Abstract
Hashimoto's thyroiditis (HT) is associated with HLA, but the associated allele is still controversial. We hypothesized that specific HLA-DR pocket-sequence variants are associated with HT and that similar variants in the murine I-E locus (homologous to HLA-DR) predispose to experimental autoimmune thyroiditis (EAT), a classical mouse model of HT. Therefore, we sequenced the polymorphic exon 2 of the HLA-DR gene in 94 HT patients and 149 controls. In addition, we sequenced exon 2 of the I-E gene in 22 strains of mice, 12 susceptible to EAT and 10 resistant. Using logistic regression analysis, we identified a pocket amino acid signature, Tyr-26, Tyr-30, Gln-70, Lys-71, strongly associated with HT (P = 6.18 × 10−5, OR = 3.73). Lys-71 showed the strongest association (P = 1.7 × 10−8, OR = 2.98). This association was seen across HLA-DR types. The 5-aa haplotype Tyr-26, Tyr-30, Gln-70, Lys-71, Arg-74 also was associated with HT (P = 3.66 × 10−4). In mice, the I-E pocket amino acids Val-28, Phe-86, and Asn-88 were strongly associated with EAT. Structural modeling studies demonstrated that pocket P4 was critical for the development of HT, and pockets P1 and P4 influenced susceptibility to EAT. Surprisingly, the structures of the HT- and EAT-susceptible pockets were different. We conclude that specific MHC II pocket amino acid signatures determine susceptibility to HT and EAT by causing structural changes in peptide-binding pockets that may influence peptide binding, selectivity, and presentation. Because the HT- and EAT-associated pockets are structurally different, it is likely that distinct antigenic peptides are associated with HT and EAT.
Keywords: gene, Hashimoto's thyroiditis, HLA, major histocompatibility complex
Hashimoto's thyroiditis (HT) is among the most common human autoimmune diseases with a population prevalence in the United States of 1–4.6% (1, 2). HT is characterized by infiltration of the thyroid by autoreactive T and B cells causing thyroid cell death and production of anti-thyroid peroxidase (TPO) and anti-thyroglobulin (Tg) antibodies (reviewed in ref. 3). Clinically, the disease manifests by hypothyroidism requiring thyroid hormone supplementation, and most patients develop goiter. The pathogenesis of HT is believed to involve a complex interaction between inborn genetic susceptibility (reviewed in ref. 4) and an external trigger such as infection (5) or iodine (6). As a result, thyroid-specific T cells become activated and infiltrate the thyroid. The thyroid-infiltrating T cells induce thyroid cell death, causing gradual destruction of the thyroid gland, hypothyroidism, and goiter (reviewed in ref. 3).
The MHC gene locus encoding the HLA glycoproteins in humans consists of a complex of genes located on chromosome 6p21 (reviewed in ref. 4). Because the HLA region is highly polymorphic and contains many immune response genes, it was the first candidate genetic region to be studied for association with HT. However, in contrast to the clear association of Graves' disease (GD) with HLA-DR3, data on HLA alleles in HT have been less consistent and thus less definitive (4). Early studies showed an association of goitrous HT with HLA-DR5 (7) and of atrophic HT with -DR3 (8). Later studies have reported associations of HT with HLA-DR3 (9, 10), -DR4 (11, 12), or -DR5 (13) in Caucasians. The prevalent assumption is that these discrepancies are caused by the wide variation in the diagnostic criteria for HT ranging from the simple presence of thyroid autoantibodies with focal lymphocytic infiltration to the presence of goitrous or atrophic thyroiditis characterized by gross thyroid failure (14). Another possible explanation is that specific sequences within the HLA-DR molecule that are present across HLA-DR types predispose to HT; therefore, no specific DR type shows significant association with HT.
In several autoimmune diseases, including type 1 diabetes (T1D) (15) and rheumatoid arthritis (16), there is persuasive evidence that the disease is associated with specific amino acid sequences within the MHC class II genes. Moreover, we recently have shown that a specific HLA-DR pocket variant containing arginine at position 74 is strongly associated with GD (17). A similar situation might exist in HT. Thus, we hypothesized that specific amino acid variants within the HLA-DR peptide-binding pocket predispose to HT, in a manner similar to that shown for other autoimmune diseases. However, the sequence of the DRB1 gene has not been investigated in HT thus far. Therefore, the aims of our study were to determine the HLA-DR amino acid sequence in a cohort of well characterized HT patients and to compare those sequences with the HLA-DR sequences in matched controls. Our results demonstrated that specific amino acid signatures within the HLA-DR peptide-binding pockets conferred strong risk for HT. Moreover, these amino acid signatures are different from those we have identified in mice that are susceptible to autoimmune thyroiditis, suggesting that different antigenic epitopes are involved in the etiology of autoimmune thyroiditis in humans and in mice.
Results
Characteristics of the Dataset.
We analyzed 94 HT patients (75 females and 19 males). The age at diagnosis ranged from 5 to 84 years, with a mean of 34.8 years. Fifty-five patients (58.5%) had a documented family history of autoimmune thyroid disease, 10 patients (10.6%) had a definitely negative family history of autoimmune thyroid disease, and in 29 patients (30.9%) the family history could not be confirmed. Goiter was present in 16 patients (17%), whereas 42 patients (44.7%) had a normal thyroid volume; in 36 patients (38.3%) definitive thyroid anatomy was not available.
HLA-DR Typing.
Typing was performed in 94 patients and 153 controls. We found a significantly higher frequency of DR3 in patients than in controls. The DR3 allele was present in 29/94 patients (15.4%) and in 22/153 controls (7.2%) [P = 2 × 10−3, odds ratio (OR) = 2.7]. In addition, the DR4 allele was more frequent in the HT patients; it was found in 37 of 94 patients (19.7%) and in 43 of 153 controls (14.1%), but this difference did not reach statistical significance (P = 0.06, OR = 1.7). DR1 and DR8 were significantly more frequent in the control group than in patients, suggesting a protective role in the development of HT. DR1 was present in 15 of 94 patients (8%) and in 44 of 153 controls (14.4%) (P = 0.02, OR = 0.47), and DR8 was found in 3 of 94 patients (1.6%) and in 17 of 153 controls (5.6%) (P = 0.03, OR = 0.3). The other DR specificities did not differ significantly between patients and controls [see supporting information (SI) Table S1].
HLA-DR Sequence Analyses in HT Patients and Controls.
Sequence analyses were performed successfully in all 94 patients and in 149 controls. Of the amino acid positions encoded by exon 2, 13 positions were polymorphic.
Significant differences in the frequencies of amino acids in patients and controls were seen at positions 26, 30, 47, 70, 71, and 74 (Table 1 and Table S2). The amino acid most strongly associated with HT was Lys at position 71 [P = 1.65 × 10−8, using the Cochran–Armitage test; OR = 2.98; 95% confidence interval (CI) = 1.98–4.51]. Tyr at position 26 was significantly more frequent in HT patients than in controls (18% vs. 8.1%, P = 6.9 × 10−4; OR = 2.52; CI = 1.4–4.6), and Tyr at position 30 also was associated with HT (79.3% vs. 64.4%, P = 7.5 × 10−4; OR = 2.11; CI = 1.35–3.30). At position 70, the presence of Gln was significantly higher in patients than in controls (P = 4.3 × 10−3; OR = 1.65; CI = 1.11–2.45). Interestingly, Arg at position 74, which we had shown to be associated with GD (17), also was associated with HT in the current cohort (P = 1.5 × 10−4; OR = 3.4; CI = 1.66–7.05) (Table 1). In contrast, Tyr at position 47 showed a protective effect, because it was significantly more frequent in controls than in patients (63.1% vs. 52.7%, P = 0.02; OR = 0.65; CI = 0.44–0.96) (Table S2).
Table 1.
HLA-DR amino acid frequencies in patients and controls at DR positions that showed association with HT
| Amino acid position | Amino acid variants | HT (%)n = 188 | Controls (%)n = 298 | P value* | OR | CI |
|---|---|---|---|---|---|---|
| 26 | Phenylalanine | 131 (69.7) | 210 (70.4) | Not significant | ||
| Tyrosine | 34 (18) | 24 (8.1) | 6.9 × 10−4 | 2.52 | 1.39–4.57 | |
| Leucine | 15 (8) | 44 (14.8) | 0.02 | 0.50 | 0.26–0.96 | |
| Undetermined† | 8 (4.3) | 20 (6.7) | Not significant | |||
| 30 | Tyrosine | 149 (79.3) | 192 (64.4) | 7.5 × 10−4 | 2.11 | 1.35–3.30 |
| Cysteine | 15 (8) | 44 (14.8) | 0.02 | 0.50 | 0.26–0.96 | |
| Histidine | 1 (0.5) | 21 (7) | 7.6 × 10−4 | 0.07 | 0–0.5 | |
| Leucine | 17 (9) | 23 (7.7) | Not significant | |||
| Glycine | 0 | 4 (1.3) | Not significant | |||
| Undetermined† | 6 (3.2) | 14 (4.7) | Not significant | |||
| 70 | Glutamine | 124 (66) | 161 (54) | 4.3 × 10−3 | 1.65 | 1.11–2.45 |
| Aspartic acid | 55 (29.3) | 104 (34.9) | Not significant | |||
| Arginine | 7 (3.7) | 26 (8.7) | 0.03 | 0.40 | 0.16–1.0 | |
| Undetermined† | 2 (1.1) | 7 (2.3) | Not significant | |||
| 71 | Lysine | 89 (47.3) | 69 (23.2) | 1.7 × 10−8 | 2.98 | 1.98–4.51 |
| Glutamic acid | 10 (5.3) | 29 (9.7) | Not significant | |||
| Alanine | 17 (9) | 27 (9.1) | Not significant | |||
| Arginine | 70 (37.2) | 166 (55.7) | 7.2 × 10−5 | 0.47 | 0.32–0.7 | |
| Undetermined† | 2 (1.1) | 7 (2.3) | Not significant | |||
| 74 | Alanine | 96 (51.1) | 144 (48.3) | Not significant | ||
| Arginine | 27 (14.4) | 14 (4.7) | 1.5 × 10−4 | 3.40 | 1.66–7.05 | |
| Glutamic acid | 23 (12.2) | 44 (14.8) | Not significant | |||
| Glutamine | 35 (18.6) | 79 (26.5) | 0.04 | 0.63 | 0.39–1.0 | |
| Leucine | 2 (1.1) | 14 (4.7) | 0.03 | 0.22 | 0.03–1.0 | |
| Undetermined† | 5 (2.7) | 3 (1) | Not significant |
*P values in the associated amino acids were calculated with the Cochran–Armitage test.
†Unequivocal determination of amino acids was not possible.
Multiple Logistic Regression Analysis.
To determine which amino acids and haplotypes are causative and to distinguish them from amino acids that are associated with HT only because of linkage disequilibrium (LD) with the causative variants, we performed a logistic regression analysis. The minimal haplotype that showed the strongest risk for HT was Tyr-26, Tyr-30, Gln-70, Lys-71 (P = 6.18 × 10−5; OR = 3.73; CI = 1.4–9.4), with the allele Lys-71 giving the main contribution to the risk. Moreover, all of the risk alleles in this haplotype contributed independently to disease. The haplotype Tyr-26, Tyr-30, Gln-70, Lys-71, Arg-74 also was associated with HT (P = 3.66 × 10−4; OR = 3.45; CI = 1.15–9.99), but the likelihood ratio test with the nested model Tyr-26, Tyr-30, Gln-70, Lys-71 was not significant, suggesting that Arg-74 had the least independent contribution to risk, because it is in tight LD with Lys-71. However, Arg-74 contributed most to the pocket structure conferring risk for HT (see below).
I-E Sequence Analysis in Mice Susceptible and Resistant to EAT.
The I-E locus of the mouse is homologous to the human HLA-DR locus. To determine whether certain amino acid signatures within the murine I-E locus are associated with susceptibility to EAT, we sequenced the variable exon 2 of the mouse I-E gene in 22 strains of mice: 12 were susceptible to EAT, and 10 were resistant. Sequencing of exon 2 of the mouse I-E gene demonstrated that 27 of the 92 amino acid positions sequenced were polymorphic (Table S3). Significant differences in the amino acid frequencies between susceptible and resistant strains were found at 14 of the 27 polymorphic amino acid positions (Table 2). The most significant positions were 86, 88, and 28. At position 86, Ser was found in 9/10 of the mouse strains resistant to EAT and was not found in the susceptible mouse strains (90% vs. 0%, P = 1.9 × 10−5). However, Phe was present in 10/12 susceptible mouse strains and in 1/10 of the resistant strains (83% vs. 10%, P = 6.1 × 10−4). Lys at position 88 was found to have a protective role, as well. It was present in 9 of the 10 resistant mouse strains and in only 2 of the 12 susceptible strains (90% vs. 17%, P = 6.1 × 10−4). In contrast, Asn at this position increased the risk for EAT (83% vs. 10%, P = 6.1 × 10−4). At position 28 Glu was present in 8/10 resistant mouse strains and in 1/12 susceptible strains (80% vs. 17%, P = 6.6 × 10−4), showing a protective role in the development of EAT. Val at the same position predisposed to EAT; it was present in 11/12 susceptible strains and in none of the resistance strains (83% vs. 0%, P = 1.8 × 10−5). Apart from these three amino acid positions, the amino acids at positions 26, 30, 47, 70, 71, and 74 also were significantly associated with EAT, although the associations were weaker (Table 2). Interestingly, these six positions showing weaker associations with EAT also were associated with human autoimmune thyroiditis (Table 1). However, at two of these key six positions (amino acid positions 26 and 74), the amino acid residues associated with human autoimmune thyroiditis (Tyr-26, Arg-74) were distinct from the residues associated with EAT (Leu-26, Gln-74). These sequence differences resulted in distinct structural and peptide-binding properties of the human and mouse MHC II pockets that were associated with thyroiditis (see below).
Table 2.
I-E amino acid frequencies in EAT-susceptible and -resistant mice
| Amino acid position | Amino acid variants | Susceptible mice (%)n = 12 | Resistant mice (%)n = 10 | Comparison | P value |
|---|---|---|---|---|---|
| 6 | Tryptophan (W) | 12 (100) | 6 (60) | W vs. R | 0.015 |
| Arginine (R) | 4 (40) | ||||
| 26 | Leucine (L) | 11 (92) | 5 (50) | L vs. F | 0.03 |
| Phenylalanine (F) | 1 (8) | 5 (50) | |||
| 28 | Glutamic acid (E) | 1 (17) | 8 (80) | E vs. others | 6.6 × 10−4 |
| Valine (V) | 11 (83) | V vs. others | 1.8 × 10−5 | ||
| Lysine (K) | 1 (10) | ||||
| Aspartic acid (D) | 1 (10) | ||||
| 30 | Tyrosine (Y) | 12 (100) | 7 (70) | Y vs. F | 0.04 |
| Phenylalanine (F) | 3 (30) | ||||
| 31 | Phenylalanine (F) | 12 (100) | 7 (70) | F vs. I | 0.04 |
| Isoleucine (I) | 3 (30) | ||||
| 34 | Leucine (L) | 12 (100) | 6 (60) | L vs. R | 0.015 |
| Arginine (R) | 4 (40) | ||||
| 47 | Phenylalanine (F) | 12 (100) | 6 (60) | F vs. Y | 0.015 |
| Tyrosine (Y) | 4 (40) | ||||
| 67 | Phenylalanine (F) | 11 (92) | 5 (50) | F vs. I | 0.03 |
| Isoleucine (I) | 1 (8) | 5 (50) | |||
| 70 | Glutamine (Q) | 12 (100) | 7 (70) | Q vs. D | 0.04 |
| Aspartic acid (D) | 3 (30) | ||||
| 71 | Lysine (K) | 11 (92) | 6 (60) | K vs. E/A | 0.04 |
| Arginine (R) | 1 (8) | 1 (10) | |||
| Glutamic acid (E)/Alanine (A) | 3 (30) | E/A vs. others | 0.04 | ||
| 74 | Glutamic acid (E) | 10 (83) | 5 (50) | E vs. S | 0.03 |
| Alanine (A) | 2 (17) | 2 (20) | |||
| Serine (S) | 3 (30) | S vs. E | 0.03 | ||
| 86 | Serine (S) | 9 (90) | S vs. others | 1.9 × 10−5 | |
| Phenylalanine (F) | 10 (83) | 1 (10) | F vs. others | 6.1 × 10−4 | |
| Leucine (L) | 2 (17) | ||||
| 88 | Lysine (K) | 2 (17) | 9 (90) | K vs. N | 6.1 × 10−4 |
| Asparagine (N) | 10 (83) | 1 (10) | |||
| 92 | Arginine (R) | 3 (25) | 9 (90) | R vs. P | 2 × 10−3 |
| Proline (P) | 9 (75) | 1 (10) |
Structural Modeling Studies
Comparison Between HT-Susceptible and -Resistant HLA-DR.
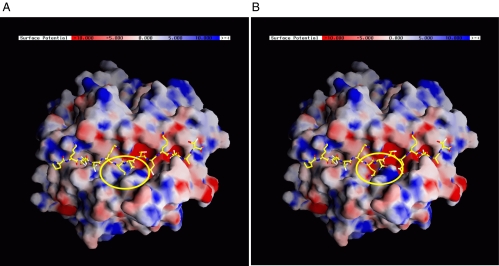
We constructed the structural models of HT-susceptible and -resistant HLA-DR based on the genetic association results. The positions of HT-associated amino acids in HLA-DR and the changes in local properties upon substitution are listed in Table 3. To compare the two structural models, we characterized the electrostatic potential on the surface of both molecules. The major difference is in the P4 pocket (Fig. 1), which is more positively charged in HT-susceptible HLA-DR (β74-Arg) than in the HT-resistant HLA-DR (β74-Gln/Leu). This change would be expected to affect the selectivity of the binding of pathogenic peptides to the HLA-DR pocket and thus could increase the risk for developing HT by altering Tg or TPO peptide binding and presentation to T cells.
Table 3.
List of HT-associated amino acid residues in HLA-DR, their positions in peptide-binding pockets, and changes upon amino acid substitutions
| Residue | MHC pocket*† | Susceptible | Resistant | Changes*‡ |
|---|---|---|---|---|
| β26 | P4 (bottom) | Tyr | Leu | Smaller in size |
| (P = 6.9 × 10−4; OR = 2.52) | (P = 0.02; OR = 0.50) | Loss of −OH | ||
| β30 | P6 (bottom) | Tyr | His | Smaller in size |
| (P = 7.5 × 10−4; OR = 2.11) | (P = 7.6 × 10−4; OR = 0.07) | Positive, polar | ||
| Loss of −OH | ||||
| β70*§ | P4 (above) | Gln | Arg | Larger in size |
| (P = 4.3 × 10−[3; OR = 1.65) | (P = 0.03; OR = 0.40) | Positive | ||
| β71 | P4 (side) | Lys | Arg | Larger in size |
| (P = 1.7 × 10−8; OR = 2.98) | (P = 7.2 × 10−5; OR = 0.47) | |||
| β74 | P4 (side) | Arg | Gln/Leu | Smaller in size |
| (P = 1.5 × 10−4; OR = 3.40) | (P = 0.04; OR = 0.63) | Uncharged |
*The location of the amino acid in the MHC pocket is given in parentheses.
†The changes described here reflect the change in the amino acid residue itself (resulting from the amino acid substitution from HT-susceptible to HT-resistant residue), not the changes in MHC pocket.
‡The amino acid residue at position β70 might interact with T cell receptor.
Fig. 1.
Structural models of HT-susceptible and -resistant HLA-DR4. (A) HT-susceptible HLA-DR4. (B) HT-resistant HLA-DR4. The electrostatic potential was calculated in each system to characterize the surface properties of the molecule. The differences are considered to be relevant to the predisposition to HT. The peptide-binding groove is shown with the influenza hemagglutinin peptide. A significant difference was found in P4 pocket (shown in the yellow ellipse). P4 pocket in HT-susceptible HLA-DR4 is more positively charged. This might affect the selectivity of binding peptides.
Comparison Between EAT-Susceptible and -Resistant I-E.
We also generated the structural models of EAT-susceptible and -resistant I-E based on the sequencing results. The positions of structurally important EAT-associated amino acids in I-E, and the changes in local properties upon substitution are summarized in Table S4. Comparison of the calculated electrostatic potentials on the surface of both molecules shows that the most significant differences are in P1 and P4 pockets (Fig. S1). In EAT-resistant I-E variants, the P1 and P4 pockets are more spacious because of β86 Phe > Ser in P1 and β71 Lys > Glu/Ala and β74 Glu > Ser in P4. These results suggest that EAT-inducing peptides also might bind to EAT-resistant I-E pockets, albeit with a lower affinity.
Comparison Between HT-Susceptible HLA-DR and EAT-Susceptible I-E.
Because EAT-susceptible mice frequently have been used to identify pathogenic Tg peptides (18–20), we investigated whether the structure of the I-E pocket in EAT-susceptible mice is similar to the HLA-DR pocket in HT. Surprisingly, the structures of the EAT-susceptible I-E pocket and the HT susceptible pocket are very different. The disease-associated DR pocket was neutral in P1, positively charged in P4, and negatively charged in P6 and P9 pockets, whereas all of the I-E pockets associated with EAT were negatively charged. Thus, inasmuch as such analysis exposes the essential characteristics of autoimmune peptide binding, it suggests that different types of autoantigenic peptides could bind and induce autoimmune thyroiditis in humans and in mice (Figs. 1 and S1).
Discussion
Data on HLA class II alleles have been less definitive in HT than in GD or other autoimmune diseases. In the current report, we show that a molecular signature of HLA-DR pocket, determined by specific amino acids, confers a significant risk for the development of HT that is higher than the risk conferred by any previously identified HT gene, such as CTLA-4 (21). The presence of the HLA-DR amino acids Tyr-26, Tyr-30, Gln-70, and Lys-71 in the pocket was strongly associated with HT in our dataset, whereas amino acids Leu-26, His-30, Arg-70, Arg-71, and Gln-74 are protective. Importantly, Arg-74, whereas in tight LD with Lys-71, contributed most to the pocket structure conferring risk for HT. Because the amino acid risk haplotype is present across several HLA-DR allelic subtypes, previous studies looking only at HLA-DR allele types in HT could not detect the effect of an amino acid group and gave mixed results. Thus, our results demonstrate the power of HLA class II sequencing in identifying specific amino acid variants that are associated with disease, as we have shown for GD (17) and now for HT. Moreover, identifying the specific three-dimensional pocket structures associated with HT enables us to dissect the potential biological effects of these variants on susceptibility to disease.
The proposed mechanisms by which HLA class II molecules confer susceptibility to autoimmune diseases focus on the immunological synapse, or the interface between antigen-presenting cells and T cells. T cells recognize and respond to an antigen by interacting with a complex composed of an antigenic peptide and an HLA molecule (reviewed in ref. 22). It is thought that different HLA pocket variants confer different affinities for autoantigenic peptides (e.g., thyroid antigens). These peptides then are recognized by T cell receptors on cells that have escaped tolerance (23). Thus, certain HLA-DR pocket variants may permit the autoantigenic peptides to fit into the antigen-binding pocket of the HLA molecule and to be recognized by the T cell receptor, whereas others may not (24). This property, namely the ability of the pocket to bind a specific peptide, can determine whether an autoimmune response to thyroid autoantigenic peptides will develop. Indeed, studies on the structure of HLA variants associated with T1D provided strong evidence in support of this hypothesis. The highest risk for T1D is conferred by HLA class II alleles DQ8 and DQ2. At the molecular level the susceptibility to T1D has been correlated with a β57 polymorphism in HLA-DQ lacking Asp residue at this position (25). Structurally, the DQ8/DQ2 pockets have particular features that allow presentation of insulin B:9-23 peptide, a peptide that is critical for the development of T1D (26–28).
The logistic regression analysis has shown that, even though Lys-71 was the pocket DR variant most strongly associated with HT, three additional variants, Tyr-26, Tyr-30, and Gln-70, contributed independently to disease risk, and Arg-74, albeit in tight LD with Lys-71, had some independent contribution to risk as well. These results are intriguing, because HT was associated with a five-pocket amino acids signature, but GD was associated with only one specific pocket amino acid variant (DR-β74). This difference may indicate that the pocket organization conferring susceptibility to HT is more stringent than the pocket organization conferring susceptibility to GD. Moreover, the amino acid variant predisposing to GD (DRβ-Arg-74) also was associated with HT. Thus, the Arg-74 pocket variant may facilitate the binding of pathogenic peptides that can trigger both GD and HT, such as Tg-derived peptides. This notion is supported by our findings that variants of the Tg gene are associated with both GD and HT (29). In contrast, the other four amino acid variants (Tyr-26, Tyr-30, Gln-70, Lys-71) that were specific for HT may influence the binding of HT-specific pathogenic peptides (e.g., TPO-derived peptides).
Our sequencing and structural modeling analyses gave us some clues about the functional consequences of the amino acid variants we found to be associated with HT. At the structural level, the major difference between the DR variants predisposing to or protecting from HT was in the P4 pocket (Fig. 1). The P4 pocket in HT-susceptible HLA-DR is more positively charged than the HT-resistant HLA-DR. These findings provide the molecular basis for future prediction of pathogenic peptides that can fit into these disease-associated pockets.
Another interesting but somewhat surprising finding of this study is that the structure of the disease-associated pockets is different in human and mouse autoimmune thyroiditis. This difference is in contrast to T1D, in which the disease-associated pocket P9 has similar structure and peptide-binding properties in humans and in mice. The P9 pocket of the human diabetes risk allele DQ8 has a positive charge because an α79-Arg residue forms a salt bridge with the Glu-21 side chain of the insulin B:9-23 peptide. Therefore, the T1D risk alleles have a preference for binding peptides with negatively charged amino acids at the P9 pocket (26). Similarly, the P9 pocket of I-Ag7, the risk allele for diabetes in nonobese diabetic mice, has Arg at the same position as DQ8 and has a preference for negatively charged residues on the insulin peptide (27, 28). In contrast, the disease-associated pocket variants in human and mouse thyroiditis have distinct structures and peptide-binding properties. The HT-associated DR peptide-binding groove is neutral in pocket P1, positively charged in P4, and negatively charged in P6 and P9, whereas the I-E pocket that is associated with EAT is characterized by a nearly uniform negative electrostatic potential. This difference suggests that mice susceptible to EAT cannot serve as a good model for identifying Tg and TPO peptides that are relevant for human autoimmune thyroiditis (HT). Previously, several studies attempted to define pathogenic human Tg epitopes in HT by determining their ability to induce EAT in susceptible mice (18–20). Our findings suggest that peptides identified using this strategy are relevant only to mouse thyroiditis (i.e., EAT), not HT.
In summary, we have identified MHC class II pocket amino acid signatures that confer significant risk for human and mouse autoimmune thyroiditis. The HLA-DR pocket variants that are associated with HT create specific pocket structures that could accommodate certain pathogenic peptides that may initiate HT. The stage now is set to identify these pathogenic peptides and to dissect the mechanisms by which they interact with the disease-associated HLA-DR pocket variants to induce autoimmune thyroiditis.
Patients and Methods
Patients and Controls.
HT patients.
The project was approved by the Institutional Review Board. Ninety-four Caucasian HT patients were studied. The diagnosis of HT was established on the basis of (i) documented clinical and biochemical hypothyroidism requiring thyroid hormone replacement and (ii) the presence of autoantibodies to TPO, and/or Tg. Anti-Tg and anti-TPO antibodies were measured by specific RIA (Kronus).
Controls.
One hundred fifty-three age- and gender frequency-matched healthy Caucasian volunteers served as controls. No controls had a personal or family history of thyroid disease or goiter on examination; they had normal thyroid functions and were negative for thyroid autoantibodies (anti-Tg and -TPO antibodies).
DNA Preparation.
DNA was extracted from whole blood using the Puregene kit (Gentra Systems).
HLA-DR Typing.
Molecular typing of HLA-DR was performed according to the requirements of the American Society for Histocompatibility as previously described (17).
Direct Sequencing of Exon 2 of the Human HLA-DRB1 Gene.
To identify HLA-DR sequences that predispose to HT, we sequenced exon 2 of the HLA-DRB1 gene that encodes the specific amino acids determining the DR specificities (for methods, see SI Text and Table S5). Unequivocal sequencing of exon 2 of the HLA-DRB1 gene was achieved in all patients (n = 94) and in 149 of the control subjects.
Sequence Alignment and Analysis.
Sequence alignment analyses were performed using the Sequencher version 3.1 program (Gene Codes). The known DR1, DR2, DR3, DR4, DR7, DR8, DR9, DR10, DR11, DR12, DR13, and DR14 subtype sequences were obtained from the ImMunoGeneTics project (IMGT)-HLA Informatics Group (HIG) database (http://www.ebi.ac.uk/imgt/hla/).
Direct Sequencing of Exon 2 of the Mouse I-E Gene in Mice Susceptible and Resistant to EAT.
The mouse I-E gene locus is homologous to the human HLA-DR gene locus. To identify mouse I-E sequences that predispose to EAT, we sequenced exon 2 of the mouse I-E gene, which encodes the specific amino acids determining the I-E specificities. Exon 2 of the mouse I-E gene was sequenced in 22 strains of mice. Of the mouse strains sequenced for the I-E gene, 12 were susceptible to EAT: CBA/J (H-2k), C3H/HeJ (H-2k), C3HeB/FeJ (H-2k), CE/J (H-2k), AKR/J (H-2k), B10.BR/SgSnJ (H-2k), MA/MyJ (H-2k), ST/bJ/R (H-2k), B6C3F1/J (H-2b, k), NOD/LJ (H-2k), SJL/J (H-2s), and C57BR/cdJ/R (H-2k). Ten strains were resistant to EAT: C57BL/6J (H-2b), 129/J/R (H-2b), C57L/J/R (H-2b), LP/J/R (H-2b), BALB/cJ (H-2d), DBA/2J (H-2d), C57BLKS/J/R (H-2d), PL/J (H-2a), DBA/1J (H-2a), and C57B/10J (H-2b). Strains were classified as susceptible or resistant based on previous studies (30–34) as follows: when EAT is induced, mouse strains showing an average histological grade ≥3 thyroiditis (in a grading system in which 1 = <25% thyroid infiltration; 2 = 25%–50% infiltration; 3 = 50%–75% infiltration; and 4 = >75% infiltration) were classified as susceptible, whereas mouse strains showing less than grade 1 thyroiditis were classified as resistant. We sequenced exon 2 of the mouse I-E gene using the following primers: forward primer GACTCGGGCATCTTGTCGGC, reverse primer CACCCTCACCGTGGTTCCGC. Sequencing was performed using the ABI Big Dye DNA sequencing kit (Applied Biosystems) as described in SI Text. Sequence alignment and analysis were performed using the Sequencher version 3.1 program.
Statistical Analyses.
The analyses for association between HT and the HLA-DR variants were performed using the χ2 and the Cochran–Armitage trend tests. Haplotypes were imputed based on multimarker predictors using the E-M algorithm embedded in a stepwise logistic regression framework. The logistic regression framework allowed us to determine which allele or combination of alleles may have a causal role in disease and which show association with disease because of LD. Likelihood ratio tests were used to test nested models. The OR and CI were used as the measure of effect of the single variant or the haplotype on the disease. Statistical analyses were performed using the statistical software Epi Info version 6.03 [Centers for Disease Control (CDC)], Stata 10 (STATA), and PLINK (http://pngu.mgh.harvard.edu/purcell/plink) (35).
Power Calculations.
Power calculations were performed by using the CDC simulation software Epi-Info, 3.3.2. We assumed the population frequency of the susceptibility amino acid variants to be 10–50% based on the population frequencies observed in our controls for the 13 polymorphic HLA-DR amino acids we tested for association with HT. Fig. S2 shows the power calculation curves. The power calculations demonstrated that our dataset of 94 HT patients and 149 controls gave our study 80% power to detect a difference between the patients and the controls resulting in ORs ≥ 2.2 with an alpha of 0.05. Our study had 90% power to detect a difference between patients and controls resulting in ORs ≥ 2.43. Therefore, our study was adequately powered to detect significant associations of HLA-DR amino acid variants with HT.
Construction of Model Structures.
The structural models of HT-susceptible and -resistant HLA-DR molecules were constructed based on the x-ray crystallographic structure of HLA-DR4-influenza viral peptide complex [Protein Data Bank (PDB) ID: 1J8H] (36). The genetically significant amino acids at positions β26, β30, β70, β71, and β74 were taken into account when generating the models. These amino acids in the original crystal structure were substituted by molecular modeling software Insight II (Accelrys) if necessary in the construction of the structural models. In HT-susceptible HLA-DR, these positions were substituted for β26(Y), β30(Y), β70(Q), β71(K), and β74(R), whereas in HT-resistant HLA-DR, these positions were substituted for β26(L), β30(H), β70(R), β71(R), and β74(Q) (based on our association results).
The structural models of EAT-susceptible and -resistant I-E variants were constructed based on the x-ray crystallographic structure of I-Ek-moth cytochrome c (MCC) peptide complex (PDB ID: 1KT2) (37). The genetically important amino acids at positions β6, β26, β28, β30, β31, β34, β47, β67, β70, β71, β74, β86, β88, and β92 were considered in designing the structural models. These amino acids in the original crystal structure were replaced if necessary (using Insight II). In EAT-susceptible I-Ek, these positions were substituted for β6(W), β26(L), β28(V), β30(Y), β31(F), β34(L), β47(F), β67(F), β70(Q), β71(K), β74(E), β86(F), β88(N), and β92(P). In EAT-resistant I-Ek, these positions were substituted for β6(R) β26(F), β28(E), β30(F), β31(I), β34(R), β47(Y), β67(I), β70(D), β71(E/A), β74(S), β86(S), β88(K), and β92(R) (based on our I-E sequencing results).
Characterization of Molecular Surfaces.
To describe the surface properties of the peptide-binding groove, we calculated the electrostatic potential in each system. The electrostatic potential was calculated using the Poisson-Boltzmann solver in the program Graphical Representation and Analysis of Surface Properties (GRASP) http://wiki.c2b2.columbia.edu/honiglab_public/index.php/Software:GRASP (38, 39) and then was displayed on the molecular surface of each molecule. The ionic strength of the system was 150 mM, and the dielectric constants of the protein and its surroundings were 2 and 80, respectively. In Figs. 1 and S1, the scale of the potential is set from −10 kT (red) to +10 kT (blue) Figs. 1 and S1 show how the surface properties differ in autoimmune thyroiditis-susceptible MHC class II and the autoimmune thyroiditis-resistant MHC, in both humans and mice. We also compared the HT-susceptible HLA-DR with the EAT-susceptible I-E.
Supplementary Material
Acknowledgments.
This work was supported in part by Grants DK61659, DK067555, and DK073681 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (to Y.T.), Grants DK71775 and NS27941 (to D.A.G.), Grant DK052464 from the NIDDK, and a grant from the David Owen Segal Endowment (T.F.D.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806584105/DCSupplemental.
References
- 1.Hollowell JG, et al. Serum TSH, T(4) and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–999. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 3.Weetman AP. In: Werner and Ingmar's the Thyroid. Braverman LE, Utiger RD, editors. Philadelphia: Lippincott Williams and Wilkins; 2000. pp. 721–732. [Google Scholar]
- 4.Tomer Y, Davies TF. Searching for the autoimmune thyroid disease susceptibility genes: From gene mapping to gene function. Endocr Rev. 2003;24:694–717. doi: 10.1210/er.2002-0030. [DOI] [PubMed] [Google Scholar]
- 5.Tomer Y, Davies TF. Infection, thyroid disease and autoimmunity. Endocr Rev. 1993;14:107–120. doi: 10.1210/edrv-14-1-107. [DOI] [PubMed] [Google Scholar]
- 6.Rose NR, Bonita R, Burek CL. Iodine: An environmental trigger of thyroiditis. Autoimmun Rev. 2002;1:97–103. doi: 10.1016/s1568-9972(01)00016-7. [DOI] [PubMed] [Google Scholar]
- 7.Farid NR, Sampson L, Moens H, Barnard JM. The association of goitrous autoimmune thyroiditis with HLA-DR5. Tissue Antigens. 1981;17:265–268. doi: 10.1111/j.1399-0039.1981.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 8.Moens H, Farid NR, Sampson L, Noel EP, Barnard JM. Hashimoto's thyroiditis is associated with HLA-DRw3. N Engl J Med. 1978;299:133–134. doi: 10.1056/NEJM197807202990306. [DOI] [PubMed] [Google Scholar]
- 9.Tandon N, Zhang L, Weetman AP. HLA associations with Hashimoto's thyroiditis. Clin Endocrinol. 1991;34:383–386. doi: 10.1111/j.1365-2265.1991.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 10.Ban Y, Davies TF, Greenberg DA, Concepcion ES, Tomer Y. The influence of human leucocyte antigen (HLA) genes on autoimmune thyroid disease (AITD): Results of studies in HLA-DR3 positive AITD families. Clin Endocrinol. 2002;57:81–88. doi: 10.1046/j.1365-2265.2002.01566.x. [DOI] [PubMed] [Google Scholar]
- 11.Badenhoop K, et al. Susceptibility to thyroid autoimmune disease: Molecular analysis of HLA-D region genes identifies new markers for goitrous Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1990;71:1131–1137. doi: 10.1210/jcem-71-5-1131. [DOI] [PubMed] [Google Scholar]
- 12.Petrone A, et al. Association of DRB1*04-DQB1*0301 haplotype and lack of association of two polymorphic sites at CTLA-4 gene with Hashimoto's thyroiditis in an Italian population. Thyroid. 2001;11:171–175. doi: 10.1089/105072501300042901. [DOI] [PubMed] [Google Scholar]
- 13.Bogner U, et al. HLA-DR/DQ gene variation in nongoitrous autoimmune thyroiditis at the serological and molecular level. Autoimmunity. 1992;14:155–158. doi: 10.3109/08916939209083135. [DOI] [PubMed] [Google Scholar]
- 14.Davies TF, Amino N. A new classification for human autoimmune thyroid disease. Thyroid. 1993;3:331–333. doi: 10.1089/thy.1993.3.331. [DOI] [PubMed] [Google Scholar]
- 15.Todd JA, Bell JI, McDevitt HO. HLA-DQbeta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 16.Winchester R. The molecular basis of susceptibility to rheumatoid arthritis. Adv Immunol. 1994;56:389–466. doi: 10.1016/s0065-2776(08)60456-3. [DOI] [PubMed] [Google Scholar]
- 17.Ban Y, et al. Arginine at position 74 of the HLA-DRb1 chain is associated with Graves' disease. Genes Immun. 2004;5:203–208. doi: 10.1038/sj.gene.6364059. [DOI] [PubMed] [Google Scholar]
- 18.Texier B, et al. Characterization and sequencing of a 40-amino-acid peptide from human thyroglobulin inducing experimental autoimmune thyroiditis. J Immunol. 1992;148:3405–3411. [PubMed] [Google Scholar]
- 19.Hoshioka A, et al. A common T-cell epitope between human thyroglobulin and human thyroid peroxidase is related to murine experimental autoimmune thyroiditis. Immunol Lett. 1993;37:235–239. doi: 10.1016/0165-2478(93)90036-2. [DOI] [PubMed] [Google Scholar]
- 20.Karras E, Carayanniotis G, Lymberi P. Induction of murine thyroiditis by a non dominant E(k)-restricted peptide of human thyroglobulin. Immunology. 2003;108:556–561. doi: 10.1046/j.1365-2567.2003.01607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donner H, et al. Codon 17 polymorphism of the cytotoxic T lymphocyte antigen 4 gene in Hashimoto's thyroiditis and Addison's disease. J Clin Endocrinol Metab. 1997;82:4130–4132. doi: 10.1210/jcem.82.12.4406. [DOI] [PubMed] [Google Scholar]
- 22.Buus S, Sette A, Grey HM. The interaction between protein-derived immunogenic peptides and Ia. Immunol Rev. 1987;98:115–141. doi: 10.1111/j.1600-065x.1987.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 23.Nelson JL, Hansen JA. Autoimmune disease and HLA. CRC Crit Rev Immunol. 1990;10:307–328. [PubMed] [Google Scholar]
- 24.Faas S, Trucco M. The genes influencing the susceptibility to IDDM in humans. J Endocrinol Invest. 1994;17:477–495. doi: 10.1007/BF03347743. [DOI] [PubMed] [Google Scholar]
- 25.Aitman TJ, Todd JA. Molecular genetics of diabetes mellitus. Baillière's Clin Endocrinol Metab. 1995;9:631–656. doi: 10.1016/s0950-351x(95)80655-5. [DOI] [PubMed] [Google Scholar]
- 26.Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immunol. 2001;2:501–507. doi: 10.1038/88694. [DOI] [PubMed] [Google Scholar]
- 27.Wucherpfennig KW. MHC-linked susceptibility to type 1 diabetes: A structural perspective. Ann NY Acad Sci. 2003;1005:119–127. doi: 10.1196/annals.1288.013. [DOI] [PubMed] [Google Scholar]
- 28.Wucherpfennig KW. Insights into autoimmunity gained from structural analysis of MHC-peptide complexes. Curr Opin Immunol. 2001;13:650–656. doi: 10.1016/s0952-7915(01)00274-6. [DOI] [PubMed] [Google Scholar]
- 29.Ban Y, et al. Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine autoimmune thyroid disease. Proc Natl Acad Sci USA. 2003;100:15119–15124. doi: 10.1073/pnas.2434175100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vladutiu AO, Rose NR. Autoimmune murine thyroiditis: Relation to histocompatibility (H-2) type. Science. 1971;174:1137–1139. doi: 10.1126/science.174.4014.1137. [DOI] [PubMed] [Google Scholar]
- 31.Tomazic V, Rose NR. Autoimmune murine thyroiditis. VIII. Role of different thyroid antigens in the induction of experimental autoimmune thyroiditis. Immunology. 1976;30:63–68. [PMC free article] [PubMed] [Google Scholar]
- 32.Kong Y, David CS, Giraldo AA, Elrehewy M, Rose NR. Regulation of autoimmune response to mouse thyroglobulin: Influence of H-2D-end genes. J Immunol. 1979;123:15–18. [PubMed] [Google Scholar]
- 33.Braley-Mullen H, Johnson M, Sharp GC, Kyriakos M. Induction of experimental autoimmune thyroiditis in mice with in vitro activated splenic T cells. Cell Immunol. 1985;93:132–143. doi: 10.1016/0008-8749(85)90394-6. [DOI] [PubMed] [Google Scholar]
- 34.Jin Z, et al. Experimental autoimmune thyroiditis in nonobese diabetic mice lacking interferon regulatory factor-1. Clin Immunol. 2004;113:187–192. doi: 10.1016/j.clim.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Purcell S, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hennecke J, Wiley DC. Structure of a complex of the human alpha/beta T cell receptor (TCR) HA1.7, influenza hemagglutinin peptide, and major histocompatibility complex class II molecule, HLA-DR4 (DRA*0101 and DRB1*0401): Insight into TCR cross-restriction and alloreactivity. J Exp Med. 2002;195:571–581. doi: 10.1084/jem.20011194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fremont DH, et al. Structural basis of cytochrome c presentation by IE(k) J Exp Med. 2002;195:1043–1052. doi: 10.1084/jem.20011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholls A, Honig B. A rapid finite difference algorithm utilizing successive overrelaxation to solve the Poisson-Boltzmann equation. J Comp Chem. 1991;12:435–445. [Google Scholar]
- 39.Honig B, Nicholls A. Classical electrostatics in biology and chemistry. Science. 1995;268:1144–1149. doi: 10.1126/science.7761829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.