Abstract
Study Objectives.
Sleep propensity and skin temperature are functionally related. In young adults, changes of skin temperature within the comfortable thermoneutral zone affect sleep-onset latency and vigilance performance. Aging is associated with both decreased thermosensitivity and poorer sleep. Our goal was to test whether subtle manipulations of core body and skin temperature affect sleep onset in elderly people without sleep complaints and in elderly insomniacs and whether the subjective perception of these mild body temperature manipulations is preserved with aging and insomnia.
Design:
In a 2-day semiconstant–routine protocol, 288 sleep-onset latencies were polysomnographically determined while manipulating core body and skin temperatures differentially in warm and cold directions within a comfortable thermoneutral range.
Setting:
Sleep laboratory of the Netherlands Institute for Neuroscience.
Patients or Participants:
Eight elderly subjects without sleep complaints (65.8 ± 2.8 years, mean ± SEM) and 8 elderly insomniacs (59.1 ± 1.9 years).
Measurements and Results:
Warming the proximal skin by 0.4°C facilitates sleep onset equally effective in healthy elderly (by 18% ie, by 1.84 minutes [95% confidence interval {CI}, 0.76-2.92]) and elderly insomniacs (28%, 2.85 minutes [CI: 2.55-3.18]). These effects were comparable to the results in healthy young subjects, in spite of a marked decrease in the subjective perception of temperature changes in elderly subjects, especially in insomniacs.
Conclusion:
The findings show that mild changes in skin temperature have an effect on sleep propensity in elderly and indicate that elderly insomniacs may have a diminished capability to recognize that a slight increase in bed temperature facilitates the initiation or reinitiation of sleep.
Citation:
Raymann RJEM; Van Someren EJW. Diminished capability to recognize the optimal temperature for sleep initiation may contribute to poor sleep in elderly people. SLEEP 2008;31(9):1301-1309.
Keywords: sleep, core body temperature, thermosensitivity, sleep-onset latency, skin temperature, circadian rhythms, human, preoptic area/anterior hypothalamus, elderly, aging, insomnia, MSLT
BOTH SKIN TEMPERATURE AND CORE BODY TEMPERATURE (CBT) SHOW A DAY-NIGHT RHYTHM THAT IS FUNCTIONALLY LINKED TO THE SLEEP-WAKE CYCLE. The circadian rhythm in CBT in humans is characterized by a relatively low temperature throughout the nocturnal sleeping period and a relatively high temperature during the day, while being awake. It is well established that CBT and sleep propensity are negatively related1 and that sleep onset is most likely to occur when CBT decline is at its maximum rate.2–4 The majority of studies on the interaction between thermoregulation and sleep-wake transitions have focused on the relationship between CBT and sleep. Skin temperature also exhibits a circadian rhythm that is reciprocal to the CBT rhythm,5–8 ie, low during the habitual wake period. Its potential role in sleep regulation was already recognized by Magnussen in 1939 but since then has been almost totally neglected.9 Recently, a renewed interest in the relationship between skin temperature and sleep has emerged. Kräuchi and colleagues have shown that the degree of heat loss at the skin of the hands and feet is the best physiologic predictor for a rapid sleep onset.10,11 Fronczek et al recently demonstrated that, under less-controlled circumstances, the proximal skin temperature (PST) predicts subsequent sleep-onset latency (SOL) even better than does distal skin temperature (DST), in both narcoleptic subjects as well as healthy controls.12 We have previously addressed the underlying mechanisms of the relationship between skin temperature and sleep propensity and have provided a neurobiologic model postulating that autonomous thermoregulatory changes in CBT and especially skin temperature could act as an input signal to modulate neuronal activity in sleep-regulating brain areas.13 For example, the activity of thermosensitive neurons in the preoptic area/anterior hypothalamus, a key area in both sleep regulation and thermoregulation, is indeed modulated more strongly by changes in skin temperature than by changes in CBT.14 In support of a feedback mechanism of skin temperature to sleep-regulating brain areas, we have shown that manipulation of the skin temperature within the normal thermophysiologic range—without activating thermoregulatory responses— modulates sleepiness in healthy young adults15 and patients with narcolepsy16 and affects sleep depth.17
Insomnia is more prevalent in the elderly.18,19 Poor sleep in elderly subjects is typically undertreated20 or treated with pharmacologic interventions with adverse consequences for daytime function (reviewed in Van Someren).21 Given the graying society, it is highly relevant to investigate alternative strategies for sleep management in elderly.22,23
The aims of the present study were 2-fold. The first aim was to evaluate whether subtle skin warming in elderly subjects without sleep complaints and in elderly insomniacs promotes sleep onset in a fashion similar to that reported earlier in healthy young adults.15 The second aim was to evaluate whether the previously reported age-related decrease in awareness of changes in temperature during the daytime is also present in a sleeping environment and equally so in good sleepers and insomniacs. Preservation of these subjective and objective responses is not trivial because thermosensitivity during wakefulness decreases with age.24
METHODS
Participants
Sixteen elderly subjects were recruited through newspaper and both magazines and Internet sites aiming at an elderly audience. Participants were 8 healthy elderly subjects without sleep complaints (mean ± SEM: 65.8 ± 2.8 years, 4 men) and 8 elderly subjects diagnosed with primary insomnia (59.1 ± 1.9 years, 4 men) according to the qualitative criteria of the International Classification of Sleep Disorders25 and the Research Diagnostic Criteria for Primary Insomnia,26 as well as according to proposed quantitative criteria by Lichstein et al.27 The groups did not differ regarding their mean age (t test, P = 0.38).
Although the study was performed prior to the recently published Recommendations for a Standard Research Assessment of Insomnia,28 it complied with the majority of the recommendations. Diagnosis was performed by accredited sleep specialists and included interviews, sleep diaries, and 2 questionnaires: a Dutch adaptation29 of the 75-item Sleep Disorders Questionnaire (SDQ)30 and the Pittsburgh Sleep Quality Index (PSQI).31 All elderly subjects suffering from primary insomnia had a PSQI score greater than 5 (10.9 ± 1.1) and an SDQ-Insomnia score greater than 2.5 (3.3. ± 0.1). All elderly subjects without sleep complaints scored within the normal range of these scales: 3.6 ± 0.4 for the PSQI and 2.0 ± 0.1 for the SDQ-Insomnia subscale. The diagnosis of insomnia was supported by sleep diaries kept over a period of 2 weeks just prior to the experiment. Sleep diary data are given in Table 1. The 2 groups differed significantly on subjective sleep efficiency, SOL, and the duration of wake after sleep onset.
Table 1.
Sleep Characteristics from Sleep Diaries of Elderly Subjects Covering 2 Weeks Prior to the Experiment
| Sleep Characteristic | Elderly |
|
|---|---|---|
| Without sleep complaints | With insomnia | |
| SOL, min | 11.23 ± 2.11 | 46.25 ± 13.50a |
| TIB, min | 475.88 ± 16.58 | 512.70 ± 20.06 |
| TST, min | 434.78 ± 18.55 | 365.72 ± 32.47b |
| WASO, min | 7.99 ± 2.15 | 50.96 ± 9.04c |
| Sleep efficiency, % | 91.4 ± 1.1 | 71.5 ± 5.5c |
Subjective sleep characteristics, presented as mean ± SEM, for elderly subjects without sleep complaints and elderly subjects with insomnia. P values are determined using independent sample t-tests.
SOL refers to sleep-onset latency; TIB, time in bed; TST, total sleep time; WASO, wake time after sleep onset.
P < 0.05;
P < 0.10;
P < 0.01
None of the subjects scored higher than the cutoff score on the SDQ subscales Narcolepsy, Apnea, Restless legs, and Psychiatry. Polysomnographic confirmation of disturbed sleep in the absence of apnea and periodic leg movements was demonstrated during the study. A history or present symptoms of medical or psychiatric disorders were furthermore excluded by interview and evaluating the Symptom Check List. All subjects were in good health, and none used hypnotic, psychotropic, or cardiovascular medication. All women were postmenopausal. The data from 8 young and healthy adults (mean ± SEM: 27.0 ± 2.4 years, 4 men) used from a previous study15 for the comparison all scored within the normal range of these scales: 4.0 ± 0.5 for the PSQI and 1.8 ± 0.1 for the SDQ-Insomnia subscale, respectively.15 All subjects participated with informed consent. The Medical Ethics Committee of the Academic Medical Center of the University of Amsterdam approved the protocol.
Design and Procedure
A previously described design was used that consisted of a modified constant-routine protocol32, 33 over 2 experimental days in which sleepiness was measured using the (Multiple Sleep Latency Test [MSLT]).34,35 The procedures have been described in detail before15 and are given here in a comprehensive way.
Participants refrained from caffeine, alcohol, and tobacco for 8 hours before arriving at the sleep laboratory at 22:00, where they were prepared for polysomnography and were fitted with a thermosuit (Coretech Cool tubesuit, Med-Eng Systems Inc., Ottawa, Canada) for skin-temperature manipulation. From midnight until 06:00, lights were turned off, and participants were allowed to sleep. The experiment started at 06:30 and consisted of a modified constant-routine protocol under dim-light (<10 lux) conditions and a fixed body-position schedule. Over 2 experimental days, 18 SOLs for each subject were determined while CBT was manipulated with food and drinks and skin temperature was manipulated with a thermosuit.
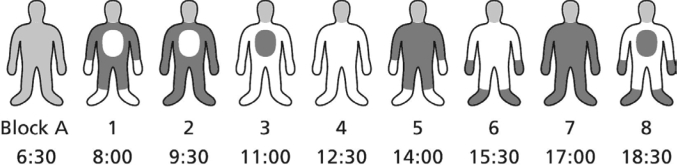
Both experimental days consisted of 9 consecutive blocks with a duration of 1.5 hours each (see Figure 1). Within the first 60 minutes of each block, CBT and skin-temperature manipulations were applied, and a computerized neuropsychological task battery had to be completed. This battery included assessment of vigilance, thermal comfort, and temperature sensation using 100-mm visual-analog scales. At 60 minutes, the lights were switched off, and the participants were asked to try to sleep. Sleep onset was determined online (MSLT),34,35 and participants were awakened directly after sleep-onset determination (see below) and stayed awake in bed with the dim light turned on. The maximum time allowed to fall asleep was 30 minutes. The results of the effects of temperature manipulation on vigilance-task performance have been reported elsewhere.36
Figure 1.
Schematic view of the temperature manipulations within one day within one subject. Core body, proximal and distal skin manipulation occurs simultaneously during every block. Within the last 30 minutes of each block the MSLT was performed. On the 2nd day, temperature manipulation combinations were the opposite of those of the 1st day to provide a protocol balanced for circadian effects. Block A: Habituation block. White represents cool; dark gray represents warm.
CBT, PST, and DST were simultaneously and independently manipulated in either a slightly warmer (+) or cooler (−) direction, within the comfortable and thermoneutral range. This brings up a 2 × 2 × 2 experimental design (CBT warm or cool [CBT+ or CBT−], PST warm or cool [PST+ or PST−], or DST or cool [DST+ or DST−]) with 8 possible manipulation combinations, which were all tested in the 8 blocks within a single day in every participant (see Figure 1 for an example of 1 subject). We have previously demonstrated that the “cool” condition can be regarded as an adequate approximation of the skin temperature during normal sleep in elderly subjects.17 The sequence of the manipulation combinations was different for each participant such that, over all participants, every manipulation combination was given once in each of the 8 blocks, and every transition from one to any other combination occurred only once. At the end of the first day, subjects went home for a normal night of sleep and returned to the laboratory the next evening for a repeated assessment according to the same procedure. However, on the second day, the temperature manipulation combinations were the opposite of those of the first day to provide a protocol balanced for circadian effects (for example: Day 1, block n: CBT+, PST−, DST+; Day 2, block n: CBT−, PST+, DST−).
Temperature Manipulations and Measurement
Temperature manipulations and measurements have been described in detail previously.15 In brief, CBT was manipulated by means of 200-mL hot (heated to 80°C, served 2 min later) or cold (0°C, crushed ice) diet (4.25 kcal), decaffeinated tea (iced tea mix [diet decaffeinated lemon], Lipton, Englewood Cliffs, NJ) together with an isocaloric hot or cold snack of the subjects' choice (200 kcal). Skin temperatures were manipulated with the use of a water-perfused thermosuit (Coretech Cool tube suit, Med-Eng Systems Inc.), connected to 2 computer-controlled bath-circulation thermostats (K6KP, Lauda, Lauda-Köningshofen, Germany). The temperatures measured at the tubes just before entering the thermosuit ranged between approximately 31°C and 34°C, a comfortable range of skin-temperature manipulations that does not trigger major thermoregulatory responses.
Ingestion of food and drinks to manipulate CBT and skin temperature were started about 50 minutes before lights off.
Body temperature was obtained using 8 thermistors (P-8432, ICBT, Tokyo, Japan). CBT was measured using a rectal thermistor. PST was measured at 3 places: right midthigh, abdomen, and the right infraclavicular area. DST was measured at 4 points: thenar eminence of the left and right hand and medial plantar aspect of the left and right foot. Temperature was digitally recorded at 1 Hz (Embla A10 and Somnologica software, Flaga hf, Reykjavik, Iceland). An automated procedure was applied to remove occasional artifacts and to calculate average DST (Tdist) and PST (Tprox) using a weighting method, as has been described previously.15 Temperature data averaged over the 5 minutes before lights off were used for further analyses.
Sleep
Polysomnographic sleep recordings consisted of electroencephalography from 2 bipolar derivations (FpzCz and PzOz),37 submental electromyography, and electrooculography. The signals were digitally recorded at 200 Hz using the Embla A10 recorder and Somnologica software (Flaga hf).
Sleep onset was determined online during the experiment according to standard criteria,38 with sleep onset defined as 3 consecutive 30-second epochs of Stage 1 sleep or one 30-second epoch of Stage 2 (or deeper) sleep.34 Real-time sleep-stage determination was aided by the use of spectral views of the EEG signal. MSLT recordings were once more visually scored offline by 2 independent scorers blind to the temperature manipulations. SOL was defined as the time between lights off and sleep onset. If the participant did not sleep during the 30 minutes, SOL was scored as 30 minutes. This occurred in 8% of the SOL determinations.
Statistical Analysis
To determine the effects of skin and core-temperature manipulation on body temperatures and subjective comfort, mixed-effect regression analysis (also known as hierarchic or multilevel analysis) was applied using the MLwiN software (Centre for Multilevel Modelling, Institute of Education, London, UK). These analyses were necessary in order to take into account the interdependence of the data points inherent to the hierarchic structure of the design, in our case the sequential sleep onset observations i that were nested within days j, once more nested within participants k.39 They were necessary as well to independently estimate the effects of the simultaneously and differentially manipulated CBT, DST, and PST, a major aim of the present study. Because the frequency distribution of SOLs is skewed, a Poisson distribution rather than normal distribution was assumed in the mixed-effect analyses on the effects of the manipulations on SOL.
We evaluated how rectal (Tre) and skin temperatures (Tprox and Tdist), subjective comfort, and SOL were affected by the manipulations. To do so, the CBT, PST, and DST manipulation levels were dummy coded as dichotomous predictor variables, using 0 for the cool and 1 for the warm manipulation in the linear models, and −0.5 and 0.5 in the Poisson model. A 3-level regression model was fitted [each block (i), nested within days (j), nested within subjects (k)]. To account for possible diurnal variation in core and skin temperature40 and a possible decrease of SOL with practice,41, 42 both time of day (Hour) and the number of repeats trying to fall asleep (Repeats) were entered in the models as covariates. Since these effects are likely to be nonlinear, their square-root and squared values were allowed in the model in addition to their possible linear contribution. Additional models were run to test for confounding effects of the prior temperature manipulations, by adding temperature manipulations of the preceding block (pCBT, pPST, and pDST) to the regression models.
Maximum likelihood was used to estimate the regression coefficients (effect sizes), which were tested for significance with the Wald test.43 Additional terms were allowed in the model only if their coefficients were significant and the residual error of the model was reduced. Two-tailed significance levels were set at 0.05.
RESULTS
Effects of Temperature Manipulations on CBT and Skin Temperatures
The effects of the temperature manipulations on CBT and skin temperatures for both groups are shown in Table 2. The overall average Tre during the 5 minutes prior to lights off was 36.86°C ± 0.09°C for elderly subjects without sleep complaints and 36.80°C ± 0.07°C for elderly insomniacs. Tre was significantly affected by the CBT manipulation, with 0.20°C and 0.16°C higher Tre in the CBT+ condition, compared with the CBT− condition for elderly subjects and elderly insomniacs, respectively. Tre also showed modulation over the time of day, accounting for 47% and 36%, respectively, of the variance. The CBT manipulation accounted for another 33% and 32% of the residual variance in Tre. The addition of preceding temperature manipulations to test for confounding effects of the previous manipulations accounted for an additional 27% (in elderly without sleep complaints, by all previous manipulations) and 20% (in elderly insomniacs, by previous PST manipulation) of the variance.
Table 2.
Effects of Manipulations on Measured Temperatures of 8 Elderly Subjects Without Sleep Complaints and 8 Elderly Subjects With Insomnia
| Tre | Tprox | Tdist | |
|---|---|---|---|
| Elderly without sleep complaints | |||
| Overall mean | 36.86 ± 0.09 | 35.03 ± 0.01 | 35.00 ± 0.15 |
| CBT | 0.20 ± 0.03a | 0.41 ± 0.06a | |
| PST | 0.71 ± 0.04 a | 0.29 ± 0.06a | |
| DST | 0.82 ± 0.06a | ||
| Elderly Insomniacs | |||
| Overall mean | 36.80 ± 0.07 | 35.00 ± 0.09 | 35.18 ± 0.11 |
| CBT | 0.16 ± 0.02a | 0.42 ± 0.05a | |
| PST | 0.73 ± 0.04a | 0.27 ± 0.05a | |
| DST | 0.09 ± 0.04b | 0.72 ± 0.05 |
Estimates of the effects, i.e. mean ± SEM difference of temperature (°C) in the warm relative to cool manipulation conditions, for core body temperature (Tre), proximal skin temperature (Tprox), and distal skin temperature (Tdist) before lights off. CBT, PST, DST manipulation were included in the model as dichotomous variables, with cool and warm coded as 0 and 1. The full regression model was as follows: Tijk = ß0ijk + ß1*Hourijk + ß2*Hou2ijk + ß3*√Hourijk+ ß4*CBTijk + ß5*PSTijk + ß6*DSTijk.Subscripts indicate the ith observation on day j for subject k. Hour = Time of day, not shown.
P < 0.001;
P < 0.05
The overall average Tprox during the 5 minutes prior to lights off was 35.03°C ± 0.01°C for elderly without sleep complaints and 35.00°C ± 0.09°C for elderly insomniacs. Tprox was significantly affected by the PST manipulation, with 0.71°C and 0.73°C higher Tprox in the PST+ condition compared with the PST− condition for elderly subjects and elderly insomniacs, respectively. Moreover, in elderly insomniacs, Tprox was significantly affected by the DST manipulation, with 0.09°C higher Tprox in the DST+ condition compared with the DST− condition. Tprox showed modulation over the time of day, accounting for 5% and 8% of the variance for elderly subjects and elderly insomniacs, respectively. The PST manipulations accounted for another 76% and 71% of the residual variance in Tprox. The addition of preceding temperature manipulations to test for the effects of the previous manipulations accounted for an additional 3% (by previous PST manipulation) of the variance, but only in the elderly without sleep complaints.
The overall average Tdist during the 5 minutes prior to lights off was 35.00°C ± 0.15°C for elderly without sleep complaints and 35.18°C ± 0.11°C for elderly insomniacs. Tdist was significantly affected by the DST manipulation, with 0.82°C and 0.72°C higher Tdist in the DST+ condition compared with the DST− condition for elderly subjects and elderly insomniacs, respectively. Tdist was also modulated by proximal skin manipulations (0.29°C, and 0.27°C higher Tdist in the PST+ condition) and CBT manipulation (0.41°C, and 0.42°C higher Tdist in the CBT+ condition). In the elderly insomniacs, Tdist also showed modulation over the time of day, accounting for 3% of the variance. The DST manipulations accounted for another 70% and 72% of the residual variance in Tdist. . The addition of preceding temperature manipulations accounted for an additional 6% (in elderly without sleep complaints, by previous skin temperature manipulations) and 21% (in elderly insomniacs, by previous PST manipulation) of the variance.
In summary, the manipulations accounted for a high proportion of the variance in body temperatures, and their effects were highly comparable between both groups. Only very limited carryover effects of manipulations in the previous block were present. Likewise, crosstalk was limited such that the largest changes of temperature were consistently in the body parts being manipulated.
Effects of Temperature Manipulations on Temperature Perception
The effects of the temperature manipulations on thermal comfort and temperature sensation for both groups are shown in Table 3. The overall average rating of thermal comfort prior to lights off was 55.6 ± 4 for elderly without sleep complaints and 49.9 ± 7.9 for elderly insomniacs. Only in the elderly without sleep problems was thermal comfort significantly affected by all of the temperature manipulations. In these subjects, comfort was maximal (71.0 ± 4.6 on the 100-mm scale ranging from 0 = uncomfortable to 100 = comfortable) when all conditions were cool (CBT−, PST−, and DST−). Thermal comfort was significantly lower in the warm conditions (−12.6 ± 2.6 for CBT+, −12.6 ± 2.6 for PST+, −5.4 ± 2.6 for DST+). In contrast, none of the temperature manipulations affected thermal comfort in elderly insomniacs.
Table 3.
Effects of Manipulation on Thermal Comfort and Temperature Sensation of 8 Elderly Subjects Without Sleep Complaints and 8 Elderly Subjects With Insomnia
| Thermal Comfort | Temperature Sensation | |
|---|---|---|
| Without sleep complaints | ||
| Overall mean | 55.6 ± 4.0 | 59.7 ± 2.8 |
| CBT | −12.5 ± 2.6 a | 12.8 ± 2.3 a |
| PST | −12.6 ± 2.6 a | 11.4 ± 2.3 a |
| DST | −5.4 ± 2.6b | |
| With insomnia | ||
| Overall mean | 49.9 ± 7.9 | 63.2 ± 4.5 |
| CBT | 9.4 ± 2.1a | |
| PST | ||
| DST | ||
| Young subjects* | ||
| Overall mean | 59.1 ± 2.3 | 62.4 ± 1.8 |
| CBT | −20.2 ± 2.8 a | 12.0 ± 1.6a |
| PST | −18.6 ± 2.8 a | 13.2 ± 1.6a |
| DST | −5.9 ± 2.8b | 4.2 ± 1.6c |
Estimates of the effects, i.e. mean ± SEM difference of the visual-analog scale scores (mm) on subjective temperature sensation and thermal comfort in the warm relative to cool manipulation conditions. Core body, proximal skin, and distal skin temperature manipulation (CBT, PST, DST) were included in the model as dichotomous variables, with cool and warm coded as 0 and 1. Thermal Comfort was measured on a visual-analog scale ranging from 0 (uncomfortable) to 100 (comfortable). Temperature sensation was measured on a visual-analog scale ranging from 0 (cool) to 100 (warm), with 50 reflecting most thermoneutral. Only the optimal models are shown, with only the significantly contributing predictors shown. The full regression model was as follows: Yijk = ß0ijk + ß1*CBTijk + ß2*PSTijk + ß3*DSTijk. Subscripts indicate the ith observation on day j for subject k.
*Data of young subjects taken from Raymann et al.15
P < 0.001;
P < 0.05;
P < 0.01
The overall average rating of temperature sensation prior to lights off was 59.7 ± 2.8 for elderly subjects and 63.2 ± 4.5 for elderly insomniacs. Temperature sensation was significantly affected by the CBT manipulation. The CBT+ condition was rated 12.84 warmer by elderly subjects and 9.35 warmer by elderly insomniacs, as compared with the CBT− condition. Only in elderly subjects without sleep complaints was temperature sensation also significantly affected by the PST manipulation. The PST+ condition was rated 11.48 warmer than the PST− condition. Of note is that, in elderly subjects, the temperature sensation was neutral (47.5 ± 3.2 on the 100-mm scale ranging from 0 = cool to 100 = warm) when CBT manipulation and proximal skin manipulation conditions were cool (CBT− and PST−). In elderly insomniacs the temperature sensation was just above neutral (58.5 ± 4.7) when the CBT manipulation was cool (CBT−).
In summary, temperature sensation was affected by the CBT manipulations in all elderly subjects, whereas the PST manipulations were experienced only as warmer than the cool by elderly without sleep complaints. DST manipulations did not affect temperature sensation in either elderly group. All temperature increases were seen as discomforting by elderly good sleepers, but none were discomforting to elderly insomniacs.
Effects of Temperature Manipulations on SOL
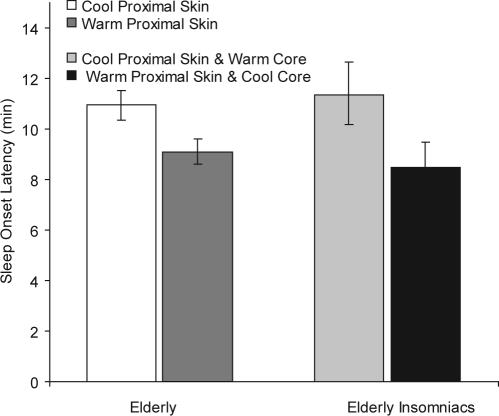
Table 4 shows the effects of the temperature manipulations on SOL. The overall average SOL was 10.43 minutes (95% confidence interval [CI]: 7.13 - 15.26) for elderly subjects and 10.10 minutes (CI: 6.96-14.65) for elderly insomniacs. In elderly good sleepers, SOL was significantly modulated by time (hour2) and affected by the number of sleep-onset repeats (Repeats and √Repeats) and by the proximal skin warming. Sleep onset was 1.84 minutes (CI: 0.76-2.92) shorter in the PST+ condition, compared with the PST− condition. Sleep latency was not significantly affected by core and distal manipulations. As compared to the cool condition, warming of the proximal skin thus resulted in an 18% decrease in the time to fall asleep (see Figure 2). In elderly insomniacs, SOL was significantly modulated by time (Hour) and affected by the number of sleep-onset repeats (Repeats and Repeats2) and by both proximal skin warming and core body cooling. Sleep onset was 1.68 minutes (CI: 0.60-2.76) shorter in the CBT− condition, compared with the CBT+ condition, and 1.16 minutes (CI: 0.08-2.24) shorter in the PST+ condition, compared with the PST− condition. Sleep latency was not significantly affected by distal manipulations. Warming of the proximal skin together with cooling of the core thus resulted in a 28% decrease in the time to fall asleep, as compared with the opposite manipulation (see Figure 2).
Table 4.
Effects of Manipulation on Sleep Latency in 8 Elderly Subjects Without Sleep Complaints and 8 Elderly Subjects With Insomnia
| Subjects |
||
|---|---|---|
| Without sleep complaints | With insomnia | |
| Overall mean | 10.43 (7.13-15.26) | 10.10 (6.96-14.65) |
| CBT | 1.68 (0.60-2.76)a | |
| PST | −1.84 (0.76-2.92)b | −1.16 (0.08-2.24)c |
| DST | ||
Estimates of the effects, i.e. mean and confidence intervals difference of sleep onset latency (min) in the warm relative to cool manipulation conditions. This table shows sleep-onset latency in minutes, calculated by taking e to the power of the coefficients, as estimated from the Poisson regression. Temperature manipulations were included in the model as dichotomous variables, with cool and warm coded as −0.5 and +0.5.
The full Poisson regression model was as follows: Ln(SOLijk) = ß0ijk + ß1*Hourijk + ß2*Hou2ijk + ß3*Repeatsijk + ß4*Repeat2sijk + ß5*√Repeatsijk + ß6*CBTijk + ß7*PSTijk + ß8*DSTijk (Subscripts indicate ith observation on day j for subject k). Hour (time), defined as the number of hours since the start of the first included sleep-latency test within each day, starting with 0 at 0900. Repeats, defined as the number of times allowed to fall asleep since day 1, starting with 1. Effects of Hour and Repeats are not shown.
CBT refers to core body temperature manipulation; PST, proximal skin temperature manipulation; DST, distal skin temperature manipulation; SOL, sleep-onset latency.
P < 0.01;
P < 0.001;
P < 0.05
Figure 2.
Sleep onset latencies (± 95% confidence intervals) in elderly and elderly insomniacs. Effect sizes follow from the regression analysis results presented in table 4.
In summary, SOLs increase with time over the day but decrease by “practice” and proximal skin warming. In insomniacs, core-body cooling also accelerates sleep onset.
Effects of Age and Insomnia on Induced Temperature Changes and Subjective Thermosensitivity
To investigate changes in subjective thermosensitivity related to insomnia, we compared the regression model of both groups. To investigate changes in subjective thermosensitivity related to aging, we compared the regression model of both groups with the models of a group of young subjects who underwent the same protocol.15 Such comparisons were possible because temperature manipulations induced changes in rectal and skin temperatures of similar magnitude in all 3 groups (all Z-tests: P > 0.10).
Between-groups comparison of the effects of the temperature manipulations on thermal comfort and temperature sensation indicated only a minor loss in temperature perception with aging, per se, and a major loss in elderly insomniacs. When comparing thermal comfort in the young and the elderly without sleep complaints, it was found that thermal comfort was affected highly similarly by PST and DST manipulations (both Z-tests: P > 0.10) and significantly less by CBT manipulations in the elderly (Z-tests: P < 0.05). However, in the elderly insomniacs, none of the temperature manipulations affected thermal comfort. When comparing temperature sensation in the young and the elderly without sleep complaints, it was found that temperature sensation was affected similarly by CBT and PST manipulations (both Z-tests P > 0.50) but not by DST, which affected temperature sensation only in young subjects. In the elderly insomniacs, only CBT manipulation affected temperature sensation, and this effect was comparable to the effect seen in the young and the elderly (both Z-tests P > 0.25).
In summary, the applied ranges of temperature manipulations affected the skin temperatures and CBT in a highly similar fashion in all 3 groups, and all manipulations affected the thermal comfort and sensation, but only in the young group. However, with age, the effect of the applied CBT range on thermal comfort attenuates, and the effect of the applied DST range on temperature sensation disappears. In elderly insomniacs, only the effect of the applied CBT range on temperature sensation holds on. It indicates that the subjective effects of thermal manipulation are slightly weakened with aging and more strongly so in elderly insomniacs.
Effects of Age and Insomnia on the Effects of Temperature Manipulations on SOL
Between-groups comparison indicated no differences on the effects of PST manipulations on SOL (all Z-Tests P > 0.05). Increases of PST resulted in decreases of SOL in all groups. Only in the elderly insomniacs did decreases of CBT result in decreases of SOL.
DISCUSSION
The aims of the present study were (1) to evaluate whether subtle manipulation of CBT and skin temperature within the range that is naturally occurring during the circadian cycle affects SOL in elderly without sleep complaints and elderly insomniacs in a similar fashion as reported earlier in healthy young adults15 and (2) to evaluate whether the previously reported age-related decrease in awareness of changes in temperature at daytime is also present in a sleeping environment. Whereas it has been shown that elderly in general show attenuated thermosensitivity24,44,45 compared with younger subjects when awake, no prior studies have addressed such possible attenuation during attempts to fall asleep and/or maintain sleep within the microclimate of the bed. Of note, our experimental approach of manipulating CBT and skin temperatures and quantifying their effect on thermal appreciation and sleep onset allowed for a cause-and-effect interpretation, in contrast with correlation studies.
With regard to the effect of skin temperature on sleep initiation, our study showed that proximal skin warming, in particular, facilitated sleep onset in elderly with and without sleep complaints. The effect was comparable in both groups of elderly and also did not differ significantly from the effect found in young adults, reported in our earlier study.15 In elderly insomniacs, core body cooling also accelerated sleep onset, an effect that was not present in either younger or older participants without sleep complaints.
The failure of distal warming to affect sleep latency is in line with the results of our previous study on foot warming and sleep onset that reported an attenuated sensitivity of sleep propensity to foot warming in elderly.46 Moreover, the normal diurnal time course of DST reaches values much lower than we have applied, and, hence, both our cool and warm DST manipulation might be interpreted as being warm. Whereas the subtle PST manipulations we applied were sufficient to affect SOL, we cannot exclude that applying DST manipulations in a slightly lower and wider range would be at least as adequate in affecting SOL.
Although the present study demonstrates a proof of principle, it remains to be addressed in a larger sample whether CBT and skin-temperature manipulations will be of clinical relevance in real-life situations. A reduction of 2.8 minutes (28%) in the SOL of elderly insomniacs, induced by the optimal combination of a warmer proximal skin in the absence of a warmer core, may not seem clinically relevant at first sight. However, it approximates the order of magnitude that can be obtained with hypnotic compounds. In adult and elderly insomniacs, comparable improvements have been reported with the administration of melatonin (eg, 17%)47 or benzodiazepines (4.2 minutes on average in a meta-analysis).48 Moreover, control of skin temperature not only affects SOL, but also sleep depth.17 Further work is necessary to evaluate the clinical efficacy of the manipulations in insomniacs with an objectively verifiable very long SOL. In the present sample of insomniacs, the daytime SOLs under strictly controlled laboratory conditions did not differ from those of elderly without sleep complaints, even though the insomniacs reported much increased sleep latencies in the diaries on their sleep at home. Such discrepancy has been reported previously for laboratory-based studies of nocturnal sleep49 and for laboratory-based studies on SOL under constant-routine conditions comparable with our present study.50
With respect to our investigation of an age-related decrease in awareness of changes in temperature in a sleeping environment, the results show that elderly insomniacs have a notable deficit in the detection of subtle changes in skin temperature—even though the temperature manipulations affected their skin temperatures and CBTs, as they did in younger and older subjects without sleep complaints. This attenuation of subjective thermosensitivity was much less in elderly without sleep complaints who, as compared with young subjects, only failed to detect the distal warming condition as being warmer than the distal cooling condition. Elderly insomniacs did not discriminate temperature changes associated with either PST or DST manipulations nor did they rate any of the manipulations of core and skin temperature as affecting subjective comfort. The only sensitivity that the elderly insomniacs preserved similar to young and elderly without sleep complaints was that they rated the core warming condition as warmer than the core cooling condition.
These findings are in agreement with those of previous studies reporting a decrease in the sensitivity of subjective thermal perception with age, especially for warm stimuli and particularly in the distal parts.51,52 Our results also show a reduced contribution of the CBT to the subjective experience of thermal comfort at advanced age. In elderly insomniacs, none of the applied temperature manipulations affected thermal comfort. This finding is in agreement with studies showing that elderly tolerate larger deviations in temperature before discomfort is noticed, at least when awake.53–56
To our knowledge, this is the first experimental finding of a pronounced attenuation of subjective thermosensitivity in elderly insomniacs within the small range of normal bed temperatures. It should be noted that our results cannot be extrapolated to a wide range of temperatures, including those outside of the thermoneutral range; it cannot be concluded that insomniacs have a generalized failure to recognize temperature changes over a range of several degrees. Since subjective thermosensitivity is better preserved in elderly without sleep complaints, we consider the possibility that an attenuated thermosensitivity could contribute to suboptimal sleep in elderly subjects. A suboptimal temperature of the bed may go unnoticed even though it could adversely affect the capability to initiate or reinitiate sleep and adequate countermeasures may not be taken.
A limitation of the study is that the manipulation of CBT, DST, and PST was not completely independent. However, the combined manipulations still did account for most of the variability observed throughout the day in CBT and skin temperatures.
A surprising finding was that the average SOL of the elderly insomniacs did not differ from that of the elderly without sleep complaints, although the groups differed in reported SOL in the 2 weeks prior to the experiment, as shown in Table 1. It may be that, in our particular protocol, the elderly insomniacs benefited from increased daytime sleepiness due to the restricted sleep allowed during both nights preceding the experimental days. Moreover, the elderly insomniacs frequently reported that the environment of the sleep laboratory, with no sound, very low light levels, and a comfortable temperature, was rather ideal to them. Also, sleep onset occurred increasingly faster throughout the 2 experimental days, supporting the previous notion of Harris et al41 that sleep onset can improve with frequently repeated training. Finally, the application of relatively high distal temperatures in both cool and warm condition might have optimized sleep-onset conditions and, thus, contributed to the elimination of possible differences in SOL between elderly groups.
Our findings may have implications for SOL in elderly and elderly insomniacs in everyday life. In both elderly and elderly insomniacs, warming the proximal skin resulted in a decrease in SOL. Hence, warming of the skin either by promoting peripheral heat loss or by subtle and feedback-controlled warming of the skin within the thermoneutral range provides a means to improve sleep onset in elderly who have trouble falling asleep in the beginning of the night or after nocturnal or early morning awakening. Since our results indicate that the sleep onset in elderly insomniacs is accelerated by CBT cooling, it is of importance that the applied skin-warming strategy is not resulting in an influx of heat to the core of the body. Application of, for instance, an electric blanket throughout the night (ie, continuous heating) results in an increase in the CBT and disturbs nocturnal sleep.57,58 Feedback control of the heating is clearly necessary to keep the microclimate in bed at such a level that the skin is warmed slightly only when skin-temperature measurement indicates that it falls below a critical value. Such procedure should be optimized to prevent an increase in CBT.
In conclusion, our results confirm that even mild changes in skin temperature, likely to occur in normal sleeping circumstances, can have an effect on sleep propensity in elderly subjects, just as we previously demonstrated in young adults.15 The sensitivity of the sleep-regulating systems in the brain to small variations in skin temperature is thus preserved at advanced age. In contrast, the subjective appreciation of subtle induced changes in skin temperature is decreased in elderly subjects, especially so in insomniac elderly. As a consequence, elderly insomniacs may lie awake awaiting sleep onset without noticing that the bed microclimate might not be supportive for falling asleep and, consequently, without taking the appropriate behavioral action, eg, a change of bedding or clothing, to optimize the bed microclimate. Especially elderly insomniacs may benefit from interventions that increase skin temperature, eg, by passive body heating before sleep (resulting in an increased drop in CBT and an elevated skin temperature)59–62 or by controlled warming of the beds microclimate. It is essential that this warming should not be timed too close to bedtime because elevated body temperature does not favor sleep63 and, only after about 2 hours after body warming, the CBT becomes lower than without warming while skin temperature is still elevated.64
ACKNOWLEDGMENTS
Research supported by NWO/ZonMw, The Hague (projects SOW 014-90-001 and Innovation Grant 016.025.041); EU FP6 Sensation Integrated Project (FP6-507231).
The authors are greatly indebted to Esther van Braak, Susan Collins, Spyros Drosopoulos, René den Haan, Gayle van Krevelen, Wesley Scheijdenberg, Ralf Vis (all NIN) and Patricia Murphy (Laboratory of Human Chronobiology, Cornell University) for their contribution to the realization of this study. We would like to thank Prof. J. Stam (Academic Medical Center, Amsterdam) for clinical surveillance during the protocol. Jenneke Kruisbrink (NIN), Henk Stoffels (NIN), Jos Twisk (VUmc), and Wilma Verweij (NIN) are kindly acknowledged for, respectively, bibliographic, graphic, statistical, and linguistic assistance.
Contributions have also been made by Braun, Rijswijk, The Netherlands; Cambridge Neurotechnology, Cambridge, UK; Itamar Medical, Ceasarea, Israel; Medcare/Flaga hf, Reykjavik, Iceland; Royal Auping, Deventer, The Netherlands; NIN, Amsterdam, The Netherlands.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Lack LC, Lushington K. The rhythms of human sleep propensity and core body temperature. J Sleep Res. 1996;5:1–11. doi: 10.1046/j.1365-2869.1996.00005.x. [DOI] [PubMed] [Google Scholar]
- 2.Campbell SS, Broughton RJ. Rapid decline in body temperature before sleep: fluffing the physiological pillow? Chronobiol Int. 1994;11:126–31. doi: 10.3109/07420529409055899. [DOI] [PubMed] [Google Scholar]
- 3.Barrett J, Lack L, Morris M. The sleep-evoked decrease of body temperature. Sleep. 1993;16:93–9. [PubMed] [Google Scholar]
- 4.Murphy PJ, Campbell SS. Nighttime drop in body temperature: a physiological trigger for sleep onset? Sleep. 1997;20:505–11. doi: 10.1093/sleep/20.7.505. [DOI] [PubMed] [Google Scholar]
- 5.Marotte H, Timbal J. Circadian rhythm of temperature in man. Comparative study with two experimental protocols. Chronobiologia. 1982;8:87–100. [PubMed] [Google Scholar]
- 6.van Marken Lichtenbelt WD, Daanen HA, Wouters L, et al. Evaluation of wireless determination of skin temperature using iButtons. Physiol Behav. 2006;88:489–97. doi: 10.1016/j.physbeh.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Van Someren EJW. Mechanisms and functions of coupling between sleep and temperature rhythms. Prog Brain Res. 2006;153:309–24. doi: 10.1016/S0079-6123(06)53018-3. [DOI] [PubMed] [Google Scholar]
- 8.Gradisar M, Lack L. Relationships between the circadian rhythms of finger temperature, core temperature, sleep latency, and subjective sleepiness. J Biol Rhythms. 2004;19:157–63. doi: 10.1177/0748730403261560. [DOI] [PubMed] [Google Scholar]
- 9.Magnussen G. Vasomotorische Veränderingen in den Extremitäten im Verhältnis zu Schlaf und Schlafbereitschaft. Acta Psychiatr Neurol. 1939;14:39–54. [Google Scholar]
- 10.Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Functional link between distal vasodilation and sleep-onset latency? Am J Physiol. 2000;278:R741–8. doi: 10.1152/ajpregu.2000.278.3.R741. [DOI] [PubMed] [Google Scholar]
- 11.Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Warm feet promote the rapid onset of sleep. Nature. 1999;401:36–7. doi: 10.1038/43366. [DOI] [PubMed] [Google Scholar]
- 12.Fronczek R, Overeem S, Lammers GJ, van Dijk JG, Van Someren EJW. Altered skin-temperature regulation in narcolepsy relates to sleep propensity. Sleep. 2006;29:1444–9. doi: 10.1093/sleep/29.11.1444. [DOI] [PubMed] [Google Scholar]
- 13.Van Someren EJW. More than a marker: interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol Int. 2000;17:313–54. doi: 10.1081/cbi-100101050. [DOI] [PubMed] [Google Scholar]
- 14.Boulant JA, Bignall KE. Hypothalamic neuronal responses to peripheral and deep-body temperatures. Am J Physiol. 1973;225:1371–4. doi: 10.1152/ajplegacy.1973.225.6.1371. [DOI] [PubMed] [Google Scholar]
- 15.Raymann RJEM, Swaab DF, Van Someren EJW. Cutaneous warming promotes sleep onset. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1589–97. doi: 10.1152/ajpregu.00492.2004. [DOI] [PubMed] [Google Scholar]
- 16.Fronczek R, Raymann RJEM, Romeijn N, et al. Manipulation of core body and skin temperature improves vigilance and maintenance of wakefulness in narcolepsy. Sleep. 2008;31:233–40. doi: 10.1093/sleep/31.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raymann RJEM, Swaab DF, Van Someren EJW. Skin deep: enhanced sleep depth by cutaneous temperature manipulation. Brain. 2008;131:500–13. doi: 10.1093/brain/awm315. [DOI] [PubMed] [Google Scholar]
- 18.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 19.Kryger M, Monjan A, Bliwise D, Ancoli-Israel S. Sleep, health, and aging. Bridging the gap between science and clinical practice. Geriatrics. 2004;59(24-6):9–30. [PubMed] [Google Scholar]
- 20.Kamel NS, Gammack JK. Insomnia in the elderly: cause, approach, and treatment. Am J Med. 2006;119:463–9. doi: 10.1016/j.amjmed.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 21.Van Someren EJW. Circadian and sleep disturbances in the elderly. Exp Gerontol. 2000;35:1229–37. doi: 10.1016/s0531-5565(00)00191-1. [DOI] [PubMed] [Google Scholar]
- 22.Sivertsen B, Omvik S, Pallesen S, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295:2851–8. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 23.Van Someren EJW, Riemersma RF, Swaab DF. Functional plasticity of the circadian timing system in old age: light exposure. Prog Brain Res. 2002;138:205–31. doi: 10.1016/S0079-6123(02)38080-4. [DOI] [PubMed] [Google Scholar]
- 24.Van Someren EJW, Raymann RJEM, Scherder EJA, Daanen HAM, Swaab DF. Circadian and age-related modulation of thermoreception and temperature regulation: mechanisms and functional implications. Ageing Res Rev. 2002;1:721–78. doi: 10.1016/s1568-1637(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 25.International Classification of Sleep Disorders: Diagnostic and Coding Manual. Rochester, MN: American Sleep Disorders Association; 1990. [Google Scholar]
- 26.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 27.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 29.Sweere Y, Kerkhof GA, De Weerd AW, Kamphuisen HA, Kemp B, Schimsheimer RJ. The validity of the Dutch Sleep Disorders Questionnaire (SDQ) J Psychosom Res. 1998;45:549–55. doi: 10.1016/s0022-3999(98)00030-0. [DOI] [PubMed] [Google Scholar]
- 30.Douglass AB, Bornstein R, Nino-Murcia G, et al. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep. 1994;17:160–7. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Mills JN, Minors D, Waterhouse J. Adaptation to abrupt time shifts of the oscillator(s) controlling human circadian rhythms. J Physiol. 1978;285:455–70. doi: 10.1113/jphysiol.1978.sp012582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czeisler CA, Brown EN, Ronda JM, Kronauer RE, RIchardson GS, Freitag WO. A clinical method to assess the endogenous circadian phase (ECP) of the he deep circadian oscillator in man. Sleep Res. 1985;14:295. [Google Scholar]
- 34.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 35.Carskadon MA, Dement WC. Multiple sleep latency tests during the constant routine. Sleep. 1992;15:396–9. doi: 10.1093/sleep/15.5.396. [DOI] [PubMed] [Google Scholar]
- 36.Raymann RJEM, Van Someren EJW. Time-on-task impairment of psychomotor vigilance is affected by mild skin warming and changes with aging and insomnia. Sleep. 2007;30:96–103. doi: 10.1093/sleep/30.1.96. [DOI] [PubMed] [Google Scholar]
- 37.van Sweden B, Kemp B, Kamphuisen HA, Van der Velde EA. Alternative electrode placement in (automatic) sleep scoring (Fpz-Cz/Pz-Oz versus C4-A1) Sleep. 1990;13:279–83. doi: 10.1093/sleep/13.3.279. [DOI] [PubMed] [Google Scholar]
- 38.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda: United States Department of Health, Education and Welfare; 1968. [Google Scholar]
- 39.Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology. Cambridge, UK: Cambridge University Press; 2003. [Google Scholar]
- 40.Kräuchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267:R819–R29. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- 41.Harris J, Lack L, Wright H, Gradisar M, Brooks A. Intensive Sleep Retraining treatment for chronic primary insomnia: a preliminary investigation. J Sleep Res. 2007;16:276–84. doi: 10.1111/j.1365-2869.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 42.Clodore M, Benoit O, Foret J, Bouard G. The Multiple Sleep Latency Test: individual variability and time of day effect in normal young adults. Sleep. 1990;13:385–94. doi: 10.1093/sleep/13.5.385. [DOI] [PubMed] [Google Scholar]
- 43.Wald A. Tests of statistical hypotheses concerning several parameters when the number of observations is large. Trans Am Math Soc. 1943;54:426–82. [Google Scholar]
- 44.Van Someren EJW. Thermoregulation and aging. Am J Physiol Regul Integr Comp Physiol. 2007;292:R99–102. doi: 10.1152/ajpregu.00557.2006. [DOI] [PubMed] [Google Scholar]
- 45.Kenney WL, Munce TA. Invited review: aging and human temperature regulation. J Appl Physiol. 2003;95:2598–603. doi: 10.1152/japplphysiol.00202.2003. [DOI] [PubMed] [Google Scholar]
- 46.Raymann RJEM, Swaab DF, Van Someren EJW. Skin temperature and sleep-onset latency: Changes with age and insomnia. Physiol Behav. 2007;90:257–66. doi: 10.1016/j.physbeh.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Lushington K, Pollard K, Lack L, Kennaway DJ, Dawson D. Daytime melatonin administration in elderly good and poor sleepers: effects on core body temperature and sleep latency. Sleep. 1997;20:1135–44. doi: 10.1093/sleep/20.12.1135. [DOI] [PubMed] [Google Scholar]
- 48.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225–33. [PMC free article] [PubMed] [Google Scholar]
- 49.Rosa RR, Bonnet MH. Reported chronic insomnia is independent of poor sleep as measured by electroencephalography. Psychosom Med. 2000;62:474–82. doi: 10.1097/00006842-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Gradisar M, Lack L, Wright H, Harris J, Brooks A. Do chronic primary insomniacs have impaired heat loss when attempting sleep? Am J Physiol Regul Integr Comp Physiol. 2006;290:R1115–21. doi: 10.1152/ajpregu.00266.2005. [DOI] [PubMed] [Google Scholar]
- 51.Kenshalo DR. Age changes in touch, vibration, temperature, kinesthesia and pain sensitivity. In: Birren JE, Schaie KW, editors. Handbook of the Psychology of Aging. New York, NY: Van Nostrand; 1977. pp. 562–79. [Google Scholar]
- 52.Kenshalo DR. Somesthetic sensitivity in young and elderly humans. J Gerontol. 1986;41:732–42. doi: 10.1093/geronj/41.6.732. [DOI] [PubMed] [Google Scholar]
- 53.Tochihara Y. Thermal comfort and blood pressure changes in the elderly. In: Werner J, Hexamer M, editors. Proceedings of the IX Conference on Environmental Ergonomics; 2000; Dortmund, Germany. 2000. pp. 243–7. [Google Scholar]
- 54.Kaji Y, Yadoguchi I, Shoyama S, Kaji M, Tochihara Y. Effects of room temperature on physiological and subjective responses to bathing of the elderly. In: Werner J, Hexamer M, editors. Proceedings of the IX Conference on Environmental Ergonomics; 2000; Dortmund, Germany. 2000. pp. 425–8. [Google Scholar]
- 55.Frank SM, Raja SN, Bulcao C, Goldstein DS. Age-related thermoregulatory differences during core cooling in humans. Am J Physiol. 2000;279:R349–54. doi: 10.1152/ajpregu.2000.279.1.R349. [DOI] [PubMed] [Google Scholar]
- 56.Collins KJ, Dore C, Exton-Smith AN, Fox RH, MacDonald IC, Woodward PM. Accidental hypothermia and impaired temperature homoeostasis in the elderly. Br Med J. 1977;1:353–6. doi: 10.1136/bmj.1.6057.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okamoto-Mizuno K, Tsuzuki K, Ohshiro Y, Mizuno K. Effects of an electric blanket on sleep stages and body temperature in young men. Ergonomics. 2005;48:749–57. doi: 10.1080/00140130500120874. [DOI] [PubMed] [Google Scholar]
- 58.Fletcher A, van den Heuvel C, Dawson D. Sleeping with an electric blanket: effects on core temperature, sleep, and melatonin in young adults. Sleep. 1999;22:313–8. doi: 10.1093/sleep/22.3.313. [DOI] [PubMed] [Google Scholar]
- 59.Kanda K, Tochihara Y, Ohnaka T. Bathing before sleep in the young and in the elderly. Eur J Appl Physiol. 1999;80:71–5. doi: 10.1007/s004210050560. [DOI] [PubMed] [Google Scholar]
- 60.Dorsey CM, Lukas SE, Cohen-Zion M, Sterfanovic L. Passive body heating vs. Zolpidem in older female insomniacs. Sleep. 1998;21(S3):255. doi: 10.1093/sleep/22.7.891. [DOI] [PubMed] [Google Scholar]
- 61.Dorsey CM, Lukas SE, Teicher MH, et al. Effects of passive body heating on sleep of older female insomniacs. J Geriatr Psychiatry Neurol. 1996;9:83–90. doi: 10.1177/089198879600900203. [DOI] [PubMed] [Google Scholar]
- 62.Dorsey CM, Teicher MH, Cohen-Zion M, et al. Core body temperature and sleep of older female insomniacs before and after passive body heating. Sleep. 1999;22:891–8. doi: 10.1093/sleep/22.7.891. [DOI] [PubMed] [Google Scholar]
- 63.Sewitch DE. Slow wave sleep deficiency insomnia: A problem in thermo-downregulation at sleep onset. Psychophysiology. 1987;24:200–15. doi: 10.1111/j.1469-8986.1987.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 64.Sung EJ, Tochihara Y. Effects of bathing and hot footbath on sleep in winter. J Physiol Anthropol Appl Hum Sci. 2000;19:21–7. doi: 10.2114/jpa.19.21. [DOI] [PubMed] [Google Scholar]