive daytime sleepiness,” frequency of “not feeling rested regardless of hours of sleep duration,” and experiencing “extreme sleepiness, beyond fatigue, that interferes with daily life.” Regression performed on each strata showed severe SDB either with or without sleepiness was associated with increased mortality: adjusted hazard ratios for mortality with severe SDB ranged from 2.0–3.9 for the “sleepy” strata, and from 2.8–3.8 for the “not sleepy” strata.
Although cardiovascular death rates were elevated for those with SDB at baseline, the adjusted hazard ratios for cardiovascular mortality with SDB were not statistically significant (Table 3).
All associations between SDB and mortality were markedly stronger after excluding 126 participants who had reported regular CPAP use (Table 6). Compared to individuals without SDB, the adjusted mortality risks were highest for those with severe SDB who had not used CPAP: the hazard ratio for all-cause mortality was 3.8 (95% CI, (1.6, 9.0) and the hazard ratio (95% CI) for cardiovascular mortality was 5.2 (1.4, 19.2).
Table 6.
| Baseline AHI category | All-cause mortality Hazard Ratio (95% CI) | Cardiovascular mortality Hazard Ratio (95% CI) |
|---|---|---|
| None: 0 – < 5 | Reference | Reference |
| Mild: 5 – < 15 | 1.4 (0.7, 2.6) | 1.3 (0.4, 4.1) |
| Moderate: 15 – < 30 | 1.7 (0.7, 4.1) | 1.5 (0.3, 7.3) |
| Severe: ≥30 | 3.8 (1.6, 9.0) | 5.2 (1.4, 19.2) |
| P trend = 0.004 | P-trend = 0.03 |
Hazard ratios adjusted for age, age2, sex, body mass index, and body mass index2
126 persons who reported usual use of continuous positive air pressure (CPAP) ≥ 4 nights per week were excluded from sample
AHI denotes number of apnea and hypopnea events per hour of sleep, CI denotes confidence interval
DISCUSSION
Mortality follow-up of the Wisconsin Sleep Cohort, comprising 20,963 person-years, indicates that severe SDB is significantly associated with a 3-fold increased all-cause mortality risk (P < 0.0008), independently of age, sex, BMI, and other potential confounders. After excluding persons who had reported using CPAP, the associations were even more striking: adjusted hazard ratios (95% CI) for all-cause mortality comparing participants with severe SDB to those without SDB were 3.8 (95% CI, [1.6, 9.0]) Similarly, after excluding persons who had used CPAP from the sample, the hazard ratio for cardiovascular mortality increased in magnitude and statistical significance, to 5.2 (1.4, 19.2). Although mortality risk was 50% greater for those with mild and moderate SDB compared to those without SDB, the estimates were not statistically significant.
Although most previous reports support a role of SDB in mortality, interpretation has been limited by concern with clinic referral biases. Our findings, based on mortality follow-up of a general population cohort with polysomnographically determined SDB at baseline, affirm mortality as a significant part of the public health burden of SDB.
In the first follow-up study of sleep apnea patients, He et al17 reported that premature death was associated with severe sleep apnea (> 20 vs < 20 apnea/hour), but survival was not decreased for patients treated with CPAP. Although limited by small sample size, clinical referral biases, and incomplete follow-up, the alarming findings of this study made further investigation of a sleep apnea-mortality link imperative. Since then, clinic studies with larger sample sizes, adjustment for confounding factors, and more complete follow-up continue to find an increased mortality risk with SDB.18,20–23 In the largest clinic sample conducted on men, the mortality risk differed by comparison group: mortality rates of clinic patients compared with population statistics were higher only for men less than 50 years of age, but the all-cause mortality hazard ratio was significantly greater for patients with > 30 vs < 10 respiratory disturbances/hour.19 Thus, concerns with referral biases and lack of optimal comparison groups remain, limiting interpretation of findings from patient samples. Although a bias favoring evaluation of patients with both SDB and significant comorbidity is of most concern, potentially causing overestimation of risk, other biases associated with time period, referral patterns, and SDB awareness may lead to underestimation of risk.
The consistency of our population-based findings of elevated mortality risk for severe SDB with the results of clinic-based studies over the past 20 years increases confidence in the validity of a significant role of SDB in mortality. In addition to supporting the findings of He et al,17 our findings are similar to those from 2 recent sleep clinic-based studies using the same polysomnographic methods and apnea and hypopnea definitions as used in our study. Yaggi et al,22 in a 3.5-year follow-up of a large sample of patients evaluated for sleep apnea, found a 2-fold increased adjusted risk for either incident stoke or death for patients diagnosed with sleep apnea (mean AHI = 35) versus not diagnosed (mean AHI = 2). Marin et al21 reported 3-fold greater odds of cardiovascular mortality in patients with severe sleep apnea, compared with community controls and patients without SDB.
We found a significant, strong increase in cardiovascular mortality (adjusted hazard ratio (95% CI) = 5.2 [1.4, 19.2]) with severe SDB only after CPAP users were excluded, suggesting that CPAP was protective particularly against cardiovascular death. This finding is consistent with results of several clinic studies reporting greater mortality risk with untreated SDB among cardiovascular and stroke patients.33–36 Similarly, recent clinical studies of patients with both SDB and cardiovascular or stroke morbidity suggest CPAP decreases heart failure and stroke hospitalization time, and improves survival.1 In addition, there is evidence that CPAP use may reduce hypertension risk.37
Although our findings are consistent with a protective effect of CPAP against mortality, it is important to note limitations in our data on CPAP use, including lack of information on consistent use over time or effectiveness of air pressure level to prevent airway closure. Participants with SDB detected in our study are not diagnosed or randomized to treatment. Participants who independently choose to seek care and treatment may have health characteristics that differ from those who do not seek care. Thus, CPAP use may be a marker of increased healthy behaviors that protect against death. Consequently, our data cannot establish how CPAP contributes to lower death rates.
We explored baseline medical disorders as possible pathways through which SDB may contribute to mortality. The association between SDB and mortality was only partly explained by hypertension and clinically diagnosed cardiovascular disease, diabetes, or stroke. However, without knowledge of appropriate latent or induction periods over which SDB may affect new or underlying morbidity, it is difficult to speculate on mechanisms through which SDB contributed to mortality in our data. The average time to death for participants with SDB was 11.8 years, indicating that SDB is not an acutely fatal disorder. Furthermore, we do not know how long participants had SDB prior to their baseline study, so we cannot validly estimate the time course from baseline to death with these data.
Our findings are based on a cohort with little ethnic diversity (95% white) and persons who were employed at recruitment. While validity in our findings with respect to white adults with adequate income and access to medical care is increased by the homogeneity of the sample, it is likely that our findings may underestimate the mortality risk of SDB in other ethnic groups or the lowest socioeconomic strata where there is poor awareness and access to health care.
The benefit of treating SDB patients who are not sleepy is controversial.38 Furthermore, studies have indicated that sleepiness is not a common SDB symptom in patients with both cardiovascular disease and SDB.39 Our analyses indicated that SDB, irrespective of excessive sleepiness symptoms, was associated with increased mortality. This finding, and the striking high cardiovascular mortality risk in untreated severe SDB, suggests that SDB treatment should not be contingent on daytime sleepiness symptoms.
CONCLUSIONS
Eighteen-year follow-up of the Wisconsin Sleep Cohort provides new insights on mortality as part of the public health burden of SDB. Participants with severe SDB at baseline had a statistically significant (P < 0.01) 3-fold greater risk (with 95% confidence interval ranging from 1.4–6.3) of all-cause mortality compared to those without SDB, independent of sex, BMI, age, and other potential confounders. However, it is possible that residual confounding due to unmeasured or inadequately measured factors contributes to bias in the estimate. Given the inevitable increase in SDB prevalence with the contemporary epidemic of obesity, the 4- to 5-fold increase in all-cause and cardiovascular mortality associated with untreated, severe SDB is of high concern. Although further studies are needed to quantify the proportion of mortality that could be lowered by prevention or treatment of SDB, the results of our study can be applied directly to current health care practice. Our findings of significant mortality risk with untreated SDB, in conjunction with prior evidence that CPAP can effectively treat severe SDB, underscore the immediate need for heightened clinical recognition and treatment of SDB, indicated by frequent episodes of apnea and hypopnea, irrespective of symptoms of sleepiness.
ACKNOWLEDGMENTS
We thank Kathryn Pluff, Linda Evans, Amanda Rasmuson, and Katherine Kenison for their technical expertise and Joyce Knapton for assistance with Wisconsin vital records.
Study performed at the University of Wisconsin-Madison, School of Medicine and Public Health, Madison WI. Financial Support: NIH grants R01:HL62252, R01:AG14124, and RR:03186.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: American Heart Association Scientific Statement. Circulation. 2008. Epub June 17. [DOI] [PubMed]
- 2.Krieger J, McNicholas WT, Levy P, et al. Public health and medicolegal implications of sleep apnoea. Eur Respir J. 2002;20:1594–609. doi: 10.1183/09031936.02.00404502. [DOI] [PubMed] [Google Scholar]
- 3.Colten HR, Altevogt BM, editors. Washington DC: Institute of Medicine /National Academies Press; 2006. Sleep disorders and sleep deprivation: an unmet public health problem/Committee on Sleep Medicine and Research. Board on Health Sciences Policy; pp. 20–32. [PubMed] [Google Scholar]
- 4.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 7.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: A long-term follow-up. Eur Respir J. 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 9.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–15. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 11.Robbins J, Redline S, Ervin A, Walsleben JA, Ding J, Nieto FJ. Association of sleep-disordered breathing and cerebral changes on MRI. J Clin Sleep Med. 2005;1:159–65. [PubMed] [Google Scholar]
- 12.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 14.Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. 2004;169:354–60. doi: 10.1164/rccm.200306-756OC. [DOI] [PubMed] [Google Scholar]
- 15.Finn L, Young T, Palta M, Fryback DG. Sleep-disordered breathing and self-reported general health status in the Wisconsin Sleep Cohort Study. Sleep. 1998;21:701–6. [PubMed] [Google Scholar]
- 16.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 17.He J, Kryger MH, Zorick FJ, Conway W, Roth T. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- 18.Young T, Finn L. Epidemiological insights into the public health burden of sleep disordered breathing: sex differences in survival among sleep clinic patients. Thorax. 1998;53(Suppl 3):S16–9. doi: 10.1136/thx.53.2008.s16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J. 2005;25:514–20. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]
- 20.Campos-Rodriguez F, Peña-Griñan N, Reyes-Nuñez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128:624–33. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 21.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 22.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 23.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 24.Bliwise DL, Partinen M, Pursley AM, Dement WC. Sleep apnea and mortality in an aged cohort. Am J Public Health. 1988;78:544–7. doi: 10.2105/ajph.78.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ancoli-Israel S, DuHamel ER, Stepnowsky C, Engler R, Cohen-Zion M, Marler M. The relationship between congestive heart failure, sleep apnea, and mortality in older men. Chest. 2003;124:1400–5. doi: 10.1378/chest.124.4.1400. [DOI] [PubMed] [Google Scholar]
- 26.Ancoli-Israel S, Klauber MR, Kripke DF, Parker L, Cobarrubias M. Sleep apnea in female patients in a nursing home. Increased risk of mortality. Chest. 1989;96:1054–8. doi: 10.1378/chest.96.5.1054. [DOI] [PubMed] [Google Scholar]
- 27.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 28.Young T, Hutton R, Finn L, Badr S, Palta M. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med. 1995;156:2445–51. [PubMed] [Google Scholar]
- 29.Lohman TG, Roche AF, Martorell R. Champaign, IL: Human Kinetics; 1988. Anthropometric Standardization reference manual. [Google Scholar]
- 30.Rechtschaffen A, Kales AA. Los Angeles, CA: U.S. Government Printing Office; 1968. A manual of standardized terminology techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 31.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 33.Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161(2 Pt 1):375–80. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–31. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 35.Cassar A, Morgenthaler TI, Lennon RJ, Rihal CS, Lerman A. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:1310–4. doi: 10.1016/j.jacc.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Olson LJ, Somers VK. Sleep apnea: implications for heart failure. Curr Heart Fail Rep. 2007;4:63–9. doi: 10.1007/s11897-007-0002-9. [DOI] [PubMed] [Google Scholar]
- 37.Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database of Syst Rev. 2006. p. CD001106. [DOI] [PubMed]
- 38.Lévy P, Pépin JL, McNicholas WT. Should all sleep apnoea patients be treated? Yes. Sleep Med Rev. 2002;6:17–26. doi: 10.1053/smrv.2002.0209. [DOI] [PubMed] [Google Scholar]
- 39.Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–22. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]