Abstract
Right ventricular (RV) dysfunction is a common long-term complication in patients after the repair of congenital heart disease. Previous investigators have examined the cellular and molecular mechanisms of left ventricular (LV) remodeling, but little is known about the stressed RV. Our purpose was to provide a detailed physiological characterization of a model of RV hypertrophy and failure, including RV-LV interaction, and to compare gene alterations between afterloaded RV versus LV. Pulmonary artery constriction was performed in 86 mice. Mice with mild and moderate pulmonary stenosis (PS) developed stable hypertrophy without decompensation. Mice with severe PS developed edema, decreased RV function, and high mortality. Tissue Doppler imaging demonstrated septal dyssynchrony and deleterious RV-LV interaction in the severe PS group. Microarray analysis showed 196 genes with increased expression and 1,114 with decreased expression. Several transcripts were differentially increased in the afterloaded RV but not in the afterloaded LV, including clusterin, neuroblastoma suppression of tumorigenicity 1, Dkk3, Sfrp2, formin binding protein, annexin A7, and lysyl oxidase. We have characterized a murine model of RV hypertrophy and failure, providing a platform for studying the physiological and molecular events of RV remodeling. Although the molecular responses of the RV and LV to afterload stress are mostly concordant, there are several key differences, which may represent targets for RV failure-specific therapy.
Keywords: gene expression, right ventricle, tetralogy of Fallot
the right ventricle is uniquely at risk in patients with complex congenital heart disease involving right-sided obstructive lesions (e.g., tetralogy of Fallot, tetralogy/pulmonary atresia) and in patients with systemic right ventricles (1, 40, 46). Increased stress on the right ventricle in the form of increased hemodynamic loading (pressure and/or volume) may result in abnormalities in cardiac structure, function, metabolism, coronary perfusion, neurohormonal activation, and molecular signaling. These stresses lead to both adaptive and maladaptive structural and molecular remodeling of the right ventricle and deleterious effects on the left ventricle through ventricular-ventricular interaction and may limit long-term survival (3, 7, 29, 42). For many of these patients, detrimental conditions for the right ventricle exist throughout life, even after successful repair or palliation. As surgical techniques for the primary repair of complex forms of congenital heart disease improve, long-term survival and quality of life will depend on our ability to preserve long-term right ventricular (RV) function. Decisions regarding the ideal time for surgical reintervention will need to be based on more quantitative rather than qualitative measures.
Developing a better understanding of the molecular mechanisms of RV remodeling will assist in developing new therapeutic modalities to diagnose, prevent, and treat RV dysfunction. Although there are considerable data on the molecular events underlying afterload-induced left ventricular (LV) remodeling, there is little information for the right ventricle and some evidence to suggest that the stress responses of the right and left ventricles may be different. In the past, it was believed that differences in global structure and loading conditions represented the main differences between the right and left ventricles. However, recent work has shown an increasing divergence in the anterior and primary heart field pathways leading to the differentiation of RV versus LV cardiomyocytes during early development (46) and chamber-specific differences in cell signaling and calcium handling, suggesting fundamental differences between the right and left ventricles at the cellular level as well (10, 32, 34, 54, 72). Recent studies suggest that standard pharmacotherapies used to treat LV dysfunction may not be as effective in patients with dysfunction of a systemic right ventricle (18). The development and characterization of a murine model of the afterloaded right ventricle will add significantly to our knowledge of chamber-specific molecular responses to stress and provide the basis for the further dissection of molecular pathways using the wide range of transgenic and gene knockouts for specific signaling components.
We utilized a murine model of RV pressure overload hypertrophy and RV failure using pulmonary artery (PA) constriction (PAC). The purpose of the current study was to, first, provide a detailed physiological characterization of this model, including a documentation of the effects of RV-LV interaction using tissue-Doppler imaging (TDI). TDI has emerged as a leading technique for the assessment of ventricular dyssynchrony using data obtained from myocardial motion (17, 57). TDI-derived strain has been shown to be an excellent index of regional myocardial function because it is less influenced by overall cardiac motion (24, 25, 61). Although the usefulness of TDI has been shown in the assessment of LV function in mice (58), data on RV function are lacking. We also sought to provide the first evaluation of genome-wide transcriptional alterations associated with RV pressure loading as well as a preliminary comparison of gene changes in the afterloaded RV versus LV.
METHODS
Model of PAC.
All mice were male FVB, aged 8 to 10 wk. Anesthesia was induced with pentobarbital sodium (50 mg/kg ip). Mice were then intubated transtracheally with a 20-gauge angiocath and ventilated artificially using a Harvard rodent ventilator (Harvard Apparatus, Holliston, MA) at a rate of 120–140 breaths/min with a tidal volume of 10 μl/g body wt. Maintenance anesthesia was provided with 1% to 2% isoflurane. A right lateral thoracotomy was then performed. The main pulmonary trunk was identified under the left atrial appendage and banded with a 7-0 suture, tied tight against a 27-gauge needle, which was then removed. The chest was then closed with 7-0 sutures around adjacent ribs, and the skin was closed with 5-0 suture. Air was evacuated to avoid the need for a chest tube postoperatively. Our laboratory has previously shown the importance of using true sham controls in studies of gene expression in the afterloaded LV (73), so all age- and strain-matched controls in this study were subjected to the same operative procedure, including the dissection of the main PA, with the sole exception of the placement of the band.
Echocardiography and TDI.
Echocardiographic images were acquired with a GE Vivid 7 ultrasound system (GE Healthcare, Milwaukee, WI) equipped with both 13- and 10-MHz transducers. Mice subjected to pulmonary banding and sham-operated controls underwent echocardiography at 7 days postoperative to evaluate the status of the surgical intervention and obtain hemodynamic data. Doppler signals analyzed included maximum pulse wave Doppler and velocity-time integral (VTI), with angle correction, in the RV outflow tract (RVOT) to estimate the peak pressure gradient (PPG) and mean pressure gradient (MPG) between the RV and PA. Cardiac output was calculated as VTI × RVOT area × heart rate (HR) (70).
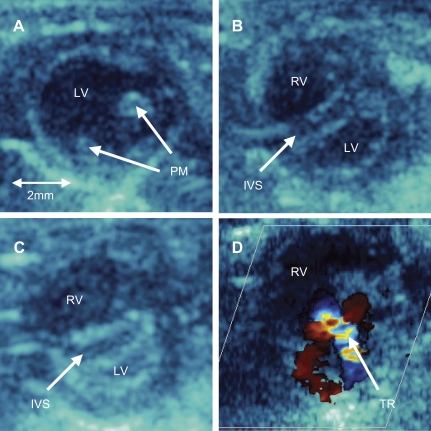
Mice were divided into three groups based on echocardiographic findings. Mice with a PPG of <20 mmHg were excluded from further study (n = 2). Mice with a PPG of 20–35 mmHg and without RV enlargement (Fig. 1A) were included in the mild pulmonary stenosis (PS) group. Mice with PPG between 35 and 60 mmHg but without evidence of flattening of the interventricular septum and without evidence of heart failure were included in the moderate PS group. Mice with an RV-PA PPG between 35 and 60 mmHg and with evidence of RV enlargement and either a flat interventricular septum or concave septal shift encroaching into the left ventricle (Fig. 1, B and C) were included in the severe PS group. As will be seen below, these mice also had evidence of right-sided heart failure [increased RV end-diastolic pressure, tricuspid regurgitation (Fig. 1D), and peripheral edema]. Peripheral edema was assessed both visually and by increases in body weight (BW) and liver weight.
Fig. 1.
A: systolic 2-dimensional image from the parasternal short-axis view at the level of the left ventricular (LV) papillary muscles (PM) in a mouse with mild pulmonary stenosis (PS). Mice with moderate PS showed similar 2-dimensional echo characteristics. B: comparable systolic 2-dimensional image in a mouse with severe PS showing a markedly enlarged right ventricular (RV) chamber and flattening of the interventricular septal (IVS). C: comparable systolic image in a mouse with severe PS showing bowing of the IVS into the LV cavity. D: color Doppler echocardiogram from parasternal short-axis view at the level of aortic valve in a mouse with severe PS showing significant tricuspid regurgitation (TR).
LV fractional shortening (FS), LV diastolic dimension, and HR were calculated from M-mode echocardiograms in the parasternal short-axis view at the level of papillary muscles. RVOT dimension and RVOT FS were measured from the RVOT view at the level of the aortic valve. TDI was performed to assess RV function and RV-LV interaction. TDI images were collected in the apical four-chamber view at frame rates of 477 frames/s and at depths of 1 cm. The region of interest was placed at the tricuspid valve annulus to evaluate RV free wall maximum velocity, and analysis was performed offline with the use of commercially available Echo Pac software (GE Healthcare).
ECG and invasive hemodynamic parameters.
ECGs were recorded to assess intra-RV conduction using Chart for Windows v4.1 (AD Instruments, Colorado Springs, CO). For invasive hemodynamic evaluation, a 1.4 F transducer-tipped micromanometer catheter (Millar Instruments, Houston, TX) was inserted via the right jugular vein to determine RV pressure and change in pressure over time (dP/dt) as described previously (56, 68). Steady-state data were measured over an average of 10 beats.
Organ weight and histopathology.
Whereas all echocardiographic measurements were performed at 7 days, all morphometric and gene expression analysis studies were performed at 10 days after PAC. This 3-day break was introduced to minimize gene changes secondary to the stress of anesthesia at the time echocardiography (73). At 10 days after PAC, the heart and liver were excised, and their weights were determined. The heart was separated into right and left ventricles and the septum. From the mid-RV and mid-LV, transverse 5-μm sections were cut and stained with wheat germ agglutinin and 4-6-diamidino-2-phenylindole to determine the myocyte cross-sectional area. The cardiomyocyte cross-sectional area was measured with the use of a Leica imaging system (Leica Microsystems, Exton, PA). At least 60 cardiomyocytes were examined in each heart (n = 3) for a total of 180 cardiomyocytes for each condition, and the data were averaged (2).
Gene microarrays.
Samples were obtained from the RV free wall 10 days after either PAC or sham operation. Mice with severe PS were used for gene expression studies (n = 16). Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA), and RNA was then reverse transcribed to double-stranded cDNA. Labeled cRNA was synthesized by the incubation of 1 g cDNA with biotin-labeled ribonucleotides and RNA polymerase for 5 h at 37°C using the BioArray High Yield RNA transcript labeling kit (Enzo Diagnostics, Farmingdale, NY). Biotin-labeled cRNAs were then fragmented by heating and hybridized onto microarrays composed of 38,467 70mer oligonucleotide probes representing over 25,000 genes, essentially the entire mouse transcriptome. The array includes 35,302 well-annotated probes targeting mouse genes and alternative exons, as well as multiple spots for biological and technical controls. For more details on the microarray platform and our methods for quality control, see Wagner et al. (71), Zhao et al. (73), and the Stanford Functional Genomics Web site (60). Microarrays were scanned on an Agilent G2565AA microarray scanner. Quantitative RT-PCR (QRT-PCR) was performed for each of the major differentially expressed genes in discussion for verification. Gene expression data from RV samples after PAC were then compared with LV samples obtained from mice after 10 days of aortic banding [transverse aortic constriction (TAC)], obtained from previous studies from our laboratory (73).
Microarray analysis.
Statistical analysis was performed using Stanford microarray database software, with subsets of the data exported to the Institute for Genome Research Multiple Array Viewer (55), significance analysis of microarrays (SAM), and classification software such as prediction analysis of microarrays (62, 67). Clustering algorithms used include two-dimensional hierarchical clustering analysis, K-means clustering, ingenuity pathway assist (Ingenuity Systems, Redwood City, CA), and self-organizing maps (59, 63). These analyses identify smaller clusters of genes with distinct expression patterns that highlight distinct characteristics of subsets of the experimental samples. Gene ontology (GO) overrepresentation analysis was used to identify biological processes which were up- or downregulated. Groups of genes identified as differentially regulated were analyzed for GO class overrepresentation using Fisher's exact test.
Model of TAC.
RV gene expression during PAC was compared with LV gene expression during TAC as published previously (73). Anesthesia was induced with 3% isoflurane and maintained with 1.5% isoflurane. TAC was performed via a left thoracotomy incision, avoiding the pleural space and, hence, the need for artificial ventilation, as described by Rockman et al. (52). A 7-0 silk suture was placed around the transverse aorta between the left common carotid artery and the brachiocephalic trunk and tied tight around both the aorta and a 27-gauge needle, which was then removed, yielding a reproducible degree of constriction. LV myocardium was obtained at 10 days postoperatively and hybridized to Affymetrix U74Av2 mouse genome arrays (Affymetrix, Santa Clara, CA), containing 12,488 known genes and expressed sequences tags (ESTs). Details of the analysis of these samples have been published previously (73).
Statistical analysis.
The XL stat software (Addinsoft) was used. Values are expressed as means ± SD. Statistical significance of differences in mean values of physiological parameters from sham-operated and PAC (mild, moderate, and severe PS) mice were assessed by one-way ANOVA with Fisher's paired least significant difference post hoc testing using Statview software (SAS, Cary, NC). A probability value ≤0.05 was considered significant.
Animal care.
All procedures were performed in accordance with National Institutes of Health standards and were approved by the Administrative Panel on Laboratory Animal Care at Stanford University.
RESULTS
Survival and evidence of right heart failure.
PAC was performed on 86 male FVB mice. In the early phase of our studies, four mice died during the procedure due to hemorrhage from the PA. Subsequent survival has been 100%. Echocardiographic evaluation to assess RV-PA PPG was performed on postoperative day 7 in 82 mice. Two mice with an RV-PA PPG of <20 mmHg were excluded, leaving 80 mice for further study. Thus, after a short initial learning curve, PAC can be successfully performed in 92% of mice.
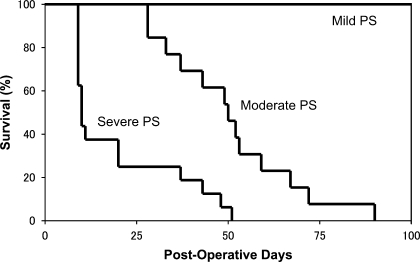
Thirty-nine mice were categorized into the severe PS group with evidence of RV enlargement and either a flat interventricular septum or concave septal shift (Fig. 1, B and C). Twenty-nine mice were categorized into the moderate PS group without a flattening of the interventricular septum, and 12 mice were categorized into the mild PS group (Fig. 1A). Within each group, one subgroup was evaluated by weekly echocardiography and survival analysis performed by a log-rank analysis of Kaplan-Meier curves (Fig. 2). There was a significant difference in survival between the mild, moderate, and severe PS groups. All mice with mild PS survived to 100 days, which was the end of our study. In contrast, the mean survival was 50.8 days in the moderate PS group and only 19.6 days in the severe PS group (P < 0.001).
Fig. 2.
Kaplan-Meier survival curves comparing mice with mild PS (n = 5), moderate PS (n = 13), and severe PS (n = 16) after pulmonary artery (PA) constriction (PAC). Survival half-time was 19.6 days in severe PS vs. 50.8 days in moderate PS. None of the mice with mild PS died by 100 days, which was the end of our study. There was a significant difference between moderate PS and severe PS survival (P < 0.001 by log-rank analysis).
There was no significant difference between groups in BW or in HR at 7 days postoperative (Table 1); however, all mice with severe PS developed signs of right heart failure with peripheral edema by 7 days postoperative (Fig. 3A). The edema was moderate in 35 mice and severe in four mice. No mice with mild or moderate PS developed peripheral edema.
Table 1.
Echocardiographic parameters of PAC and sham-operated controls at 1 wk postoperative
| Control | Mild | Moderate | Severe | ANOVA | |
|---|---|---|---|---|---|
| n | 16 | 5 | 13 | 16 | |
| BW, g | 27.7±3.6 | 27.9±1.4 | 27.8±1.6 | 27.7±3.6 | NS |
| HR, beats/min | 449.2±36.2 | 457.4±37.4 | 451.8±34.0 | 462.0±38.2 | NS |
| LVDd, mm | 3.18±0.15 | 3.16±0.19 | 3.14±0.24 | 1.89±0.21* | P < 0.001 |
| LV FS, % | 44.65±2.46 | 43.26±2.82 | 44.94±3.30 | 63.01±5.49* | P < 0.001 |
| RVOTDd, mm | 1.19±0.07 | 1.20±0.07 | 1.40±0.11* | 1.71±0.14* | P < 0.001 |
| RVOT FS, % | 32.77±4.44 | 33.55±6.77 | 29.52±3.11 | 16.39±3.92* | P < 0.001 |
| PPG/RV-PA, mmHg | 2.55±1.56 | 26.35±3.47* | 41.45±4.39* | 47.54±3.67* | P < 0.001 |
| VTI, cm | 5.59±0.51 | 5.74±0.38 | 4.19±0.41* | 1.86±0.22* | P < 0.001 |
| CO, ml/min | 28.08±4.97 | 29.77±4.57 | 29.46±6.41 | 19.80±3.48* | P < 0.001 |
Values are means ± SD; n, no. of mice/group.
P < 0.001 vs. control. PAC, pulmonary artery (PA) constriction; BW, body weight; HR, heart rate; LVDd, left ventricular (LV) diastolic dimension; FS, fractioning shortening; RVOTDd, right ventricular (RV) outflow tract (RVOT) diastolic dimension; PPG, peak pressure gradient; VTI, velocity-time integral; CO, cardiac output; NS, not significant.
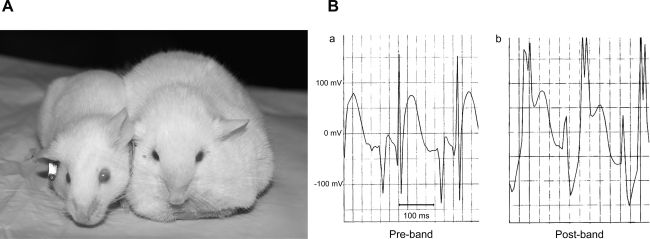
Fig. 3.
A: a mouse with generalized edema 2 wk after banding (right) compared with a sham-operated control (left). B: representative ECGs before (a) and after (b) PAC. Mice with severe PS showed ECG findings of a widened QRS with rsR′ pattern, consistent with an incomplete right bundle branch block, as well as ST-T elevations within 7 days of PAC. Mice with mild or moderate PS did not show these ECG findings.
Electrocardiographic findings 1 wk after PAC.
ECGs were performed 1 wk after PAC in five mice with mild PS, in 13 mice with moderate PS, and in 16 mice with severe PS. Eleven of the 16 (69%) mice with severe PS developed a widened QRS with an rsR′ pattern [incomplete right bundle branch block (iRBBB)] along with ST-T elevations (Fig. 3B). None of the mice with mild or moderate PS developed ECG abnormalities.
Echocardiographic findings 1 wk after PAC.
Characteristics of each PAC group and sham-operated controls at postoperative day 7 are shown in Table 1. Mice with moderate PS showed a mild enlargement of the RV (as assessed by the short-axis RVOT diameter) versus that of mice with mild PS and sham-operated controls. In contrast, mice with severe PS showed a markedly enlarged RV chamber (increased by 44%) and either flattening (Fig. 1B) of the septum or bowing of the septum into the LV, with the LV assuming a crescent-shaped appearance in the short-axis (Fig. 1C). In all mice with severe PS, there was also evidence of tricuspid regurgitation (Fig. 1D), which was not present in mice with mild or moderate PS.
RV-LV interaction and LV dyssynchrony.
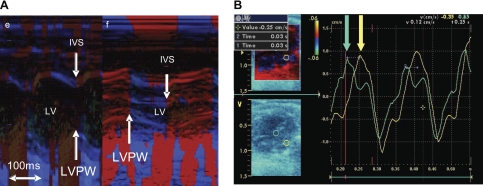
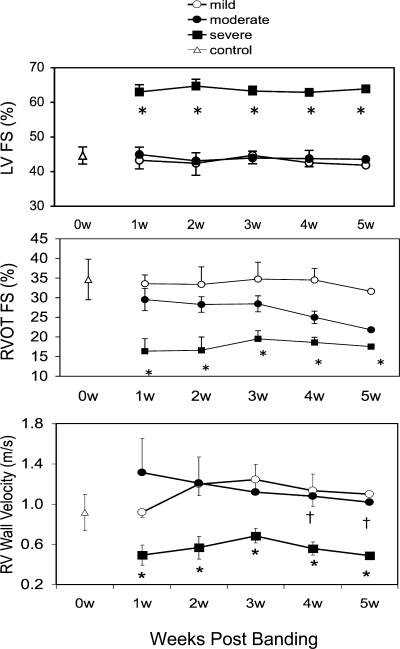
Color M-mode echocardiograms in mild and moderate PS showed that interventricular septal (IVS) motion was coordinated with the LV free wall, whereas there was paradoxical septal motion during systole in severe PS (Fig. 4A). Combined with the right bundle branch block, this resulted in intraleft ventricular dyssynchrony, similar to that seen in many patients with repaired tetralogy of Fallot. The delay between anterior LV free wall and posterior septal maximal excursion averaged 33.3 ± 15.6 ms in mice with severe PS. TDI also showed significant septal-LV free wall dyssynchrony, which was present only in mice with severe PS (Fig. 4B). Left ventricle diastolic dimension (LVDd) was significantly lower in severe PS versus the other groups (Table 1). Because of the marked decrease in LV volume, M-mode-derived LV FS was increased; however, RVOT FS was reduced in the severe PS group (see Serial changes in LV and RV function), and cardiac output (CO), derived by Doppler echocardiography, was significantly reduced in mice with severe PS (Table 1).
Fig. 4.
A: representative color M-mode echocardiogram from a mouse with moderate PS (e) compared with severe PS (f). In moderate PS, the IVS motion is coordinated with the LV posterior wall (LVPW), whereas in severe PS, there is paradoxical IVS motion during systole and a time delay between LVPW motion and IVS motion (arrows). B: representative tissue Doppler image (TDI) showing LV dyssynchrony in a mouse with severe PS. Doppler regions of interest are marked on the 2-dimensional echo image (left) with a green circle (IVS) and yellow circle (LVPW). The corresponding wall motion plots show the time differential between the IVS (green plot and green arrow) and the LVPW (yellow plot and yellow arrow).
Serial changes in LV and RV function.
LV FS was increased at 1 wk in severe PS, as LV dimensions were decreased secondary to the septal shift (Fig. 5, top). LV FS was not different from control in mild and moderate PS. LV FS did not change significantly over the course of 5 wk in any of the three groups. In contrast, RVOT FS was significantly reduced at all time points in the severe PS group (P < 0.001 vs. mild and moderate PS). In the moderate PS group, RVOT FS was initially not different from control, but by 4 wk after PA banding began to decrease (P < 0.01 vs. control; Fig. 5, middle). In the mild PS group, RVOT FS remained at normal control levels (based on data obtained from nonbanded mice of similar strain and age in our laboratory) throughout the study period.
Fig. 5.
Serial evaluation of LV and RV function in mice with PAC. Top: LV fractional shortening (FS) was increased at 1 wk (w) in severe PS (primarily due to reduction in LV cavity size) and normal in mild and moderate PS. There was no significant difference between 1 and 4 wk in each group. Middle: RV outflow tract (RVOT) FS (in %) was decreased at 1 wk in severe PS and normal in mild and moderate PS. There was no further decline in RV FS in severe PS mice; however, RV FS began to decrease in mice with moderate PS by 3 wk. Bottom: RV wall velocity was decreased at 1 wk in severe PS and normal in mild and moderate PS. As with RVOT FS, mice with moderate PS showed a decrease in RV wall velocity by 3 wk. †P < 0.01 vs. 1 wk; *P < 0.01 vs. mild and moderate. Data obtained from 12 control mice before any surgical procedure are also shown.
Although used with good results in humans and larger animals, TDI has not been utilized extensively in the mouse. We found that RV wall velocity obtained from TDI can be performed with good reliability in this species. Inter- and intraobserver variability for TDI were 8.4 ± 3.1% and 7.2 ± 4.2%, respectively. Serial measurements of RV wall velocity were recorded in mild, moderate, and severe PS groups (Fig. 5, bottom). RV wall velocity was significantly reduced at all time points in severe PS group compared with control (P < 0.001). RV wall velocity started out at control levels and then significantly fell at 4 and 5 wk in the moderate PS group (P < 0.05). RV wall velocity remained at control levels in the mild PS group.
Invasive hemodynamics.
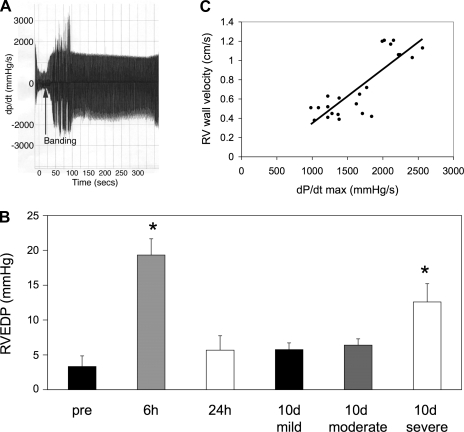
To correlate RV wall velocity obtained from TDI with invasive measurement of RV function, 23 mice representing varying degrees of RV banding (5 controls without PAC, 2 with mild PS, 9 with moderate PS, and 7 with severe PS) were catheterized at different time points after PAC. Echocardiographic and hemodynamic studies were done concurrently under the same anesthetic conditions. RV systolic pressure and dP/dt increased immediately after the placement of the PA band (Fig. 6A). RV end-diastolic pressure (RVEDP) was increased at 6 h after PAC (vs. pre, P < 0.01) and recovered by 24 h. At 10 days after PAC, mice with mild and moderate PS had mild elevations in RVEDP. In contrast, those with severe PS had significant elevations in RVEDP (P < 0.01; Fig. 6B). There was an excellent correlation (r = 0.833; P < 0.0001) between noninvasive measurements of RV free wall velocity and invasive measurements of RV maximal dP/dt (dP/dtmax; Fig. 6C), indicating that RV free wall velocity is a reasonable surrogate for measurements of RV dP/dt when serial measurements are required.
Fig. 6.
Invasive hemodynamics. A: maximal change in pressure over time (dP/dtmax) increases immediately after PA banding (arrow). Tracing shows continuous measurements over 5 min. B: RV end-diastolic pressure (RVEDP) was significantly elevated at 6 h after banding and returned to baseline levels by 24 h. At 10 days (d), in mice with severe PS, RVEDP was significantly increased compared with that of pre-PAC (*P < 0.01) and compared with that of mild and moderate PS (*P < 0.01). Prebanding, n = 3 at 6 h, n = 3 at 24 h, n = 3 at 10 day; mild, n = 4 at 10 day; moderate, n = 5 at 10 day; severe, n = 5 at 10 day. C: there was a good correlation between RV free wall velocity obtained from TDI and dP/dtmax measured at various time intervals from 6 h to 10 day (r = 0.833; P < 0.0001; n = 23).
Pathology and morphometrics.
Table 2 shows heart weight data from each of the three groups at 10 days postoperative. In the severe PS group, heart weight and heart weight-to-BW ratio were markedly increased. All of this increase in weight was due to the increased weight of the RV, which increased by 80%, with no significant change in LV weight. The RV-to-BW ratio was also significantly increased in the moderate PS group. Additional evidence of RV failure in the severe PS group was manifested by an increase in liver weight/BW by 22%, which was not present in the mild and moderate PS groups.
Table 2.
Morphometric characteristics of PAC and sham-control mice at 10 days postoperative
| Sham | Mild | Moderate | Severe | ANOVA | |
|---|---|---|---|---|---|
| n | 16 | 7 | 7 | 16 | |
| BW, g | 27.74±1.98 | 28.66±1.08 | 28.10±0.88 | 27.34±3.49 | NS |
| HW, mg | 122.93±10.78 | 127.04±9.41 | 133.60±4.60 | 142.79±15.86* | <0.001 |
| HW/BW, mg/g | 4.45±0.34 | 4.43±0.26 | 4.76±0.22 | 5.25±0.50* | <0.001 |
| LVW/BW, mg/g | 1.95±0.25 | 1.79±0.13 | 1.81±0.09 | 1.87±0.24 | NS |
| RVW/BW, mg/g | 0.94±0.10 | 1.05±0.08 | 1.41±0.15* | 1.70±0.28* | <0.001 |
| Sep/BW, mg/g | 1.18±0.18 | 1.12±0.11 | 1.15±0.14 | 1.29±0.21 | NS |
| Liver/BW, mg/g | 36.35±7.10 | 35.01±2.91 | 36.20±3.41 | 44.33±6.83* | <0.001 |
Values are means ± SD; n, no. of mice/group. These sham and severe pulmonary stenosis (PS) groups were used for gene microarray studies.
P < 0.001 vs. control. HW, heart weight; LVW, LV weight; RVW, RV weight; Sep, interventricular septal weight.
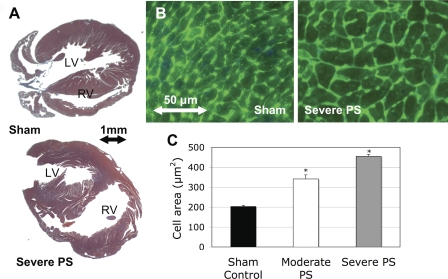
A microscopic evaluation showed that the RV cavity was significantly dilated at 10 days in the severe PS group (Fig. 7A). The average area of RV myocytes was significantly increased in moderate PS versus control (340.9 ± 20.7 vs. 203.0 ± 6.2 μm2; P < 0.01) and even further increased in severe PS (454.5 ± 10.2 μm2; P < 0.01; Fig. 7, B and C). In all mice with PS, the degree of PS correlated well with the RV-to-BW ratio (r = 0.692) and to histological evidence of cardiomyocyte hypertrophy (r = 0.768). There was no evidence of fibrosis or inflammation.
Fig. 7.
A: low-power microscopic examination (Masson trichrome stain) showing increased RV free wall and cavity dimensions in severe PS compared with sham control. B: high-power microscopic examination (wheat germ agglutinin with 4-6-diamidino-2-phenylindole) showing increased myocyte cross-sectional areas in severe PS compared with sham control. C: cell area was significantly increased in moderate and severe PS groups compared with sham. *P < 0.05 vs. sham control.
Gene expression in the afterload-stressed RV.
RV samples from 16 severe PS mice at 10 days postoperative and from 16 sham controls were hybridized to gene microarrays, and data were analyzed using SAM and Fisher's exact test algorithms to rigorously identify differentially expressed genes and GO biological processes. Relative gene expression was expressed as the fold difference in gene expression in PAC RV versus sham RV. One-hundred ninety-six genes were upregulated and 1,114 genes were downregulated in the PAC RV compared with those of controls (Tables 3 and 4). Fisher's exact test was applied to the 8,773 unique GO annotated genes on the array to identify statistically significantly enriched and depleted GO groups in the PAC RV (Table 5). Among the most significantly upregulated processes were phosphate transport, regulation of coagulation, inorganic anion transport, and cell adhesion, which includes most of the collagens as well as other extracellular matrix (ECM) genes. Downregulated processes were dominated by energy pathways.
Table 3.
135 genes significantly upregulated >1.5-fold in PAC RV versus sham RV*
| Gene Name | Gene Description | GenBank ID | Score(d) | Fold Change |
|---|---|---|---|---|
| Postn | Periostin | AV084876 | 3.056 | 16.04 |
| Fnbp4 | Formin binding protein 4 | AA240645 | 4.962 | 11.61 |
| Lox | Lysyl oxidase | AV014751 | 3.472 | 7.68 |
| Mfap4 | Microfibrillar associated protein 4 | BG074573 | 4.417 | 7.48 |
| Sfrp2 | Secreted frizzled related protein 2 | AV021712 | 2.267 | 7.48 |
| Uchl1 | Ubiquitin carboxy-terminal hydrolase | BG074009 | 3.031 | 7.23 |
| Aspn | Asporin | AV020793 | 3.454 | 6.21 |
| Col3a1 | Procollagen, type III, alpha 1 | BG073709 | 2.808 | 4.16 |
| Dkk3 | Dickkopf homolog 3 | BG075561 | 5.263 | 4.16 |
| Serpinb1a | Serine (or cysteine) peptidase inhibitor, clade B, member 1a | BG073257 | 2.816 | 4.13 |
| Col5a2 | Procollagen, type V, alpha 2 | AV089281 | 3.118 | 4.02 |
| Col5a1 | Procollagen, type V, alpha 1 | BG067011 | 3.574 | 3.88 |
| Col3a1 | Procollagen, type III, alpha 1 | BG074327 | 3.437 | 3.86 |
| Fstl1 | Follistatin-like 1 | AV024220 | 5.335 | 3.71 |
| Set | Set translocation, myeloid leukemia-associated | AV031220 | 4.968 | 3.63 |
| Traf4 | Tnf receptor associated factor 4 | AV024412 | 4.870 | 3.60 |
| Sparc | Secreted acidic cysteine rich glycoprotein | AV094848 | 4.116 | 3.51 |
| Ccnd2 | Cyclin D2 | AV087918 | 4.030 | 3.51 |
| Mgp | Matrix Gla protein | BG074366 | 4.218 | 3.50 |
| Fstl1 | Follistatin-like 1 | BG073316 | 4.191 | 3.36 |
| Sssca1 | Sjogren's syndrome/scleroderma autoantigen 1 homolog | AV023779 | 3.433 | 3.35 |
| Lpp | LIM domain containing preferred translocation partner in lipoma | BG068912 | 3.110 | 3.31 |
| Nupr1 | P8 protein | AV087068 | 3.237 | 3.30 |
| Rps20 | Ribosomal protein S20 | AV170826 | 6.675 | 3.23 |
| Bgn | Biglycan | BG073809 | 5.770 | 3.17 |
| Prss23 | Protease, serine, 23 | AV073989 | 4.318 | 3.17 |
| Ubox5 | U-box domain containing 5 | BG072221 | 3.956 | 3.16 |
| Rbp1 | Retinol-binding protein 1 | AV074050 | 3.565 | 3.15 |
| Sparc | Secreted acidic cysteine rich glycoprotein | AV104148 | 2.859 | 3.15 |
| Anxa7 | Annexin A7 | BG076013 | 4.491 | 3.03 |
| Pola1 | Polymerase (DNA directed), alpha 1 | AV095001 | 4.679 | 3.03 |
| Nbl1 | Neuroblastoma candidate region, suppression of tumorigenicity 1 | AV078033 | 5.377 | 2.83 |
| Col14a1 | Procollagen, type XIV, alpha 1 | AV017616 | 2.809 | 2.80 |
| Tacstd1 | Tumor-associated calcium signal transducer 1 | AV089835 | 5.118 | 2.80 |
| Col3a1 | Collagen, type III, alpha-1 | BG072787 | 2.802 | 2.80 |
| Serpinb1a | Serine (or cysteine) peptidase inhibitor, clade B, member 1a | AV061227 | 2.578 | 2.77 |
| Gpx3 | Glutathione peroxidase 3 | AV038358 | 6.227 | 2.76 |
| Cd48 | CD48 antigen | AV056452 | 2.879 | 2.75 |
| Rbp1 | Retinol-binding protein 1 | AV146205 | 3.359 | 2.71 |
| Rkhd2 | Mex3 homolog C (C. elegans) | AV024424 | 4.460 | 2.70 |
| Gata1 | GATA binding protein 1 | AV024112 | 3.820 | 2.67 |
| Shc1 | Src homology 2 domain-containing transforming protein C1 | BG070010 | 3.863 | 2.65 |
| Pdgfrl | Platelet-derived growth factor receptor-like | AV013190 | 3.206 | 2.64 |
| Gpx3 | Glutathione peroxidase 3 | AV137417 | 3.153 | 2.63 |
| Cyb5r3 | Cytochrome b5 reductase 3 | BG067095 | 2.821 | 2.63 |
| Loxl1 | Lysyl oxidase-like 1 | AV094998 | 5.053 | 2.60 |
| Hexb | Hexosaminidase B | BG069642 | 2.816 | 2.57 |
| Proz | Protein Z, vitamin K-dependent plasma glycoprotein | AV078387 | 2.846 | 2.56 |
| Fndc1 | Fibronectin type III domain containing 1 | AV010532 | 2.959 | 2.54 |
| Clu | Clusterin | AV149922 | 6.506 | 2.53 |
| Nt5dc2 | 5′-nucleotidase domain containing 2 | AW547246 | 3.488 | 2.53 |
| Rtn4 | Reticulon 4 | BG064276 | 3.027 | 2.48 |
| Col4a1 | Procollagen, type IV, alpha 1 | AA162273 | 4.510 | 2.46 |
| Snx10 | Sorting nexin 10 | AV095218 | 3.147 | 2.43 |
| Fhl1 | Four and a half LIM domains 1 | AV083596 | 3.399 | 2.42 |
| Clu | Clusterin | AV074721 | 4.009 | 2.39 |
| Traf5 | Tnf receptor-associated factor 5 | AV091488 | 4.721 | 2.37 |
| Anxa1 | Annexin A1 | AV037865 | 3.434 | 2.33 |
| Nupr1 | P8 protein | AV087698 | 3.090 | 2.31 |
| Cd24a | CD24a antigen | AV105800 | 2.997 | 2.31 |
| Rbp1 | Retinol-binding protein 1 | AV140184 | 8.093 | 2.30 |
| Zfp87 | Zinc finger protein 87 | BG075308 | 3.210 | 2.30 |
| Rbbp7 | Retinoblastoma-binding protein 7 | AW544081 | 6.911 | 2.29 |
| Vim | Vimentin | AV113424 | 4.562 | 2.27 |
| Zfp364 | Zinc finger protein 364 | BG072444 | 3.726 | 2.26 |
| Col4a2 | Procollagen, type IV, alpha 2 | AV010312 | 2.755 | 2.26 |
| Ryr2 | Ryanodine receptor 2 | BG074010 | 2.827 | 2.24 |
| H13 | Histocompatibility 13 | AV055621 | 2.646 | 2.22 |
| Dstn | Destrin | BG073428 | 3.185 | 2.21 |
| Synpo21 | Synaptopodin 2-like | AV015246 | 5.243 | 2.21 |
| Cola2 | Collagen alpha-2(I) chain precursor | AV009300 | 2.797 | 2.20 |
| Arl6ip2 | ADP-ribosylation-like factor 6-interacting protein 2 | AV015499 | 3.031 | 2.18 |
| Plp2 | Proteolipid protein 2 | AV133831 | 4.596 | 2.15 |
| Cdv3 | Carnitine deficiency-associated gene expressed in ventricle 3 | AV072373 | 3.324 | 2.11 |
| Dstn | Destrin | AV050410 | 4.280 | 2.10 |
| Angpt2 | Angiopoietin 2 | AA020573 | 4.174 | 2.09 |
| Rhoc | Ras homolog gene family, member C | AV140333 | 3.965 | 2.08 |
| Arpc1b | Actin related protein 2/3 complex, subunit 1B | AV000246 | 4.745 | 2.07 |
| Anxa5 | Annexin A5 | AV087971 | 2.870 | 2.04 |
| Gnb1 | Guanine nucleotide binding protein, beta 1 | AV078383 | 2.626 | 2.03 |
| Pmp22 | Peripheral myelin protein 22 | AV087039 | 3.746 | 2.02 |
| Serpine2 | Serine (or cysteine) peptidase inhibitor, clade E, member 2 | AV017162 | 3.403 | 2.01 |
| Anxa3 | Annexin A3 | AV218319 | 5.386 | 1.98 |
| Mif | Similar to macrophage migration inhibitory factor | BG064410 | 3.676 | 1.96 |
| Anxa4 | Annexin A4 | AV103319 | 2.660 | 1.96 |
| Rdh5 | Biogenesis of lysosome-related organelles complex-1, subunit 1 | AV083165 | 3.014 | 1.95 |
| Cd63 | Cd63 antigen | AV093530 | 3.026 | 1.94 |
| Clu | Clusterin | BG072209 | 2.952 | 1.94 |
| Vim | Vimentin | AV123111 | 2.969 | 1.93 |
| Atp6v1a | ATPase, H+ transporting, lysosomal V1 subunit A | BG064589 | 2.882 | 1.93 |
| Arf2 | ADP-ADP-ribosylation factor 2 | AV030860 | 2.722 | 1.92 |
| Plk2 | similar to XP_001102530.1 polo-like kinase 2 (Drosophila) isoform 2 | AV049483 | 3.516 | 1.91 |
| Nid2 | Nidogen 2 | AV013588 | 2.732 | 1.90 |
| Tmem176b | Transmembrane protein 176B | AV013352 | 4.584 | 1.88 |
| Cnn2 | Calponin 2 | AV025199 | 3.051 | 1.88 |
| Prkar1a | Protein kinase, cAMP-dependent, regulatory, type I, alpha | BB566556 | 4.444 | 1.87 |
| Cst3 | Cystatin 3 | AV153101 | 3.212 | 1.86 |
| Anxa5 | Annexin A5 | AA137915 | 2.698 | 1.86 |
| Rell1 | RELT-like 1 | AV031438 | 3.319 | 1.84 |
| Cd34 | CD34 antigen | AV086521 | 3.544 | 1.83 |
| Pmp22 | Peripheral myelin protein 22 | AV113888 | 3.100 | 1.83 |
| Azin1 | Antizyme inhibitor 1 | AV030927 | 4.074 | 1.82 |
| Cald1 | Caldesmon 1 | BG064630 | 2.635 | 1.81 |
| Dstn | Destrin | AV087224 | 2.677 | 1.80 |
| Ccnd2 | Cyclin D2 | AV140268 | 3.939 | 1.79 |
| Gfm2 | G elongation factor, mitochondrial 2 | AV095236 | 3.684 | 1.77 |
| Pik3ca | Phosphatidylinositol 3-kinase, catalytic, alpha polypeptide | BG076256 | 3.014 | 1.77 |
| Mtdh | Metadherin | AV083741 | 2.761 | 1.76 |
| Tusc3 | strongly similar to XP_001094069.1 similar to tumor suppressor candidate 3 isoform a isoform 4 | AV031846 | 2.741 | 1.74 |
| Pkm2 | Pyruvate kinase, muscle, 2 | AV094449 | 3.977 | 1.70 |
| Actn4 | Actinin alpha 4 | AI836968 | 3.079 | 1.69 |
| Hn1 | Hematological and neurological expressed sequence 1 | AV094890 | 3.892 | 1.69 |
| Ifitm2 | Interferon-induced transmembrane protein 2 | AV049395 | 2.671 | 1.66 |
| Ppgb | Beta-galactosidase protective protein | AV088011 | 2.791 | 1.65 |
| Ntan1 | N-terminal Asn amidase | AV058809 | 2.852 | 1.63 |
| Tmem43 | Transmembrane protein 43 | BG073951 | 2.763 | 1.61 |
| Akt1 | V-AKT murine thymoma viral oncogene homolog 1 | AV058304 | 2.705 | 1.60 |
| Pigq | Phosphatidylinositol glycan anchor biosynthesis, class Q | AV006019 | 3.635 | 1.59 |
| Ugp2 | UDP-glucose pyrophosphorylase 2 | AV086208 | 3.047 | 1.58 |
| Sec13l1 | SEC13 homolog | BG065187 | 3.118 | 1.57 |
| Eif4ebp1 | Eukaryotic translation initiation factor 4E-binding protein 1 | AV087438 | 2.733 | 1.56 |
| Wif1 | WNT Inhibitory factor 1 | AV032229 | 3.266 | 1.56 |
| Gmfb | Glia maturation factor, beta | AV162369 | 2.669 | 1.55 |
| S100a16 | S100 calcium binding protein A16 | AV088022 | 2.573 | 1.55 |
| Gusb | Beta-glucuronidase | AV111448 | 3.314 | 1.52 |
| Gas6 | Growth arrest-specific 6 | BG076011 | 2.738 | 1.52 |
| Rdbp | Complement factor B | AV133629 | 2.624 | 1.52 |
| Btg2 | B-cell translocation gene 2 | AV086968 | 2.673 | 1.52 |
| Taf10 | Transcribed locus, strongly similar to XP_001109041.1 similar to integrin-linked kinase isoform 3 | BG072693 | 2.902 | 1.50 |
| Sptlc1 | Serine palmitoyltransferase, long chain base subunit 1 | AV062462 | 2.930 | 1.47 |
| Nptn | Neuroplastin | AV088324 | 3.211 | 1.46 |
| Mgmt | Methylguanine-DNA methyltransferase | AV087599 | 2.696 | 1.44 |
| Ehd1 | EH-domain containing 1 | AV024484 | 2.590 | 1.39 |
| Jund1 | Jun proto-oncogene related gene d1 | AV014760 | 2.668 | 1.31 |
| Dazap2 | DAZ-associated protein 2 | AV082449 | 3.466 | 1.31 |
The significance analysis of microarrays (SAM) algorithm was employed to identify genes with statistically different expression levels between PA-banded and sham tissues. SAM incorporates a false discovery rate (FDR) correction for multiple testing errors and calculates a d statistic for each gene based on the ratio of change in gene expression to SD in the data for that gene (Ref. 18).
Out of a total of 196 significantly upregulated genes and expressed sequence tags (ESTs).
Table 4.
250 genes significantly downregulated >2.0-fold in PAC RV versus sham RV*
| Gene Name | Gene Description | Gene ID | Score(d) | Fold Down |
|---|---|---|---|---|
| Fkbp4 | FK506 binding protein 4 | BG065656 | −2.193 | 6.54 |
| Mospd1 | Motile sperm domain containing 1 | BG068741 | −2.395 | 5.38 |
| Acaa2 | Acetyl-Coenzyme A acyltransferase 2 (mitochondrial 3-oxoacyl-Coenzyme A thiolase) | BG072552 | −2.856 | 5.28 |
| Sybl1 | Synaptobrevin-like 1 | AV113528 | −4.873 | 5.00 |
| Ces3 | Carboxylesterase 3 | BG072503 | −2.156 | 4.93 |
| A2bp1 | Ataxin 2-binding protein 1 | AV029909 | −6.284 | 4.86 |
| Stk16 | Serine/threonine kinase 16 | AV119666 | −2.037 | 4.83 |
| Dgat2 | Diacylglycerol O-acyltransferase 2 | AI847556 | −2.031 | 4.61 |
| Aes | Amino-terminal enhancer of split | BG074671 | −2.295 | 4.09 |
| Hod | HOP homeobox | AI840878 | −3.112 | 4.07 |
| Ppil1 | Peptidylprolyl isomerase (cyclophilin)-like 1 | AV015645 | −2.154 | 3.92 |
| Hod | HOP homeobox | AV065655 | −2.097 | 3.60 |
| Serpinb11 | Serine (or cysteine) peptidase inhibitor, clade B (ovalbumin), member 11 | AV082348 | −2.206 | 3.53 |
| Prdx3 | Peroxiredoxin 3 | BG074871 | −2.300 | 3.50 |
| Dci | Dodecenoyl-Coenzyme A delta isomerase (3,2 trans-enoyl-Coenyme A isomerase) | AV106338 | −3.290 | 3.49 |
| Dci | Dodecenoyl-Coenzyme A delta isomerase (3,2 trans-enoyl-Coenyme A isomerase) | BG069423 | −8.829 | 3.43 |
| Tuba8 | Tubulin, alpha 8 | AA063914 | −3.529 | 3.35 |
| Hadh2 | Hydroxysteroid (17-beta) dehydrogenase 10 | BG073539 | −2.043 | 3.32 |
| Hibadh | 3-hydroxyisobutyrate dehydrogenase | AI854120 | −2.658 | 3.28 |
| Aarsd1 | Alanyl-tRNA synthetase domain containing 1 | AI847872 | −2.278 | 3.27 |
| Acat1 | Acetyl-Coenzyme A acetyltransferase 1 | AV084664 | −2.558 | 3.24 |
| Auh | AU RNA binding protein/enoyl-coenzyme A hydratase | AV095181 | −2.413 | 3.21 |
| Ndufb7 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 7 | BG075930 | −2.110 | 3.18 |
| Sf3b2 | Splicing factor 3b, subunit 2 | AV065784 | −2.965 | 3.17 |
| Csnk2b | Casein kinase 2, beta polypeptide | BG075196 | −2.013 | 3.16 |
| Ythdf2 | YTH domain family 2 | AV084848 | −1.994 | 3.13 |
| Hadhb | Hydroxyacyl-Coenzyme A dehydrogenase/3-ketoacyl-Coenzyme A thiolase/enoyl-Coenzyme A hydratase (trifunctional protein), beta subunit | BG064090 | −2.257 | 3.12 |
| Ndufa3 | NADH-ubiquinone oxidoreductase 1 alpha subcomplex, 3 | AV054731 | −2.424 | 3.12 |
| Tuba4 | Tubulin, alpha 4A | AI840604 | −7.166 | 3.09 |
| Arhgap12 | Rho GTPase activating protein 12 | BG064038 | −1.991 | 3.07 |
| Atp5 g2 | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit c (subunit 9), isoform 2 | AV056821 | −3.260 | 3.06 |
| Hdlbp | High density lipoprotein (HDL) binding protein | AV056261 | −2.815 | 3.04 |
| Eno3 | Enolase 3, beta muscle | AI841640 | −6.989 | 3.04 |
| Mapk14 | Mitogen activated protein kinase 14 | AA544997 | −5.407 | 3.03 |
| Mrpl32 | Mitochondrial ribosomal protein L32 | AV035121 | −2.995 | 3.02 |
| Hod | HOP homeobox | AV081983 | −2.911 | 3.00 |
| Hadhsc | Hydroxyacyl-Coenzyme A dehydrogenase | AV013144 | −2.652 | 2.92 |
| Rnf6 | Ring finger protein 6 | AV015385 | −2.035 | 2.92 |
| Gpsn2 | Glycoprotein, synaptic 2 | AV106079 | −2.173 | 2.91 |
| Acaa2 | Acetyl-Coenzyme A acyltransferase 2 (mitochondrial 3-oxoacyl-Coenzyme A thiolase) | AV084156 | −5.371 | 2.90 |
| Hadha | Hydroxyacyl-Coenzyme A dehydrogenase/3-ketoacyl-Coenzyme A thiolase/enoyl-Coenzyme A hydratase (trifunctional protein), alpha subunit | BG074757 | −2.412 | 2.90 |
| Dcps | Decapping enzyme, scavenger | BG063230 | −4.204 | 2.90 |
| Hrc | Histidine rich calcium binding protein | BG073810 | −2.371 | 2.88 |
| Pex11b | Peroxisomal biogenesis factor 11b | AV006229 | −4.785 | 2.86 |
| Pipox | Pipecolic acid oxidase | AV069402 | −3.867 | 2.85 |
| Ckmt2 | Creatine kinase, mitochondrial 2 | AV085004 | −2.245 | 2.85 |
| Acadm | Acyl-Coenzyme A dehydrogenase, medium chain | AI840666 | −3.584 | 2.85 |
| Etfb | Electron transferring flavoprotein, beta polypeptide | AV086609 | −6.881 | 2.84 |
| Sdha | Succinate dehydrogenase complex, subunit A, flavoprotein (Fp) | AV074725 | −2.909 | 2.84 |
| Tmem176b | Transmembrane protein 176B | BI076417 | −2.922 | 2.83 |
| Hspd1 | Heat shock protein 1 (chaperonin) | BG064728 | −3.015 | 2.83 |
| Ak2 | Adenylate kinase 2 | AV088727 | −2.390 | 2.81 |
| Synj2 | Synaptojanin 2 | AV083725 | −2.900 | 2.81 |
| Tcea3 | Transcription elongation factor A (SII), 3 | BG071507 | −7.615 | 2.77 |
| Dscr3 | Down syndrome critical region gene 3 | AV111492 | −2.132 | 2.77 |
| Ndufv1 | NADH dehydrogenase (ubiquinone) flavoprotein 1 | BG076088 | −2.697 | 2.77 |
| Cox8b | Cytochrome c oxidase, subunit VIIIb | AV083848 | −2.834 | 2.76 |
| Fkbp4 | FK506 binding protein 4 | BG064128 | −3.876 | 2.76 |
| Etfa | Electron transferring flavoprotein, alpha polypeptide | AA139719 | −6.798 | 2.76 |
| Vdac1 | Voltage-dependent anion channel 1 | AV095022 | −2.426 | 2.76 |
| Fahd1 | Fumarylacetoacetate hydrolase domain containing 1 | AV061746 | −2.902 | 2.74 |
| Tuba4 | alpha-tubulin TUBA4 | AV032310 | −4.484 | 2.73 |
| Ech1 | Enoyl coenzyme A hydratase 1, peroxisomal | AV000529 | −4.862 | 2.72 |
| Txndc1 | Thioredoxin domain containing 1 | BG064124 | −2.833 | 2.70 |
| Zan | Zonadhesin | AV326603 | −3.831 | 2.68 |
| Eed | Embryonic ectoderm development | BG068740 | −2.137 | 2.66 |
| Zfp644 | Zinc finger protein 644 | BG069858 | −3.025 | 2.66 |
| Idh2 | Isocitrate dehydrogenase 2 (NADP+), mitochondrial | AV006267 | −3.493 | 2.65 |
| Mrps17 | Mitochondrial ribosomal protein S17 | BG073811 | −3.625 | 2.64 |
| Kif4 | Kinesin family member 4 | AV355663 | −2.785 | 2.64 |
| Txn2 | Thioredoxin 2 | AA116866 | −2.667 | 2.62 |
| Nudt4 | Nudix (nucleoside diphosphate linked moiety X)-type motif 4 | AI854103 | −2.131 | 2.60 |
| Fyco1 | FYVE and coiled-coil domain containing 1 | AV006218 | −3.682 | 2.59 |
| Dld | Dihydrolipoamide dehydrogenase | AI847502 | −2.849 | 2.59 |
| Hfe2 | Hemochromatosis type 2 (juvenile) (human homolog) | AV087892 | −3.091 | 2.59 |
| Rabepk | Rab9 effector protein with kelch motifs | BG075188 | −2.783 | 2.59 |
| Lrpprc | Leucine-rich PPR-motif containing | AV162299 | −3.973 | 2.57 |
| Acsl1 | Acyl-CoA synthetase long-chain family member 1 | AV005791 | −4.637 | 2.56 |
| Slc2a4 | Solute carrier family 2 (facilitated glucose transporter), member 4 | AV005800 | −3.023 | 2.56 |
| Idh3b | Isocitrate dehydrogenase 3 (NAD+) beta | AI838687 | −3.678 | 2.56 |
| Hspd1 | Heat shock 60-KD protein 1 | BG073067 | −3.334 | 2.55 |
| Armet | Arginine-rich, mutated in early stage tumors | BG063580 | −2.050 | 2.55 |
| Slc25a11 | Solute carrier family 25 (mitochondrial carrier, oxoglutarate carrier), member11 | AV089747 | −3.883 | 2.54 |
| Acadm | Acyl-Coenzyme A dehydrogenase, medium chain | AV086733 | −7.705 | 2.54 |
| Zbtb20 | Zinc finger and BTB domain containing 20 | BG073885 | −4.558 | 2.53 |
| Acads | ACYL-CoA dehydrogenase, short-chain | AV093663 | −2.256 | 2.52 |
| Tceb1 | Transcription elongation factor B (SIII), polypeptide 1 | BG071546 | −3.545 | 2.52 |
| Suclg2 | Succinate-CoA ligase, GDP-forming, beta subunit | AV087975 | −2.577 | 2.52 |
| Etfa | Electron transferring flavoprotein, alpha polypeptide | BG074876 | −3.079 | 2.52 |
| Fkbp4 | FK506 binding protein 4 | AV111500 | −2.614 | 2.52 |
| Sec31l1 | SEC31 homolog A (S. cerevisiae) | BG074808 | −2.125 | 2.52 |
| Atp5d | ATP synthase, H+ transporting, mitochondrial F1 complex, delta subunit | AI841365 | −2.169 | 2.52 |
| Eif5b | Eukaryotic translation initiation factor 5B | BG067345 | −2.028 | 2.47 |
| Nnt | Nicotinamide nucleotide transhydrogenase | AV006306 | −6.495 | 2.47 |
| Il12b | Interleukin 12b | AA267353 | −2.568 | 2.46 |
| Atp5f1 | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit b, isoform 1 | AA119394 | −3.970 | 2.46 |
| Exosc10 | Exosome component 10 | BG063453 | −2.972 | 2.46 |
| Prss2 | Transcribed locus, weakly similar to NP_062562.1 factor VII precursor, isoform b | AV005299 | −2.481 | 2.45 |
| Uqcrc1 | Ubiquinol-cytochrome c reductase core protein I | AI841290 | −2.836 | 2.43 |
| Magmas | Mitochondria-associated protein involved in granulocyte-macrophage colony-stimulating factor signal transduction | AV066653 | −2.234 | 2.43 |
| Atrx | Alpha thalassemia/mental retardation syndrome X-linked homolog | BG068630 | −3.518 | 2.42 |
| Slc35b1 | Solute carrier family 35, member B1 | AV060614 | −2.117 | 2.42 |
| Mrps28 | Mitochondrial ribosomal protein S28 | AV107441 | −2.906 | 2.41 |
| Cox5b | Cytochrome c oxidase, subunit Vb | AV066262 | −2.118 | 2.41 |
| D19Ertd678e | Coiled-coil domain containing 86 | AV094491 | −3.703 | 2.41 |
| Ndufa3 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 3 | AV104160 | −2.149 | 2.40 |
| Mrpl15 | Mitochondrial ribosomal protein L15 | AV133566 | −2.081 | 2.40 |
| Mas1 | MAS1 oncogene | AV028487 | −2.911 | 2.40 |
| Gsn | Gelsolin | AV006010 | −3.244 | 2.39 |
| Fusip1 | FUS-interacting protein 1 | AV057448 | −2.949 | 2.38 |
| Extl3 | Exostoses (multiple)-like 3 | AI840748 | −2.089 | 2.37 |
| Vamp8 | Vesicle-associated membrane protein 8 | AV053401 | −2.194 | 2.37 |
| Gtpbp2 | GTP binding protein 2 | BG071130 | −2.897 | 2.37 |
| Asnsd1 | Asparagine synthetase domain containing 1 | AV134139 | −2.278 | 2.36 |
| BC038328 | Zinc finger protein 708 | AV133851 | −2.237 | 2.36 |
| Vdac1 | Voltage-dependent anion channel 1 | AV037195 | −2.069 | 2.36 |
| Arhgap12 | Rho GTPase activating protein 12 | AV083597 | −2.178 | 2.35 |
| Atp5b | ATP synthase, H+ transporting mitochondrial F1 complex, beta subunit | AV005999 | −2.755 | 2.35 |
| Ndufs3 | NADH-ubiquinone oxidoreductase Fe-S proetin 3 | AV066283 | −2.025 | 2.34 |
| Hspd1 | Heat shock 60-KD protein 1 | BG065342 | −2.757 | 2.33 |
| Arl8b | ADP-ribosylation factor-like 8B | AV041040 | −2.236 | 2.33 |
| Kcnv1 | Potassium channel, subfamily V, member 1 | AV088564 | −2.224 | 2.32 |
| Anxa6 | Annexin A6 | AV094561 | −3.076 | 2.32 |
| Cacna1e | Calcium channel, voltage-dependent, alpha-1E subunit | AA855859 | −4.131 | 2.31 |
| Ggnbp2 | Gametogenetin binding protein 2 | AI841660 | −2.008 | 2.31 |
| Slc25a22 | Solute carrier family 25 (mitochondrial carrier, glutamate), member 22 | AV030675 | −4.129 | 2.29 |
| Atp5c1 | ATP synthase, H+ transporting, mitochondrial F1 complex, gamma polypeptide 1 | BG074111 | −2.175 | 2.29 |
| Hdlbp | High density lipoprotein (HDL) binding protein | AV012382 | −4.346 | 2.29 |
| Hsp110 | Heat shock protein 110 | AV031906 | −2.049 | 2.28 |
| Uqcrq | Ubiquinol-cytochrome c reductase, complex III subunit VII | AV006451 | −2.836 | 2.27 |
| Hod | Homeobox only domain | AV068725 | −2.289 | 2.27 |
| Mrpl37 | Mitochondrial ribosomal protein L37 | AV087163 | −3.247 | 2.27 |
| Amotl1 | Angiomotin-like 1 | AV085162 | −2.246 | 2.26 |
| Reep5 | Receptor accessory protein 5 | AV006204 | −4.009 | 2.26 |
| Pcmt1 | Protein-L-isoaspartate (d-aspartate) O-methyltransferase 1 | BG073964 | −2.019 | 2.25 |
| Vps16 | Vacuolar protein sorting 16 | BG072913 | −2.276 | 2.25 |
| Pygm | Muscle glycogen phosphorylase | AV006273 | −2.789 | 2.25 |
| Adh7 | Alcohol dehydrogenase 7 (class IV), mu or sigma polypeptide | AV087904 | −2.085 | 2.25 |
| Eno3 | Enolase 3, beta muscle | AV006198 | −4.186 | 2.25 |
| Ndufb9 | NADH-ubiquinone oxidoreductase 1 beta subcomplex, 9 | AV086909 | −3.681 | 2.24 |
| Tssk2 | Testis-specific serine kinase 2 | AV042468 | −2.455 | 2.23 |
| Vdac3 | Voltage-dependent anion channel 3 | AV104389 | −3.656 | 2.23 |
| Idh3b | Isocitrate dehydrogenase 3 (NAD+) beta | AV170829 | −5.063 | 2.22 |
| Rapgef4 | Rap guanine nucleotide exchange factor (GEF) 4 | AA388005 | −3.119 | 2.21 |
| Hadhsc | Hydroxyacyl-Coenzyme A dehydrogenase | BG074015 | −2.336 | 2.21 |
| Pygm | Muscle glycogen phosphorylase | AI324008 | −3.632 | 2.21 |
| Ghr | Growth hormone receptor | BG072812 | −3.885 | 2.21 |
| Ech1 | Enoyl-CoA hydratase, peroxisomal | AV088052 | −4.343 | 2.21 |
| Usp15 | Ubiquitin specific peptidase 15 | AV039076 | −3.803 | 2.21 |
| Agl | Amylo-1,6-glucosidase, 4-alpha-glucanotransferase | AV016572 | −2.862 | 2.21 |
| Impa1 | Inositol (myo)-1(or 4)-monophosphatase 1 | AA152733 | −2.280 | 2.21 |
| Atp5 g1 | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit c (subunit 9), isoform 1 | BG073112 | −2.273 | 2.20 |
| Rbpsuh | Recombination signal-binding protein suppressor of hairless, drosophila, homolog of | AV094997 | −3.744 | 2.20 |
| Hadh2 | Hydroxyacyl-Coenzyme A dehydrogenase, type II | AV077923 | −2.823 | 2.20 |
| Aqp1 | Aquaporin 1 | AI838965 | −3.040 | 2.20 |
| Rpe | Ribulose-5-phosphate-3-epimerase | AV085827 | −2.305 | 2.19 |
| Mtap7 | Microtubule-associated protein 7 | AV041269 | −2.660 | 2.19 |
| Slc25a4 | Solute carrier family 25 (mitochondrial carrier, adenine nucleotide translocator), member 4 | AI841357 | −7.234 | 2.19 |
| Tagln2 | Transgelin 2 | AV084804 | −2.140 | 2.18 |
| Ptov1 | Prostate tumor over expressed gene 1 | BG073526 | −2.280 | 2.17 |
| Hadhb | Hydroxyacyl-Coenzyme A dehydrogenase/3-ketoacyl-Coenzyme A thiolase/enoyl-Coenzyme A hydratase (trifunctional protein), beta subunit | AV088068 | −3.908 | 2.17 |
| Mllt4 | Myeloid/lymphoid or mixed lineage leukemia, translocated to, 4 | AV010160 | −3.132 | 2.17 |
| 5Arc | Activity regulated cytoskeletal-associated protein | BG519376 | −3.426 | 2.16 |
| Park7 | Parkinson disease (autosomal recessive, early onset) 7 | BG075688 | −2.226 | 2.16 |
| B3 gat3 | Beta-1,3-glucuronyltransferase 3 (glucuronosyltransferase I) | AV085924 | −2.795 | 2.16 |
| Il11ra1 | Interleukin 11 receptor, alpha chain 1 | AV083410 | −2.887 | 2.16 |
| Rab14 | RAB14, member RAS oncogene family | AV055902 | −7.178 | 2.16 |
| Ldhb | Lactate dehydrogenase B | AV006303 | −2.436 | 2.16 |
| Spop | Speckle-type poz protein | BG072804 | −5.541 | 2.16 |
| Cox7c | Cytochrome c oxidase, subunit VIIc | BG063960 | −2.744 | 2.15 |
| NGFB | Nerve growth factor, beta polypeptide | W46522 | −2.071 | 2.15 |
| Capza3 | Capping protein (actin filament) muscle Z-line, alpha 3 | AV039134 | −3.081 | 2.14 |
| Ctsf | Cathepsin F | AV085152 | −1.999 | 2.14 |
| Atg7 | Autophagy-related 7 | AW539206 | −2.411 | 2.14 |
| Sbk1 | SH3-binding kinase 1 | BG063893 | −5.401 | 2.13 |
| Nexn | Nexilin | BG072510 | −3.305 | 2.13 |
| Atp5a1 | ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit, isoform 1 | AI841258 | −2.089 | 2.13 |
| Cox7c | Cytochrome c oxidase, subunit VIIc | AV015972 | −4.189 | 2.13 |
| Ide | Insulin degrading enzyme | AV006006 | −2.321 | 2.12 |
| Orc5l | Origin recognition complex, subunit 5 | AV133637 | −4.868 | 2.12 |
| Nol7 | Nucleolar protein 7 | BG066700 | −2.335 | 2.12 |
| Plekhg1 | Pleckstrin homology domain containing, family G (with RhoGef domain) member 1 | AV084852 | −3.538 | 2.12 |
| Vldlr | Very low density lipoprotein receptor | AV018220 | −4.117 | 2.12 |
| Fasr | Fas receptor | AV134967 | −2.567 | 2.12 |
| Hspa4 | Heat shock protein 4 | BG065493 | −2.672 | 2.11 |
| Lpl | Lipoprotein lipase | AA049917 | −2.519 | 2.11 |
| Cops7a | COP9 (constitutive photomorphogenic) homolog, subunit 7a (Arabidopsis thaliana) | AV087105 | −3.114 | 2.11 |
| Acadvl | Acyl-Coenzyme A dehydrogenase, very long chain | AI839605 | −2.388 | 2.10 |
| Camk4 | Calcium/calmodulin-dependent protein kinase IV | AV028684 | −2.577 | 2.10 |
| Atp5 g1 | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit c (subunit 9), isoform 1 | AV112952 | −3.004 | 2.10 |
| Pex19 | Peroxisome biogenesis factor 19 | BG072565 | −2.160 | 2.10 |
| Chpt1 | Synaptonemal complex protein 3 | AV062575 | −6.037 | 2.10 |
| Mgst3 | Glutathione S-transferase, microsomal, 3 | AV056432 | −2.480 | 2.10 |
| Akt2 | Thymoma viral proto-oncogene 2 | BG073895 | −2.055 | 2.10 |
| Fdft1 | Farnesyldiphosphate farnesyltransferase 1 | AV095240 | −2.501 | 2.09 |
| Sdhc | Succinate dehydrogenase complex, subunit C, integral membrane protein, 15-KD | AV103143 | −4.511 | 2.09 |
| Raly | HnRNP-associated with lethal yellow | AV171686 | −2.495 | 2.09 |
| Pink1 | PTEN-induced putative kinase 1 | AV087434 | −2.613 | 2.09 |
| Etfdh | Electron transfer flavoprotein dehydrogenase | AV087820 | −2.759 | 2.09 |
| Mrpl36 | Mitochondrial ribosomal protein L36 | AV061668 | −2.173 | 2.09 |
| Bzw2 | Basic leucine zipper and W2 domains 2 | BG064378 | −2.187 | 2.08 |
| Aga | Aspartylglucosaminidase | AV056679 | −2.161 | 2.08 |
| Ldhb | Lactate dehydrogenase B | AV032980 | −2.810 | 2.08 |
| Tcof1 | Treacher Collins Franceschetti syndrome 1, homolog | BG064056 | −3.181 | 2.08 |
| Mrpl45 | Mitochondrial ribosomal protein L45 | BG074455 | −2.815 | 2.08 |
| Upf3b | UPF3 regulator of nonsense transcripts homolog B | BG066789 | −2.032 | 2.07 |
| Sdha | Succinate dehydrogenase complex, subunit A, flavoprotein (Fp) | AV087966 | −3.372 | 2.07 |
| Lzts2 | Leucine zipper, putative tumor suppressor 2 | BG063967 | −3.201 | 2.07 |
| Atp5f1 | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit b, isoform 1 | AI836064 | −6.161 | 2.07 |
| Seh1l | SEH1-like (S. cerevisiae) | AV025108 | −2.554 | 2.07 |
| Ebpl | Emopamil binding protein-like | AV094562 | −2.563 | 2.06 |
| Ptp4a3 | Protein tyrosine phosphatase, type 4A, 3 | AV087012 | −2.477 | 2.06 |
| Aptx | Aprataxin | AV085680 | −2.627 | 2.06 |
| Mdh1 | Malate dehydrogenase 1, NAD (soluble) | BG067903 | −2.618 | 2.06 |
| Mapk14 | Mitogen-activated protein kinase 14 | BG074842 | −2.419 | 2.06 |
| Ncf1 | Neutrophil cytosolic factor 1 | AV074152 | −2.431 | 2 |
| Parn | Poly(A)-specific ribonuclease (deadenylation nuclease) | AA408467 | −2.817 | 2.06 |
| Smurf1 | SMAD specific E3 ubiquitin protein ligase 1 | BG076120 | −2.769 | 2.05 |
| Rgs2 | Regulator of G-protein signaling 2 | BG067321 | −3.334 | 2.05 |
| Pnn | Pinin | AV135835 | −2.050 | 2.05 |
| Cacnb2 | Calcium channel, voltage-dependent, beta 2 subunit | BG076402 | −2.234 | 2.05 |
| Cs | Citrate synthase, mitochondrial | AV006265 | −3.596 | 2.05 |
| Stat1 | Signal transducer and activator of transcription 1 | AA170538 | −2.378 | 2.04 |
| Tgfb1 | Transforming growth factor, beta 1 | AA049522 | −2.692 | 2.04 |
| Mfng | MFNG O-fucosylpeptide 3-beta-N-acetylglucosaminyltransferase | AA116377 | −2.013 | 2.04 |
| Hdlbp | High density lipoprotein (HDL) binding protein | BG064469 | −2.713 | 2.04 |
| Gstm1 | Glutathione S-transferase, MU-1 | AV149857 | −2.256 | 2.04 |
| Pik3r4 | Phosphatidylinositol 3 kinase, regulatory subunit, polypeptide 4, p150 | AV074828 | −2.566 | 2.04 |
| Abl1 | V-abl Abelson murine leukemia oncogene 1 | BG064435 | −2.074 | 2.04 |
| Plrg1 | Pleiotropic regulator 1, PRL1 homolog (Arabidopsis) | C80566 | −3.591 | 2.03 |
| Abcd3 | ATP-binding cassette, sub-family D (ALD), member 3 | AV140550 | −2.499 | 2.03 |
| Drg2 | Developmentally regulated GTP binding protein 2 | AV084970 | −2.727 | 2.03 |
| Chmp2a | Chromatin modifying protein 2A | BG075465 | −2.278 | 2.02 |
| Ccbl2 | Cysteine conjugate-beta lyase 2 | AV103749 | −2.449 | 2.02 |
| Hspd1 | Chaperonin | BB610862 | −2.971 | 2.02 |
| Farslb | Phenylalanyl-tRNA synthetase, beta subunit | BG064281 | −2.473 | 2.02 |
| Etfdh | Electron transferring flavoprotein, dehydrogenase | BG070637 | −2.472 | 2.02 |
| Farp2 | FERM, RhoGEF and pleckstrin domain protein 2 | AA146115 | −2.968 | 2.01 |
| Hnrpa1 | Heterogeneous nuclear ribonucleoprotein A1 | BG072533 | −2.335 | 2.01 |
| Mbnl2 | Muscleblind-like 2 | BG072107 | −2.174 | 2.01 |
| Hbld2 | Iron-sulfur cluster assembly 1, | AI851055 | −2.122 | 2.01 |
| Smpd2 | Sphingomyelin phosphodiesterase 2, neutral | AV005649 | −2.332 | 2.01 |
| Fyn | Fyn proto-oncogene | AV051790 | −4.000 | 2.01 |
| Abcb7 | ATP-binding cassette, subfamily B, member 7 | BG074307 | −2.087 | 2.01 |
| Ndufb10 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 10 | AI836006 | −4.215 | 2.01 |
| Ndufs6 | NADH-ubiquinone oxidoreductase Fe-S proetin 6 | AV071159 | −2.816 | 2.01 |
| Alad | Aminolevulinate, delta-, dehydratase | BG063937 | −2.467 | 2.00 |
| Aco2 | Aconitase, mitochondrial | AV085145 | −2.255 | 2.00 |
| Stx17 | Syntaxin 17 | AV015430 | −2.102 | 2.00 |
| Gabarapl1 | Gamma-aminobutyric acid (GABA(A)) receptor-associated protein-like 1 | AV150053 | −2.393 | 2.00 |
The SAM algorithm was employed to identify genes with statistically different expression levels between PA-banded and sham tissues. SAM incorporates an FDR correction for multiple testing errors and calculates a d statistic for each gene based on the ratio of change in gene expression to SD in the data for that gene (Ref. 18).
Out of a total of 1,114 significantly downregulated genes and ESTs.
Table 5.
Gene ontology category transcripts upregulated in severe PS RV versus sham RV
| Gene Ontology Category | Total Genes | Changed Genes | P Value (Log10) |
|---|---|---|---|
| Phosphate transport | 38 | 7 | −4.78 |
| Negative regulation of coagulation | 5 | 3 | −3.97 |
| Inorganic anion transport | 51 | 7 | −3.92 |
| Regulation of coagulation | 6 | 3 | −3.68 |
| Cell adhesion | 239 | 15 | −3.63 |
| Anion transport | 64 | 7 | −3.30 |
| Skeletal development | 84 | 7 | −2.59 |
| Organismal physiological process | 634 | 25 | −2.58 |
| Neuromuscular physiological process | 4 | 2 | −2.54 |
| Ion transport | 224 | 12 | −2.40 |
| Tyrosine kinase signaling pathway | 91 | 7 | −2.40 |
| Coagulation | 32 | 4 | −2.28 |
| Negative regulation of Wnt receptor signaling pathway | 6 | 2 | −2.15 |
| Negative regulation of apoptosis | 81 | 6 | −2.04 |
| Negative regulation of programmed cell death | 81 | 6 | −2.04 |
| Sensory perception | 107 | 7 | −2.01 |
| Organismal movement | 7 | 2 | −2.01 |
| Histone acetylation | 8 | 2 | −1.89 |
| Regulation of apoptosis | 199 | 10 | −1.89 |
| Apoptosis | 261 | 12 | −1.88 |
| Regulation of programmed cell death | 200 | 10 | −1.87 |
| Death | 300 | 13 | −1.81 |
| Ionic insulation of neurons by glial cells | 9 | 2 | −1.79 |
| Myelination | 9 | 2 | −1.79 |
| Programmed cell death | 269 | 12 | −1.79 |
| Regulation of physiological process | 1,366 | 41 | −1.79 |
| Smooth muscle contraction | 10 | 2 | −1.70 |
| Adult locomotory behavior | 10 | 2 | −1.70 |
| Negative regulation of histone acetylation | 1 | 1 | −1.65 |
| Regulation of Wnt receptor signaling pathway | 11 | 2 | −1.62 |
| Enzyme-linked receptor protein signaling pathway | 130 | 7 | −1.59 |
| Cell surface receptor-linked signal transduction | 397 | 15 | −1.52 |
| Calcium ion homeostasis | 32 | 3 | −1.47 |
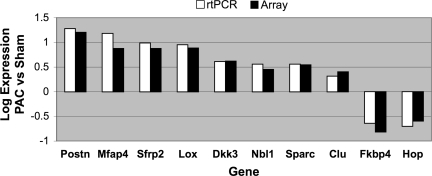
Some interesting genes related to cardiac function were increased in severe PS versus sham RV. For each of these genes, altered expression was verified by QRT-PCR (Fig. 8). Periostin (16.0 fold) was the most dramatically increased in the PAC RV. This gene is highly expressed in the myocardium in patients with heart failure (30). Periostin expression is positively regulated by transforming growth factor (TGF)-β (27), and Ingenuity Pathway Assist gene pathway analysis demonstrated a pattern of both the up- and downregulation of multiple transcripts in the TGF-β signaling pathway, which could be the subject of future study once verified by PCR. Other transcripts with marked upregulation in the PAC RV included lysyl oxidase (LOX; 7.7 fold), microfibrillar-associated protein 4 (7.5 fold), and secreted acidic cysteine rich glycoprotein (3.5 fold), related to ECM proteins and their cross-linking enzyme.
Fig. 8.
Comparison of RT-PCR vs. array for 10 differentially expressed genes in PAC RV vs. sham RV. For each of these genes, there was excellent agreement between both methods.
We next compared gene expression data from mice after PAC with data from our previous study of mice after 10 days of LV afterload stress using TAC (73). Many genes were upregulated in both the PAC RV and TAC LV, since many of the same processes of matrix remodeling, metabolic changes, and actin cytoskeletal alterations must necessarily occur in both models of afterload stress. However, there were several transcripts that showed significant differential regulation in the afterload stressed RV but not in the LV (Table 6). Those which were upregulated in the PAC RV but not in the TAC LV included three from the upregulated GO biological process Wnt signaling: Dickkopf 3, Sfrp2, and Wif1. Other important RV-specific upregulated genes include annexin A7, clusterin/apolipoprotein J, neuroblastoma suppression of tumorigenicity 1 (Nbl1), formin binding protein (Fnbp4), and LOX.
Table 6.
Transcripts that showed significant upregulation in the afterload-stressed RV but not in the afterload-stressed LV
| Gene | Function | Fold Change |
|---|---|---|
| Fnbp4 | Regulated by p53 and involved in apoptosis | 11.6 |
| Uchl1 | Involved in the processing of ubiquitin precursors | 7.2 |
| Dkk3 | Inhibitor of Wnt signaling pathway | 4.2 |
| Nbl1 | TGF-β antagonist that modulates BMP2 signaling | 2.8 |
| Tacstd1 | Ga733 tumor-associated antigen gene family | 2.8 |
| Clusterin/apolipoprotein J | Involved in apoptosis | 2.5 |
| Annexin A7 | Calcium/phospholipid-binding protein | 3 |
| Wif1 | Inhibitor of Wnt signaling pathway | 1.6 |
| Sfrp2 | Inhibitor of Wnt signaling pathway | 7.5 |
| LOX | Regulates cell migration and actin polymerization | 7.7 |
DISCUSSION
RV dysfunction is a common cause of long-term morbidity in many patients with congenital heart defects, especially those producing RV pressure overload (RVOT obstructions such as tetralogy of Fallot) and those with systemic right ventricles (e.g., hypoplastic left heart syndrome) (21, 41, 42). For many of these patients, despite incredible progress in surgical repair or palliation, long-term survival and quality of life will depend on our ability to preserve long-term RV function. Although there are considerable data on the molecular events underlying afterload-induced LV remodeling, there is little information on molecular events in the afterloaded RV. The complex geometry of the RV and differences in physiology with respect to the LV, however, make RV failure difficult to assess both clinically and in the laboratory. The response of the RV to increased afterload consists of both hypertrophy and enlargement, dilation of the tricuspid valve annulus leading to tricuspid regurgitation, and leftward displacement of IVS, leading to alterations in LV diastolic function, which can be significantly compromised by deleterious ventricular-ventricular interaction.
We have adapted a previously described model of RV afterload stress (51), with updated and detailed physiological and genomic characterization. This model shows many characteristics of both acute and chronic RV failure encountered in clinical settings: RV dilation, right bundle branch block, tricuspid regurgitation, leftward septal shift, right-sided heart failure, and decreased survival. Although there are a few previous reports of PAC in mice and rats (8, 20, 51, 53, 64), none has characterized the physiological response as clearly. We analyzed our PAC model by dividing subjects into three groups based on the degree of pressure gradient between the RV and PA and the presence or absence of IVS shift. Our results show that mice with severe PS have significantly decreased survival compared with those with mild PS, with the moderate group having a survival intermediate between the other two groups. These data suggest that our mild and moderate PS groups represent a reasonable model of chronic RV dysfunction and that our severe group represents a good model of acute decompensated RV function. This reconstructed natural history is also similar to those models of LV outflow tract obstruction (TAC) associated with LV failure (37). Although it could be argued that mice in our severe group would not meet the clinical criteria used in humans to qualify for severe PS based on outflow tract gradient alone, mice in this group did have evidence of RV failure, including significant RV dilation, decreased RV wall motion, elevation of RVEDP, decreased CO, clinical evidence of right-sided heart failure, and decreased survival. In these animals, the lack of a higher RV-PA gradient may reflect the lower CO in the severe PS group, as well as the temporal difference between an artificial animal model (with the acute onset of RV outflow obstruction) versus that in humans with congenital heart disease (with chronic RV outflow obstruction allowing the gradual development of RV hypertrophy and chronic adaptation).
In all groups of mice with PS, the degree of PS correlated well with RV weight-to-BW ratio and to histological evidence of cardiomyocyte hypertrophy. Previous reports in PA-banded rats (8, 20, 53) describe the doubling of RV weight after banding for 3 wk (8), although no histological data were presented. There are two previous reports describing the technique of PAC in mice (51, 64); however, these reports provide minimal details concerning physiological variables.
An accurate estimation of RV diastolic dimension by ultrasound is difficult not only in mice but in humans as well. We used RVOT dimension and RVOT FS to quantify RV dilation and function (43). These data show that RV function is preserved in mild and moderate PS and significantly decreased in severe PS. All mice with severe PS had evidence of tricuspid regurgitation, which also differentiated them from the mild and moderate groups. Tricuspid regurgitation is used clinically as a sign of RV dilation and dysfunction in humans with congenital heart disease and RV outflow obstruction (42, 50). Increased RV size, whether due to increased preload (atrial septal defect) or afterload (sleep apnea, primary pulmonary hypertension, pulmonary embolus), leads to iRBBB in humans. Mice with severe PS showed iRBBB, also differentiating them from mice with mild or moderate PS. Other signs of right heart failure in the severe PS group included increases in liver weight to BW and the development of peripheral edema.
The clinical assessment of RV failure has been based primarily on echocardiography, although magnetic resonance angiography is playing an increasingly important role (31). Recent human studies show that TDI holds promise for providing an accurate assessment of RV function, e.g., peak systolic velocity at the basal tricuspid annulus (9, 26, 39). In this study, we demonstrate that RV wall velocity can be obtained noninvasively in mice using TDI and that TDI-derived RV wall velocity correlates well with dP/dtmax obtained from invasive hemodynamic studies. This suggests that TDI can be useful in the serial evaluation of murine models or RV failure, in which the chronic micromanometer catheterization of the RV is not feasible. The relationship between LV wall velocity and dP/dtmax in mice has already been established and shows a similarly strong correlation (58).
One of the major problems in RV failure is the negative ventricular-ventricular interaction mediated in part through septal shift, encroaching into the LV cavity, and impairing LV diastolic function (6, 28, 69). Our model of severe PS accurately recapitulates this process, with elevation of RVEDP, shift of the septum into the LV, and decreased CO. Using echocardiography, we were able to quantify the degree of intra-LV dyssynchrony by using TDI to measure the offset between septal and LV free wall contraction. Also interventricular dyssynchrony could be measured from the time difference of peak systolic velocity at the base of the tricuspid and mitral valves.
There have been multiple previous studies of increased RV afterload in many mammalian models, including cats (15, 47), dogs (22, 45), pigs (4, 16), rabbits (5, 19, 23), and rats (8, 11, 20, 33, 36, 53). The advantages of a well-characterized murine model, particularly in the ability to alter murine gene expression, are well known. Rockman et al. (51) published a similar model of PAC in the mouse, although the degree of physiological characterization was less and the examination of gene expression changes was limited to a standard panel of heart failure genes.
There have been few prior studies of gene expression changes in the stressed RV. Several have examined RV gene expression associated with pulmonary hypertension (11, 33). In rats with monocrotaline-induced pulmonary hypertension, Buermans et al. (11) compared gene expression by microarray in compensated or decompensated RV hypertrophy. Ventricles destined to progress to failure showed an activation of proapoptotic pathways, particularly related to mitochondria, whereas the group with compensated hypertrophy showed blocked pro-death effector signaling via p38-MAPK, through the upregulation of MAPK phosphatase-1. Kogler et al. (33) also using a monocrotoline model, studied the altered regulation of calcium regulatory genes, finding a downregulation of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a, phospholamban (PLB), and the ryanodine receptor. LekanneDeprez et al. (36) used a PA banding model in the rat to investigate alterations in the expression of selected genes during the transition from compensated RV hypertrophy to failure. PA banding resulted in an induction of atrial natriuretic peptide, a moderate increase in collagen III α1, and a decrease in SERCA2 and PLB (36). However, in contrast to the current study, these authors did not utilize a genome-wide approach.
We found that in the afterload stressed RV, 196 genes were upregulated and 1,114 genes were downregulated compared with sham-operated controls. The importance of comparing surgical subjects with sham controls has been well established. Our laboratory has previously described the marked and prolonged gene expression changes that can be introduced even by sham operation (73).
The RV and LV share many common pathways, which are either up- or downregulated during the development of pressure overload, since many of the same processes of matrix remodeling, metabolic changes, and actin cytoskeletal alterations must necessarily occur in both. GO analysis, to identify significantly enriched and depleted groups, showed the most significantly upregulated processes were in the categories of phosphate transport, regulation of coagulation, inorganic anion transport, and cell adhesion. Downregulated processes were dominated by energy pathways. Periostin, the transcript with the greatest fold change (16.0 fold) in the PAC RV, is also increased in the TAC LV. Periostin is a secreted protein involved in bone formation and cell-cell adhesion. It is upregulated in the Syrian hamster model of heart failure (69) and expressed in cardiac fibroblasts and implicated in cardiac dysfunction (38). The overexpression of periostin causes LV dilation and an increase in collagen deposition (30). In contrast, the inhibition of periostin improves LV function in Dahl salt-sensitive rats (30). Periostin knockout mice also show less fibrosis and hypertrophy after TAC or myocardial infarction (44). LOX (upregulated 7.7 fold), microfibrillar-associated protein 4 (7.5 fold), and secreted acidic cysteine rich glycoprotein (3.5 fold) are additional ECM proteins that may contribute to progressive ventricular remodeling, dilation, and heart failure (48).
Despite these commonly expressed gene programs, there are several important differences between the afterload stressed RV and LV. Several transcripts showed a significant differential upregulation in the RV but not in the LV, including three from the Wnt signaling pathway (12, 14), recognized as one of the major families of developmentally regulated signaling molecules. These included the Wnt inhibitors Dickkopf 3 (4.2 fold), Sfrp2 (7.5 fold), and Wif1 (1.6 fold). Other important RV-specific upregulated genes include annexin A7 (3 fold), which has been associated with the regulation of calcium handling in cardiomyoctes (13), and clusterin/apolipoprotein J (2.5 fold), which has a nearly ubiquitous expression pattern in human tissues. Clusterin is differentially regulated in many severe physiological disturbances including cell death, aging, cancer progression, and various neurological diseases (65). Clusterin protects cardiomyocytes against ischemic cell death via a complement independent pathway (35); however, the function of clusterin remains an enigma, due to its intriguingly distinct and often opposite functions in different cell types.
Several additional genes were differentially regulated in the RV versus LV. Nbl1 is upregulated in the PAC RV (2.8 fold) but slightly downregulated in the TAC LV. Nbl1 is a TGF-β antagonist that modulates bone morphogenetic protein (BMP)2 signaling and prevents cells from entering the G1/S phase of the cell cycle. In LV hypertrophy, the activation of the first part of the G1 phase (cyclin D and E) occurs but without progression to the S phase. Nbl1 null mice have no gross phenotype unless crossbred with a Noggin (BMP antagonist) heterozygote, where there are skeletal but no known cardiac defects (49). The Fnbp4/formin binding protein was upregulated 11.6 fold in the RV compared with only twofold in the TAC LV. Formin is an actin regulator that plays a role in limb morphogenesis. In thymocytes, Fnbp4 is regulated by p53 and involved in cell death pathways. LOX/Lysyl oxidase (5.4 fold) regulates cell migration and actin polymerization through the focal adhesion kinase/Src signaling complex. LOX is also involved in the cross-linking of fibrillar collagens and elastin and is upregulated in myocardial ischemia (66).
There are several limitations in our methodology, some of which hold true for any small animal model of human disease. Although murine studies are a powerful platform by which to study genetic alterations in cardiac disease, substantial species differences must be acknowledged and findings confirmed in larger mammalian models. We chose a model of PA banding because residual RVOT obstruction is one of the major long-term sequelae in patients with repaired congenital heart disease, including tetralogy of Fallot, corrected transposition, and other complex congenital heart lesions where the RV is at risk. Other models, including pulmonary hypertension, add additional variables (altered pulmonary vascular pathology) aside from increased RV afterload, which could result in different gene expression patterns in the stressed RV. Other experimental manipulations required to produce pulmonary hypertension in rodents (e.g., hypoxia) could also have independent effects on the RV myocardium.
There are other limitations inherent to any microarray-based study. These transcriptional profiles provide a snapshot look at gene expression changes, which will vary with time after banding and with a multitude of other physiological changes. They also do not account for the myriad of posttranscriptional changes, which for many signaling pathways involved in hypertrophy and heart failure are the dominant regulators. Gene microarrays are also less sensitive in detecting small changes in gene expression that may still be biologically meaningful. We have used a strategy designed to minimize the number of false negative calls by using four replicate arrays per condition, but it is inevitable that some genes with small but meaningful changes in gene expression will not be identified using our stringent statistical analyses. Our laboratory has also shown previously that the accuracy of gene microarrays is more limited for genes with very low expression levels (73). Finally, there are limitations to our study in our comparison of gene expression in the RV with the LV. Although we obtained samples at similar time points after PAC and TAC, it is not possible to totally equalize the degrees of afterload stress between the two ventricles, given that the LV normally is exposed to systemic arterial pressure and the RV to the considerably lower PA pressure. Further investigation of RV-LV differential gene expression is thus warranted, with multiple additional time points as well as varied degrees of afterload stress, well beyond the scope of the present methodological study. Ultimately, one or more of these genes may provide utility as a biomarker for the early prediction of clinical outcome as well as a potential therapeutic target for patients with RV failure. A physiologically well-characterized murine model of PS should provide a valuable platform for these studies.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-061535 (to D. Bernstein).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe T, Komatsu S. Long-term follow-up studies in 211 patients with tetralogy of Fallot after corrective surgery over 10 years. [In Japanese.] Kyobu Geka 43: 598–604, 1990. [PubMed]
- 2.Arimoto T, Takeishi Y, Takahashi H, Shishido T, Niizeki T, Koyama Y, Shiga R, Nozaki N, Nakajima O, Nishimaru K, Abe J, Endoh M, Walsh RA, Goto K, Kubota I. Cardiac-specific overexpression of diacylglycerol kinase zeta prevents Gq protein-coupled receptor agonist-induced cardiac hypertrophy in transgenic mice. Circulation 113: 60–66, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Awariefe SO, Clarke DR, Pappas G. Surgical approach to critical pulmonary valve stenosis in infants less than six months of age. J Thorac Cardiovasc Surg 85: 375–387, 1983. [PubMed] [Google Scholar]
- 4.Bauer EP, Kuki S, Zimmermann R, Schaper W. Upregulated and downregulated transcription of myocardial genes after pulmonary artery banding in pigs. Ann Thorac Surg 66: 527–531, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Bishop JE, Rhodes S, Laurent GJ, Low RB, Stirewalt WS. Increased collagen synthesis and decreased collagen degradation in right ventricular hypertrophy induced by pressure overload. Cardiovasc Res 28: 1581–1585, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Bleeker GB, Schalij MJ, Nihoyannopoulos P, Steendijk P, Molhoek SG, van Erven L, Bootsma M, Holman ER, van der Wall EE, Bax JJ. Left ventricular dyssynchrony predicts right ventricular remodeling after cardiac resynchronization therapy. J Am Coll Cardiol 46: 2264–2269, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Borowski A, Ghodsizad A, Litmathe J, Lawrenz W, Schmidt KG, Gams E. Severe pulmonary regurgitation late after total repair of tetralogy of Fallot: surgical considerations. Pediatr Cardiol 25: 466–471, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Braun MU, Szalai P, Strasser RH, Borst MM. Right ventricular hypertrophy and apoptosis after pulmonary artery banding: regulation of PKC isozymes. Cardiovasc Res 59: 658–667, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Brili S, Alexopoulos N, Latsios G, Aggeli C, Barbetseas J, Pitsavos C, Vyssoulis G, Stefanadis C. Tissue Doppler imaging and brain natriuretic peptide levels in adults with repaired tetralogy of Fallot. J Am Soc Echocardiogr 18: 1149–1154, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol 559: 103–120, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buermans HP, Redout EM, Schiel AE, Musters RJ, Zuidwijk M, Eijk PP, van Hardeveld C, Kasanmoentalib S, Visser FC, Ylstra B, Simonides WS. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics 21: 314–323, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev 11: 3286–3305, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Camors E, Monceau V, Charlemagne D. Annexins and Ca2+ handling in the heart. Cardiovasc Res 65: 793–802, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Christodoulides C, Laudes M, Cawthorn WP, Schinner S, Soos M, O′Rahilly S, Sethi JK, Vidal-Puig A. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci 119: 2613–2620, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper G 4th, Tomanek RJ, Ehrhardt JC, Marcus ML. Chronic progressive pressure overload of the cat right ventricle. Circ Res 48: 488–497, 1981. [DOI] [PubMed] [Google Scholar]
- 16.Cusimano RJ, Ashe KA, Abel JG, Lichtenstein SV, Salerno TA. A simple model of right ventricular hypertrophy. J Invest Surg 1: 45–53, 1988. [DOI] [PubMed] [Google Scholar]
- 17.D′Andrea A, Caso P, Sarubbi B, D′Alto M, Giovanna Russo M, Scherillo M, Cotrufo M, Calabro R. Right ventricular myocardial activation delay in adult patients with right bundle branch block late after repair of Tetralogy of Fallot. Eur J Echocardiogr 5: 123–131, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Dore A, Houde C, Chan KL, Ducharme A, Khairy P, Juneau M, Marcotte F, Mercier LA. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation 112: 2411–2416, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Emani SM, Shah AS, Bowman MK, White DC, Emani S, Glower DD, Koch WJ. Right ventricular targeted gene transfer of a beta-adrenergic receptor kinase inhibitor improves ventricular performance after pulmonary artery banding. J Thorac Cardiovasc Surg 127: 787–793, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Faber MJ, Dalinghaus M, Lankhuizen IM, Steendijk P, Hop WC, Schoemaker RG, Duncker DJ, Lamers JM, Helbing WA. Right and left ventricular function after chronic pulmonary artery banding in rats assessed with biventricular pressure-volume loops. Am J Physiol Heart Circ Physiol 291: H1580–H1586, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Friedberg MK, Silverman NH, Dubin AM, Rosenthal DN. Right ventricular mechanical dyssynchrony in children with hypoplastic left heart syndrome. J Am Soc Echocardiogr 20: 1073–1079, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Gaynor SL, Maniar HS, Bloch JB, Steendijk P, Moon MR. Right atrial and ventricular adaptation to chronic right ventricular pressure overload. Circulation 112: I212–I218, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs CL, Wendt IR, Kotsanas G, Young IR, Woolley G. Mechanical, energetic, and biochemical changes in long-term pressure overload of rabbit heart. Am J Physiol Heart Circ Physiol 259: H849–H859, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Gilman G, Khandheria BK, Hagen ME, Abraham TP, Seward JB, Belohlavek M. Strain rate and strain: a step-by-step approach to image and data acquisition. J Am Soc Echocardiogr 17: 1011–1020, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto I, Li X, Hejmadi Bhat A, Jones M, Zetts AD, Sahn DJ. Myocardial strain rate is a superior method for evaluation of left ventricular subendocardial function compared with tissue Doppler imaging. J Am Coll Cardiol 42: 1574–1583, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Hsiao SH, Lin SK, Wang WC, Yang SH, Gin PL, Liu CP. Severe tricuspid regurgitation shows significant impact in the relationship among peak systolic tricuspid annular velocity, tricuspid annular plane systolic excursion, and right ventricular ejection fraction. J Am Soc Echocardiogr 19: 902–910, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Iekushi K, Taniyama Y, Azuma J, Katsuragi N, Dosaka N, Sanada F, Koibuchi N, Nagao K, Ogihara T, Morishita R. Novel mechanisms of valsartan on the treatment of acute myocardial infarction through inhibition of the antiadhesion molecule periostin. Hypertension 49: 1409–1414, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Janousek J, Tomek V, Chaloupecky VA, Reich O, Gebauer RA, Kautzner J, Hucin B. Cardiac resynchronization therapy: a novel adjunct to the treatment and prevention of systemic right ventricular failure. J Am Coll Cardiol 44: 1927–1931, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Kang IS, Redington AN, Benson LN, Macgowan C, Valsangiacomo ER, Roman K, Kellenberger CJ, Yoo SJ. Differential regurgitation in branch pulmonary arteries after repair of tetralogy of Fallot: a phase-contrast cine magnetic resonance study. Circulation 107: 2938–2943, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Katsuragi N, Morishita R, Nakamura N, Ochiai T, Taniyama Y, Hasegawa Y, Kawashima K, Kaneda Y, Ogihara T, Sugimura K. Periostin as a novel factor responsible for ventricular dilation. Circulation 110: 1806–1813, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Kjaergaard J, Petersen CL, Kjaer A, Schaadt BK, Oh JK, Hassager C. Evaluation of right ventricular volume and function by 2D and 3D echocardiography compared with MRI. Eur J Echocardiogr 7: 430–438, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Knollmann BC, Katchman AN, Franz MR. Monophasic action potential recordings from intact mouse heart: validation, regional heterogeneity, and relation to refractoriness. J Cardiovasc Electrophysiol 12: 1286–1294, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Kogler H, Hartmann O, Leineweber K, Nguyen van P, Schott P, Brodde OE, Hasenfuss G. Mechanical load-dependent regulation of gene expression in monocrotaline-induced right ventricular hypertrophy in the rat. Circ Res 93: 230–237, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Kondo RP, Dederko DA, Teutsch C, Chrast J, Catalucci D, Chien KR, Giles WR. Comparison of contraction and calcium handling between right and left ventricular myocytes from adult mouse heart: a role for repolarization waveform. J Physiol 571: 131–146, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krijnen PA, Cillessen SA, Manoe R, Muller A, Visser CA, Meijer CJ, Musters RJ, Hack CE, Aarden LA, Niessen HW. Clusterin: a protective mediator for ischemic cardiomyocytes? Am J Physiol Heart Circ Physiol 289: H2193–H2202, 2005. [DOI] [PubMed] [Google Scholar]
- 36.LekanneDeprez RH, van den Hoff MJ, de Boer PA, Ruijter PM, Maas AA, Chamuleau RA, Lamers WH, Moorman AF. Changing patterns of gene expression in the pulmonary trunk-banded rat heart. J Mol Cell Cardiol 30: 1877–1888, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Liao Y, Ishikura F, Beppu S, Asakura M, Takashima S, Asanuma H, Sanada S, Kim J, Ogita H, Kuzuya T, Node K, Kitakaze M, Hori M. Echocardiographic assessment of LV hypertrophy and function in aortic-banded mice: necropsy validation. Am J Physiol Heart Circ Physiol 282: H1703–H1708, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Litvin J, Blagg A, Mu A, Matiwala S, Montgomery M, Berretta R, Houser S, Margulies K. Periostin and periostin-like factor in the human heart: possible therapeutic targets. Cardiovasc Pathol 15: 24–32, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Meluzin J, Spinarova L, Bakala J, Toman J, Krejci J, Hude P, Kara T, Soucek M. Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion. A new, rapid, and non-invasive method of evaluating right ventricular systolic function. Eur Heart J 22: 340–348, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Meyns B, Van Garsse L, Boshoff D, Eyskens B, Mertens L, Gewillig M, Fieuws S, Verbeken E, Daenen W. The Contegra conduit in the right ventricular outflow tract induces supravalvular stenosis. J Thorac Cardiovasc Surg 128: 834–840, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Nakazawa M, Katayama H, Imai Y, Nojima K, Nakanishi T, Kurosawa H, Takao A, Okuda H. A quantitative analysis of hemodynamic effects of the right ventricle included in the circulation of the Fontan procedure. Circulation 83: 822–826, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Nollert G, Fischlein T, Bouterwek S, Bohmer C, Klinner W, Reichart B. Long-term survival in patients with repair of tetralogy of Fallot: 36-year follow-up of 490 survivors of the first year after surgical repair. J Am Coll Cardiol 30: 1374–1383, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Oelberg DA, Marcotte F, Kreisman H, Wolkove N, Langleben D, Small D. Evaluation of right ventricular systolic pressure during incremental exercise by Doppler echocardiography in adults with atrial septal defect. Chest 113: 1459–1465, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 101: 313–321, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orito K, Yamane T, Kanai T, Fujii Y, Wakao Y, Matsuda H. Time course sequences of angiotensin converting enzyme and chymase-like activities during development of right ventricular hypertrophy induced by pulmonary artery constriction in dogs. Life Sci 75: 1135–1145, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Pigula FA, Khalil PN, Mayer JE, del Nido PJ, Jonas RA. Repair of tetralogy of Fallot in neonates and young infants. Circulation 100: II157–II161, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Quaile MP, Rossman EI, Berretta RM, Bratinov G, Kubo H, Houser SR, Margulies KB. Reduced sarcoplasmic reticulum Ca2+ load mediates impaired contractile reserve in right ventricular pressure overload. J Mol Cell Cardiol 43: 552–563, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol 103: 416–419, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Reddi AH Interplay between bone morphogenetic proteins and cognate binding proteins in bone and cartilage development: noggin, chordin and DAN. Arthritis Res 3: 1–5, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynertson SI, Kundur R, Mullen GM, Costanzo MR, McKiernan TL, Louie EK. Asymmetry of right ventricular enlargement in response to tricuspid regurgitation. Circulation 100: 465–467, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Rockman HA, Ono S, Ross RS, Jones LR, Karimi M, Bhargava V, Ross J Jr, Chien KR. Molecular and physiological alterations in murine ventricular dysfunction. Proc Natl Acad Sci USA 91: 2694–2698, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA 88: 8277–8281, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roncon-Albuquerque R Jr, Vasconcelos M, Lourenco AP, Brandao-Nogueira A, Teles A, Henriques-Coelho T, Leite-Moreira AF. Acute changes of biventricular gene expression in volume and right ventricular pressure overload. Life Sci 78: 2633–2642, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Sathish V, Xu A, Karmazyn M, Sims SM, Narayanan N. Mechanistic basis of differences in Ca2+-handling properties of sarcoplasmic reticulum in right and left ventricles of normal rat myocardium. Am J Physiol Heart Circ Physiol 291: H88–H96, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Sawkins MC, Farmer AD, Hoisington D, Sullivan J, Tolopko A, Jiang Z, Ribaut JM. Comparative map and trait viewer (CMTV): an integrated bioinformatic tool to construct consensus maps and compare QTL and functional genomics data across genomes and experiments. Plant Mol Biol 56: 465–480, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vancon AC, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation 104: 1286–1291, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Schuster P, Faerestrand S, Ohm OJ. Color Doppler tissue velocity imaging can disclose systolic left ventricular asynchrony independent of the QRS morphology in patients with severe heart failure. Pacing Clin Electrophysiol 27: 460–467, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Sebag IA, Handschumacher MD, Ichinose F, Morgan JG, Hataishi R, Rodrigues AC, Guerrero JL, Steudel W, Raher MJ, Halpern EF, Derumeaux G, Bloch KD, Picard MH, Scherrer-Crosbie M. Quantitative assessment of regional myocardial function in mice by tissue Doppler imaging: comparison with hemodynamics and sonomicrometry. Circulation 111: 2611–2616, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Soukas A, Cohen P, Socci ND, Friedman JM. Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev 14: 963–980, 2000. [PMC free article] [PubMed] [Google Scholar]
- 60.Stanford. Functional Genomics (Online). http://www.microarray.org/sfgf/meebo.do.
- 61.Sutherland GR, Di Salvo G, Claus P, D′Hooge J, Bijnens B. Strain and strain rate imaging: a new clinical approach to quantifying regional myocardial function. J Am Soc Echocardiogr 17: 788–802, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Tabibiazar R, Wagner RA, Liao A, Quertermous T. Transcriptional profiling of the heart reveals chamber-specific gene expression patterns. Circ Res 93: 1193–1201, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Tamayo P, Slonim D, Mesirov J, Zhu Q, Kitareewan S, Dmitrovsky E, Lander ES, Golub TR. Interpreting patterns of gene expression with self-organizing maps: methods and application to hematopoietic differentiation. Proc Natl Acad Sci USA 96: 2907–2912, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics 16: 349–360, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Trougakos IP, Lourda M, Agiostratidou G, Kletsas D, Gonos ES. Differential effects of clusterin/apolipoprotein J on cellular growth and survival. Free Radic Biol Med 38: 436–449, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Tsuda T, Gao E, Evangelisti L, Markova D, Ma X, Chu ML. Post-ischemic myocardial fibrosis occurs independent of hemodynamic changes. Cardiovasc Res 59: 926–933, 2003. [DOI] [PubMed] [Google Scholar]
- 67.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ullrich R, Scherrer-Crosbie M, Bloch KD, Ichinose F, Nakajima H, Picard MH, Zapol WM, Quezado ZM. Congenital deficiency of nitric oxide synthase 2 protects against endotoxin-induced myocardial dysfunction in mice. Circulation 102: 1440–1446, 2000. [DOI] [PubMed] [Google Scholar]
- 69.Urasawa K, Yoshida I, Takagi C, Onozuka H, Mikami T, Kawaguchi H, Kitabatake A. Enhanced expression of beta-adrenergic receptor kinase 1 in the hearts of cardiomyopathic Syrian hamsters, BIO53.58. Biochem Biophys Res Commun 219: 26–30, 1996. [DOI] [PubMed] [Google Scholar]
- 70.Vieillard-Baron A, Loubieres Y, Schmitt JM, Page B, Dubourg O, Jardin F. Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol 87: 1644–1650, 1999. [DOI] [PubMed] [Google Scholar]
- 71.Wagner RA, Tabibiazar R, Powers J, Bernstein D, Quertermous T. Genome-wide expression profiling of a cardiac pressure overload model identifies major metabolic and signaling pathway responses. J Mol Cell Cardiol 37: 1159–1170, 2004. [DOI] [PubMed] [Google Scholar]
- 72.Watanabe T, Delbridge LM, Bustamante JO, McDonald TF. Heterogeneity of the action potential in isolated rat ventricular myocytes and tissue. Circ Res 52: 280–290, 1983. [DOI] [PubMed] [Google Scholar]
- 73.Zhao M, Chow A, Powers J, Fajardo G, Bernstein D. Microarray analysis of gene expression after transverse aortic constriction in mice. Physiol Genomics 19: 93–105, 2004. [DOI] [PubMed] [Google Scholar]