Abstract
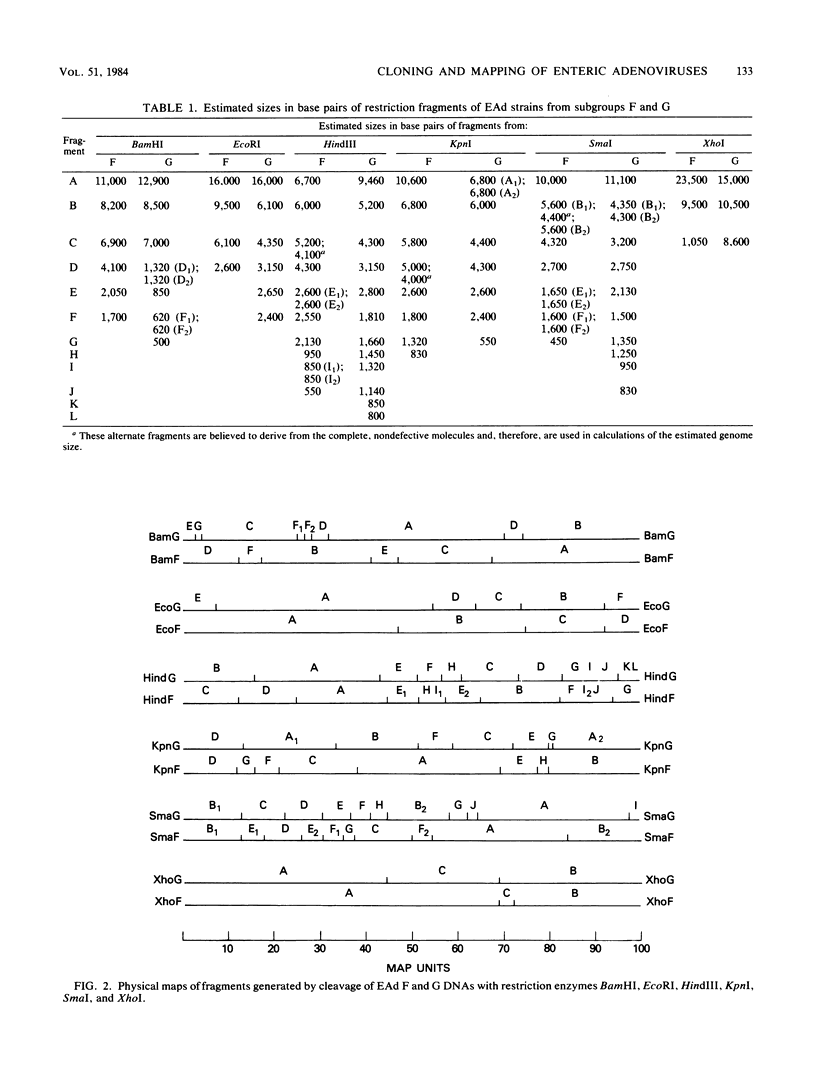
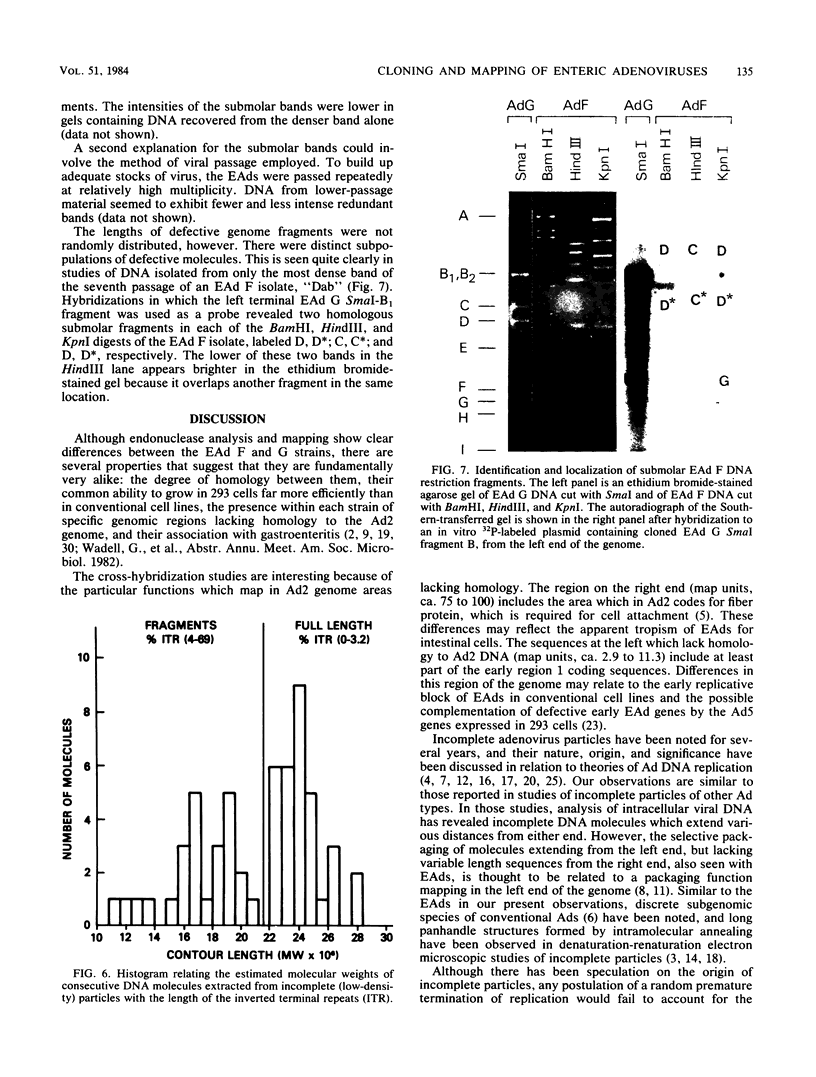
We have studied the DNAs of fastidious enteric adenoviruses recovered from the stools of infants with gastroenteritis. By endonuclease analysis, the strains examined represent candidate adenovirus types 40 and 41, which are thought to comprise new adenovirus subgroups F and G. Cloning of DNA from representative enteric adenovirus isolates, together with hybridization and subcleavage analysis, permitted the mapping of restriction enzyme cleavage sites. Although the restriction profiles are different for the two strains, they appear to have several cleavage sites in common. Cross hybridization studies show considerable homology between the subgroup F and G strains but much less homology to adenovirus 2. In addition, regions on both ends of enteric adenovirus genomes (map units, 2.9 to 11.3 and 75 to 100) possess little or no homology to adenovirus 2. Restriction enzyme digests reveal submolar fragments that map to the terminal regions of the genome. Electron micrographic studies of denatured and renatured DNA strands suggest that the submolar fragments may derive from cleavage of defective molecules. Inverted terminal repeat sequences were shown to comprise 0 to 3.2% of the length of complete (greater than or equal to 22 megadaltons) enteric adenovirus DNA molecules but 4 to 69% of incomplete-length (less than 22-megadalton) molecules.
Full text
PDF
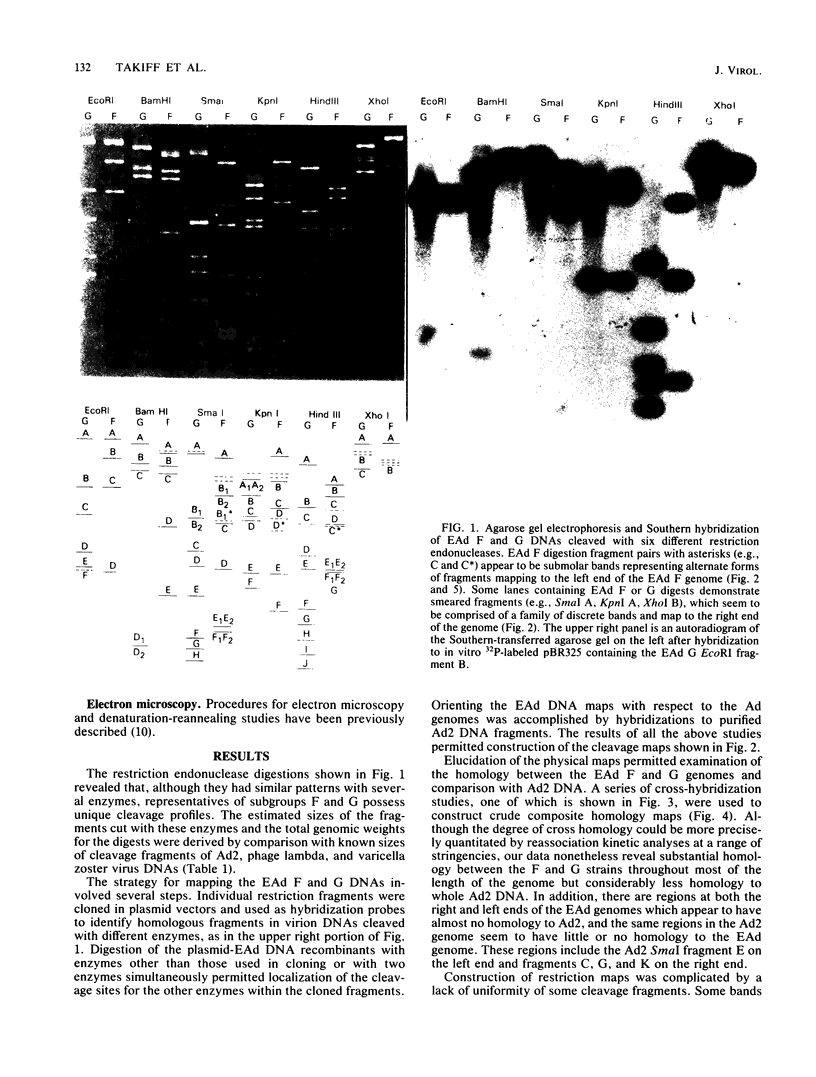


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Yolken R. H., Kapikian A. Z., Arrobio J. O., Rodriguez W. J., Wyatt R. G., Chanock R. M., Parrott R. H. Comparative epidemiology of two rotavirus serotypes and other viral agents associated with pediatric gastroenteritis. Am J Epidemiol. 1979 Sep;110(3):243–254. doi: 10.1093/oxfordjournals.aje.a112809. [DOI] [PubMed] [Google Scholar]
- Brown M., Weber J. Discrete subgenomic DNA fragments in incomplete particles of adenovirus type 2. J Gen Virol. 1982 Sep;62(Pt 1):81–89. doi: 10.1099/0022-1317-62-1-81. [DOI] [PubMed] [Google Scholar]
- Burlingham B. T., Brown D. T., Doerfler W. Incomplete particles of adenovirus. I. Characteristics of the DNA associated with incomplete adenovirions of types 2 and 12. Virology. 1974 Aug;60(2):419–430. doi: 10.1016/0042-6822(74)90336-5. [DOI] [PubMed] [Google Scholar]
- Carlson D. P., Raskas H. J. Structure and metabolism of adenovirus RNAs containing sequences from the fiber gene. J Mol Biol. 1980 Aug 15;141(3):249–265. doi: 10.1016/0022-2836(80)90180-1. [DOI] [PubMed] [Google Scholar]
- Daniell E. Genome structure of incomplete particles of adenovirus. J Virol. 1976 Aug;19(2):685–708. doi: 10.1128/jvi.19.2.685-708.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E., Mullenbach T. Synthesis of defective viral DNA in HeLa cells infected with adenovirus type 3. J Virol. 1978 Apr;26(1):61–70. doi: 10.1128/jvi.26.1.61-70.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E. Subgenomic viral DNA species synthesized in simian cells by human and simian adenoviruses. J Virol. 1981 Feb;37(2):620–627. doi: 10.1128/jvi.37.2.620-627.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F. Electron microscopy of nucleic acids. Gene Amplif Anal. 1981;2:573–589. [PubMed] [Google Scholar]
- Hammarskjöld M. L., Winberg G. Encapsidation of adenovirus 16 DNA is directed by a small DNA sequence at the left end of the genome. Cell. 1980 Jul;20(3):787–795. doi: 10.1016/0092-8674(80)90325-6. [DOI] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Winberg G., Norrby E., Wadell G. Isolation of incomplete adenovirus 16 particles containing viral and host cell DNA. Virology. 1977 Oct 15;82(2):449–461. doi: 10.1016/0042-6822(77)90018-6. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Khittoo G., Weber J. M. The nature of the DNA associated with incomplete particles of adenovirus type 2. J Gen Virol. 1981 Jun;54(Pt 2):343–355. doi: 10.1099/0022-1317-54-2-343. [DOI] [PubMed] [Google Scholar]
- Mak I., Ezoe H., Mak S. Structure and function of adenovirus type 12 defective virions. J Virol. 1979 Oct;32(1):240–250. doi: 10.1128/jvi.32.1.240-250.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prage L., Höglund S., Philipson L. Structural proteins of adenoviruses. 8. Characterization of incomplete particles of adenovirus type 3. Virology. 1972 Sep;49(3):745–757. doi: 10.1016/0042-6822(72)90531-4. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Chinnadurai G. A unique subgenomic species of adenovirus 2 DNA generated under high multiplicities of infection. Nucleic Acids Res. 1979 Nov 10;7(5):1163–1174. doi: 10.1093/nar/7.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retter M., Middleton P. J., Tam J. S., Petric M. Enteric adenoviruses: detection, replication, and significance. J Clin Microbiol. 1979 Oct;10(4):574–578. doi: 10.1128/jcm.10.4.574-578.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwirth B., Tjia S., Westphal M., Doerfler W. Incomplete particles of adenovirus. II. Kinetics of formation and polypeptide composition of adenovirus type 2. Virology. 1974 Aug;60(2):431–437. doi: 10.1016/0042-6822(74)90337-7. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Ginsberg H. S., Rose J. A. DNA-minus temperature-sensitive mutants of adenovirus type 5 help adenovirus-associated virus replication. J Virol. 1975 Jan;17(1):140–148. doi: 10.1128/jvi.17.1.140-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Owens J., Ruyechan W. T., Takiff H. E., Casey T. A., Vande Woude G. F., Hay J. Molecular cloning and physical mapping of varicella-zoster virus DNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):993–997. doi: 10.1073/pnas.79.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiff H. E., Straus S. E., Garon C. F. Propagation and in vitro studies of previously non-cultivable enteral adenoviruses in 293 cells. Lancet. 1981 Oct 17;2(8251):832–834. doi: 10.1016/s0140-6736(81)91104-1. [DOI] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Tibbetts C., Johansson K., Philipson L. Hydroxyapatite chromatography and formamide denaturation of adenovirus DNA. J Virol. 1973 Aug;12(2):218–225. doi: 10.1128/jvi.12.2.218-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C. Viral DNA sequences from incomplete particles of human adenovirus type 7. Cell. 1977 Sep;12(1):243–249. doi: 10.1016/0092-8674(77)90202-1. [DOI] [PubMed] [Google Scholar]
- Wadell G., Hammarskjöld M. L., Winberg G., Varsanyi T. M., Sundell G. Genetic variability of adenoviruses. Ann N Y Acad Sci. 1980;354:16–42. doi: 10.1111/j.1749-6632.1980.tb27955.x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Lawrence F., Leister F., Takiff H. E., Strauss S. E. Gastroenteritis associated with enteric type adenovirus in hospitalized infants. J Pediatr. 1982 Jul;101(1):21–26. doi: 10.1016/s0022-3476(82)80173-x. [DOI] [PubMed] [Google Scholar]
- de Jong J. C., Wigand R., Kidd A. H., Wadell G., Kapsenberg J. G., Muzerie C. J., Wermenbol A. G., Firtzlaff R. G. Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stool. J Med Virol. 1983;11(3):215–231. doi: 10.1002/jmv.1890110305. [DOI] [PubMed] [Google Scholar]