Abstract
Antibiotic resistance and emm gene types were examined from 692 Group A streptococci isolates from eight United States military basic training sites between 1998 and 2001. Macrolide resistance was associated with geographic sites and emm type. These data are useful for vaccine development initiatives and antimicrobial treatment considerations.
Group A streptococci (GAS; Streptococcus pyogenes) are responsible for a variety of illnesses that affect humans (14). In the 1980s both United States civilian and military personnel experienced numerous outbreaks of acute rheumatic fever, the first for the United States military in over 20 years (4, 30, 31). Epidemiological data suggest that these epidemics may have been due to emergent, potentially more virulent GAS strains (3, 12, 27).
In response to these concerns, GAS surveillance was established at eight military basic training sites throughout the United States (1, 5, 26). Between January 1998 and December 2001, GAS pharyngeal culture isolates from symptomatic military recruit trainees were systematically collected along with basic demographic data (Table 1).
TABLE 1.
Characteristics of military trainees and multivariable logistic regression modeling of antibiotic resistance among GAS isolates
| Variable | Total no. of samples (%) | No. of erythromycin- resistant isolates (%) | OR (95% CI)a | No. of tetracycline- resistant isolates (%) | OR (95% CI)a |
|---|---|---|---|---|---|
| Genderb | |||||
| Malec | 592 (85.9) | 34 (5.7) | 25 (4.2) | ||
| Female | 97 (14.1) | 10 (10.3) | NSS | 9 (9.3) | 2.3 (1.0-5.2) |
| Age (yrs)d | |||||
| 17-18c | 196 (28.6) | 13 (6.6) | 11 (5.6) | ||
| 19-20 | 281 (41.0) | 20 (7.1) | NSS | 11 (3.9) | NSS |
| 21-22 | 99 (14.5) | 6 (6.1) | NSS | 5 (5.1) | NSS |
| ≥23 | 109 (15.9) | 5 (4.6) | NSS | 6 (5.5) | NSS |
| Season | |||||
| Summer (June-August)c | 73 (10.6) | 5 (6.9) | 4 (5.5) | ||
| Fall (September-November) | 252 (36.4) | 7 (2.8) | NSS | 18 (7.1) | NSS |
| Winter (December-February) | 161 (23.3) | 10 (6.2) | 1.5 (0.6-3.8) | 5 (3.1) | NSS |
| Spring (March-May) | 206 (29.7) | 22 (10.7) | 2.2 (1.1-4.9) | 7 (3.4) | NSS |
| Training site | |||||
| Navy, Ill.c | 222 (32.1) | 7 (3.2) | 5 (2.3) | ||
| Marines, Calif. | 3 (0.4) | 0 (0.0) | NSS | 0 (0.0) | NSS |
| Marines, S. C. | 282 (40.7) | 1 (0.4) | NSS | 10 (3.6) | NSS |
| Army, S. C. | 4 (0.6) | 0 (0.0) | NSS | 1 (25.0) | 11.5 (1.1-117.8) |
| Army, Ky. | 23 (3.3) | 2 (8.7) | 5.4 (1.1-27.3) | 4 (17.4) | 6.7 (2.0-21.9) |
| Army, Mo. | 37 (5.4) | 4 (10.8) | 7.1 (2.0-25.0) | 4 (10.8) | 3.4 (1.1-10.9) |
| Army, Okla. | 19 (2.8) | 0 (0.0) | NSS | 2 (10.5) | NSS |
| Air Force, Tex. | 102 (14.7) | 30 (29.4) | 25.1 (11.0-57.1) | 8 (7.8) | 2.4 (1.0-5.7) |
OR, multivariable adjusted odds ratio; CI, confidence interval; NSS, not statistically significant.
Missing gender data from three patients.
Reference category for multivariable exact logistic regression.
Missing age data from seven patients. Variable not statistically significant at the univariate level; not included in multivariable model.
This research has been conducted in compliance with all applicable federal regulations governing the protection of human subjects in research, under protocol no. 31230.
Training sites preserved GAS specimens in tryptic soy broth with 15% glycerol at −70°C. Isolates were reconfirmed as GAS by colony morphology, bacitracin sensitivity, and a positive latex agglutination test (Hardy Diagnostics, Santa Maria, Calif.). MICs of penicillin, erythromycin, clindamycin, tetracycline, levofloxacin, and vancomycin were performed on confirmed isolates with E-test methodology (AB Biodisk, Piscataway, N.J.). Plates were incubated for 18 to 24 h at 36°C with 5% CO2. National Committee for Clinical Laboratory Standards (NCCLS) breakpoints for agar dilution of streptococci were used to interpret MIC results and to define criteria for resistant, intermediate susceptible, and susceptible isolates (21, 22). For quality control, Streptococcus pneumoniae ATCC 49619 was tested with each batch of samples.
GAS isolates were emm typed by using procedures adapted from those of the Centers for Disease Control and Prevention (2, 6). Sequencing products were analyzed with an ABI Prism 3100 sequencer as described by the manufacturer (Applied Biosystems, Foster City, Calif.). DNA sequences were submitted to a BLAST search, requiring 95% or greater homology with a reference emm gene sequence for emm type assignment (2).
Univariate analyses were initially performed to assess possible associations between demographic variables and antibiotic resistance or emm type. Variables associated with the outcome of interest (P ≤ 0.15) were included in subsequent exact multivariable logistic regression model analyses. Analyses and final models were conducted by using SAS and included only those variables independently associated with the outcome of interest with P values of ≤0.05.
From January 1998 to December 2001, 692 GAS samples were received from eight military basic training sites (Table 1). Susceptibility to penicillin, levofloxacin, and vancomycin was seen in 100% of samples. Forty-four isolates (6.4%) were resistant to erythromycin, 34 (4.9%) were resistant to tetracycline, 4 (0.58%) were resistant to clindamycin, and 10 (1.45%) were multidrug resistant. Sustained temporal trends in antibiotic resistance over the duration of the study were not seen (data not shown).
Gender and training site were associated with antibiotic resistance (Table 1). Women were more likely to be resistant to tetracycline in multivariable modeling. In addition, isolates from the Air Force site were much more likely to be resistant to erythromycin, while isolates from Army sites were more likely to be resistant to tetracycline.
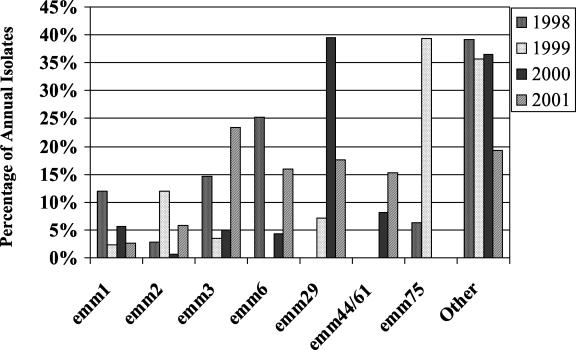
Over 30 different emm types were identified, with the most prevalent being emm29 (18.0%), emm3 (15.2%), emm6 (13.5%), emm44/61 (9.1%), emm2 (7.3%), emm75 (6.4%), and emm1 (4.8%) (Table 2). Univariate analyses demonstrated that training site was statistically associated with emm type. The Navy's Illinois site had proportionally more emm6 and emm44/61. Both emm29 and emm3 types were more prevalent at the Marine's site in South Carolina. The Air Force's site had a predominance of emm75 among its GAS isolates. Additionally, the prevalence of emm types varied from year to year (Fig. 1). An association between emm types and antibiotic resistance was also observed. Erythromycin resistance was strongly associated with emm75 isolates (P < 0.001). Tetracycline resistance was associated with emm29 and emm1 isolates.
TABLE 2.
Factors associated with emm gene type of GAS isolates
| Variable | Total no. of isolates | % of isolates of type:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| emm1 (n = 33) | emm2 (n = 50) | emm29 (n = 123) | emm3 (n = 104) | emm44/61 (n = 62) | emm6 (n = 92) | emm75 (n = 44) | Othera (n = 175) | Univariate χ2P valueb | ||
| Gender | ||||||||||
| Male | 587 | 4.94 | 8.01 | 18.6 | 16.2 | 8.86 | 13.8 | 6.47 | 23.17 | 0.018 |
| Female | 96 | 4.17 | 3.13 | 14.6 | 9.38 | 10.4 | 11.5 | 6.25 | 40.63 | |
| Age (yrs) | ||||||||||
| 17-18 | 196 | 1.02 | 8.16 | 20.9 | 20.4 | 5.61 | 10.2 | 5.61 | 28.06 | 0.002 |
| 19-20 | 278 | 4.68 | 6.83 | 17.99 | 16.19 | 8.99 | 15.83 | 7.91 | 21.58 | |
| 21-22 | 98 | 4.08 | 6.12 | 18.37 | 11.22 | 13.3 | 13.27 | 6.12 | 27.55 | |
| ≥23 | 107 | 11.2 | 8.41 | 12.2 | 7.48 | 12.2 | 14.02 | 4.67 | 29.91 | |
| Season | ||||||||||
| Summer (June-August) | 73 | 4.11 | 2.74 | 27.4 | 6.85 | 2.74 | 8.22 | 6.85 | 41.10 | <0.001 |
| Fall (September-November) | 250 | 3.6 | 12.4 | 25.6 | 23.6 | 5.20 | 2.80 | 0.80 | 26.00 | |
| Winter (December-February) | 159 | 6.92 | 3.77 | 16.4 | 10.1 | 13.8 | 21.4 | 9.43 | 18.24 | |
| Spring (March-May) | 204 | 5.88 | 5.39 | 6.37 | 11.8 | 12.3 | 22.1 | 10.8 | 25.49 | |
| Training site | ||||||||||
| Navy, Ill. | 222 | 2.70 | 8.56 | 0.0 | 8.11 | 27.0 | 32.9 | 0.90 | 19.82 | <0.001 |
| Marines, Calif. | 3 | 66.7 | 33.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Marines, S. C. | 280 | 3.57 | 8.21 | 43.2 | 25.7 | 0.0 | 1.43 | 0.36 | 17.50 | |
| Army, S. C. | 4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 25.0 | 0.0 | 75.0 | |
| Army, Ky. | 22 | 0.0 | 4.6 | 0.0 | 4.6 | 0.0 | 4.6 | 36.4 | 50.0 | |
| Army, Mo. | 36 | 2.78 | 8.33 | 5.56 | 30.6 | 5.56 | 2.78 | 5.56 | 38.89 | |
| Army, Okla. | 18 | 33.3 | 11.1 | 0.0 | 5.6 | 0.0 | 0.0 | 0.0 | 50.00 | |
| Air Force, Tex. | 101 | 9.90 | 0.99 | 0.0 | 0.99 | 0.0 | 11.9 | 30.7 | 45.54 | |
| Antibiotic resistance | ||||||||||
| Erythromycin | 43 | 4.65 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 65.12 | 30.23 | <0.001 |
| Tetracycline | 31 | 3.23 | 0.0 | 3.23 | 0.0 | 0.0 | 0.0 | 0.0 | 93.54 | <0.001 |
Other category includes the following emm types: emm12 (n = 22), emm22 (n = 13), emm11 (n = 19), emm5 (n = 14), emm89 (n = 15), emm28 (n = 19), emm18 (n = 10), emm27L/77 (n = 14), emm4 (n = 9), emm58 (n = 3), emm73 (n = 6), emm82 (n = 2), emm96 (n = 3), emm9 (n = 6), and 20 additional types with n = 1 each.
P values are based on the Monte Carlo estimate for the Pearson chi-square exact test.
FIG. 1.
Most common emm types of GAS isolates identified in military basic training surveillance, 1998-2001.
Discussion.
The growth of antibiotic resistance in GAS is a major concern for both military and civilian populations (1, 5, 19). We noted a prevalence of 6.4% erythromycin resistance and 4.9% tetracycline resistance among our population. Mass penicillin prophylaxis among recruits has proven effective in decreasing the frequency of outbreaks (11, 28). This practice is seasonal and/or dynamic and is modified depending upon local surveillance indicators. Prior research has demonstrated that prophylaxis of the penicillin-allergic individuals with erythromycin is useful in further reducing outbreak occurrences (9). However, this practice is site specific and variable. Due to the observed erythromycin resistance rate, antibiotic prophylaxis among the recruits at the training sites was examined. No apparent correlation with erythromycin resistance and a history of erythromycin use at the sites was noted; however, other researchers have shown the reality of this concern (10, 25).
From the molecular perspective, several GAS virulence factors are suggested to be associated with disease prevalence (13, 20, 24). The M protein is one of these virulence factors that offers GAS several mechanisms of defense against the host system (7, 8, 23, 29). Many common emm types reported by the Centers for Disease Control and Prevention's Active Bacterial Core Surveillance for invasive GAS were found in our surveillance of noninvasive illnesses: emm1, emm3, emm12, emm28, and emm89 (see Table 2). These emm types from noninvasive illnesses are comparable to those seen in civilian populations and reaffirm the concern that virulent strains of GAS are circulating in more benign presentations and potentially could escalate into invasive disease trends and mortality if not properly managed (16, 18). Furthermore, the heterogeneity and shifts of emm types observed reflects the importance of considering multiple emm types in designing GAS vaccines (17). Since the M protein is presently described as a major virulence factor in GAS infections, a number of present vaccine constructs are composed of multivalent M protein sequences specific to particular diseases or geographic regions (15). Our surveillance data may help to guide these constructs.
In conclusion, surveillance for GAS isolates among United States military trainees has revealed a significant prevalence of macrolide resistance. The emm type distribution varied across military training sites, with emm75 strongly correlated with erythromycin resistance at the Air Force site. Use of alternative antibiotics in penicillin-allergic individuals should be considered at sites with a background of increased macrolide resistance. Continued laboratory-based monitoring of GAS infections is important for the appropriate use of antibiotics and development of vaccines, thus minimizing streptococcal disease morbidity in both civilian and military populations.
Acknowledgments
This work represents report 03-01, supported by the Department of Defense, under work unit no. 6609.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
This surveillance is largely a credit to the personnel of our Streptococcal pyogenes Surveillance Group at the following institutions: Naval Recruit Training Center, Great Lakes, Ill.; Marine Corps Recruit Depot, San Diego, Calif.; Marine Corps Recruit Depot, Parris Island, S. C.; Army Basic Training Centers in Fort Jackson, S. C., Fort Knox, Ky., Fort Leonard Wood, Mo., and Fort Sill, Okla.; and Lackland Air Force Base, Tex. We also are grateful for the support and guidance of Ed Kaplan and Dwight Johnson of the University of Minnesota; Pat Kelley and Joel Gaydos of the Department of Defense Global Emerging Infections Surveillance program; Gary Gackstetter and Tonie Hooper of USUHS; and Mike Dove and the team at DMDC.
Footnotes
This research was presented in part at the American Society of Microbiology General Meeting, Salt Lake City, Utah, May 2002.
REFERENCES
- 1.Bassetti, M., G. Manno, A. Collida, A. Ferrando, G. Gatti, E. Ugolotti, M. Cruciani, and D. Bassetti. 2000. Erythromycin resistance in Streptococcus pyogenes in Italy. Emerg. Infect. Dis. 6:180-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beall, B., R. Facklam, and T. Thompson. 1996. Sequencing emm-specific polymerase chain reaction products for routine and accurate typing of group A streptococci. J. Clin. Microbiol. 34:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brundage, J. F., J. D. Gunzenhauser, J. N. Longfield, M. Rubertone, S. Ludwig, F. Rubin, et al. 1996. Epidemiology and control of acute respiratory diseases with emphasis on group A beta-hemolytic streptococcus: a decade of U. S. Army experience. Pediatrics 97:964-970. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1988. Acute rheumatic fever among Army trainees—Fort Leonard Wood, Missouri. Morb. Mortal. Wkly. Rep. 37:519-522. [PubMed] [Google Scholar]
- 5.Cha, S., H. Lee, K. Lee, K. Hwang, S. Bae, and Y. Lee. 2001. The emergence of erythromycin-resistant Streptococcus pyogenes in Seoul, Korea. J. Infect. Chemother. 7:81-86. [DOI] [PubMed] [Google Scholar]
- 6.Facklam, R., B. Beall, A. Efstratiou, V. Fischetti, D. Johnson, E. Kaplan, P. Kriz, M. Lovgren, D. Martin, B. Schwartz, A. Totolian, D. Bessen, S. Hollingshead, F. Rubin, J. Scott, and G. Tyrrell. 1999. Emm typing and validation of provisional M types for group A streptococci. Emerg. Infect. Dis. 5:247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Facklam, R. F., D. R. Martin, M. Lovgren, D. R. Johnson, A. Efstratiou, T. A. Thompson, S. Gowan, P. Kriz, G. J. Tyrrell, E. Kaplan, and B. Beall. 2002. Extension of the Lancefield classification for group A streptococci by addition of 22 new M protein gene sequence types from clinical isolates: emm103 to emm124. Clin. Infect. Dis. 34:28-38. [DOI] [PubMed] [Google Scholar]
- 8.Fischetti, V., R. Horstmann, and V. Pancholi. 1995. Location of the complement factor H binding site on streptococcal M6 protein. Infect. Immun. 63:149-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujikawa, J., J. P. Struewing, K. C. Hyams, E. L. Kaplan, A. K. Tupponce, and G. C. Gray. 1992. Oral erythromycin prophylaxis against Streptococcus pyogenes infection in penicillin-allergic military recruits: a randomized clinical trial. J. Infect. Dis. 166:162-165. [DOI] [PubMed] [Google Scholar]
- 10.Granizo, J. J., L. Aguilar, J. Casal, R. Dal-Re, and F. Baquero. 2000. Streptococcus pyogenes resistance to erythromycin in relation to macrolide consumption in Spain (1986-1997). J. Antimicrob. Chemother. 46:959-964. [DOI] [PubMed] [Google Scholar]
- 11.Gunzenhauser, J. D., J. F. Brundage, J. G. McNeil, and R. N. Miller. 1992. Broad and persistent effects of benzathine penicillin G in the prevention of febrile, acute respiratory disease. J. Infect. Dis. 166:365-373. [DOI] [PubMed] [Google Scholar]
- 12.Gunzenhauser, J. D., J. N. Longfield, J. F. Brundage, E. L. Kaplan, R. N. Miller, and C. A. Brandt. 1995. Epidemic streptococcal disease among Army trainees, July 1989 through June 1991. J. Infect. Dis. 172:124-131. [DOI] [PubMed] [Google Scholar]
- 13.Hoban, D. J. 2002. Prevalence and characterization of macrolide resistance in clinical isolates of Streptococcus pneumoniae and Streptococcus pyogenes from North America. J. Chemother. 14(Suppl. 3):25-30. [DOI] [PubMed] [Google Scholar]
- 14.Hoge, C. W., B. Schwartz, D. F. Talkington, R. F. Breiman, E. M. MacNeil, and S. J. Englender. 1993. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome. JAMA 269:384-389. [PubMed] [Google Scholar]
- 15.Hu, M. C., M. A. Walls, S. D. Stroop, M. A. Reddish, B. Beall, and J. B. Dale. 2002. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect. Immun. 70:2171-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, D. R., D. L. Stevens, and E. L. Kaplan. 1992. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J. Infect. Dis. 166:374-382. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan, E. L., J. T. Wotton, and D. R. Johnson. 2001. Dynamic epidemiology of group A streptococcal serotypes associated with pharyngitis. Lancet 358:1334-1337. [DOI] [PubMed] [Google Scholar]
- 18.Kiska, D. L., B. Thiede, J. Caracciolo, M. Jordan, D. Johnson, E. L. Kaplan, R. P. Gruninger, J. A. Lohr, P. H. Gilligan, and F. W. Denny, Jr. 1997. Invasive group A streptococcal infections in North Carolina: epidemiology, clinical features, and genetic and serotype analysis of causative organisms. J. Infect. Dis. 176:992-1000. [DOI] [PubMed] [Google Scholar]
- 19.Martin, J. M., M. Green, K. Barbadora, and E. R. Wald. 2002. Erythromycin-resistant group A streptococci in schoolchildren in Pittsburgh. N. Engl. J. Med. 346:1200-1206. [DOI] [PubMed] [Google Scholar]
- 20.Murakami, J., S. Kawabata, Y. Terao, K. Kikuchi, K. Totsuka, A. Tamaru, C. Katsukawa, K. Moriya, I. Nakagawa, I. Morisaki, and S. Hamada. 2002. Distribution of emm genotypes and superantigen genes of Streptococcus pyogenes isolated in Japan, 1994-9. Epidemiol. Infect. 128:397-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NCCLS. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS Document M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 22.NCCLS. 2001. Performance standards for antimicrobial susceptibility testing; eleventh informational supplement. NCCLS Document M100-S11. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.Peterson, P., D. Schmeling, P. Cleary, B. Wilkinson, Y. Kim, and P. Quie. 1979. Inhibition of alternative complement pathway opsonization by group A streptococcal M protein. J. Infect. Dis. 139:575-585. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz, B., R. Facklam, and R. Breiman. 1990. Changing epidemiology of group A streptococcal infection in the USA. Lancet i:1167-1171. [DOI] [PubMed] [Google Scholar]
- 25.Seppala, H., T. Klaukka, R. Lehtonen, E. Nenonen, and P. Huovinen. 1995. Outpatient use of erythromycin: link to increased erythromycin resistance in group A streptococci. Clin. Infect. Dis. 21:1378-1385. [DOI] [PubMed] [Google Scholar]
- 26.Seppala, H., A. Nissinen, H. Jarvinen, S. Huovinen, T. Henriksson, E. Herva, S. E. Holm, M. Jahkola, M. L. Katila, T. Klaukka, et al. 1992. Resistance to erythromycin in group A streptococci. N. Engl. J. Med. 5:292-297. [DOI] [PubMed] [Google Scholar]
- 27.Stevens, D., M. Tanner, J. Winship, R. Swarts, K. Ries, P. Schlievert, and E. Kaplan. 1989. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N. Engl. J. Med. 321:1-7. [DOI] [PubMed] [Google Scholar]
- 28.Thomas, R. J., D. E. Conwill, D. E. Morton, T. J. Brooks, C. K. Holmes, and W. B. Mahaffey. 1988. Penicillin prophylaxis for streptococcal infections in United States Navy and Marine Corps recruit camps, 1951-1985. Rev. Infect. Dis. 10:125-130. [DOI] [PubMed] [Google Scholar]
- 29.Tran, P., D. Johnson, and E. Kaplan. 1994. The presence of M protein in nontypeable group A streptococcal upper respiratory tract isolates from southeast Asia. J. Infect. Dis. 169:658-661. [DOI] [PubMed] [Google Scholar]
- 30.Veasy, L., S. Wiedmeier, G. Orsmond, H. Ruttenberg, M. Boucek, S. Roth, V. Tait, J. Thompson, J. Daly, E. Kaplan, and H. Hill. 1987. Resurgence of acute rheumatic fever in the intermountain area of the United States. N. Engl. J. Med. 316:421-427. [DOI] [PubMed] [Google Scholar]
- 31.Wallace, M. R., P. D. Garst, T. J. Papadimos, and E. C. Oldfield. 1989. The return of acute rheumatic fever in young adults. JAMA 262:2557-2561. [PubMed] [Google Scholar]