Abstract
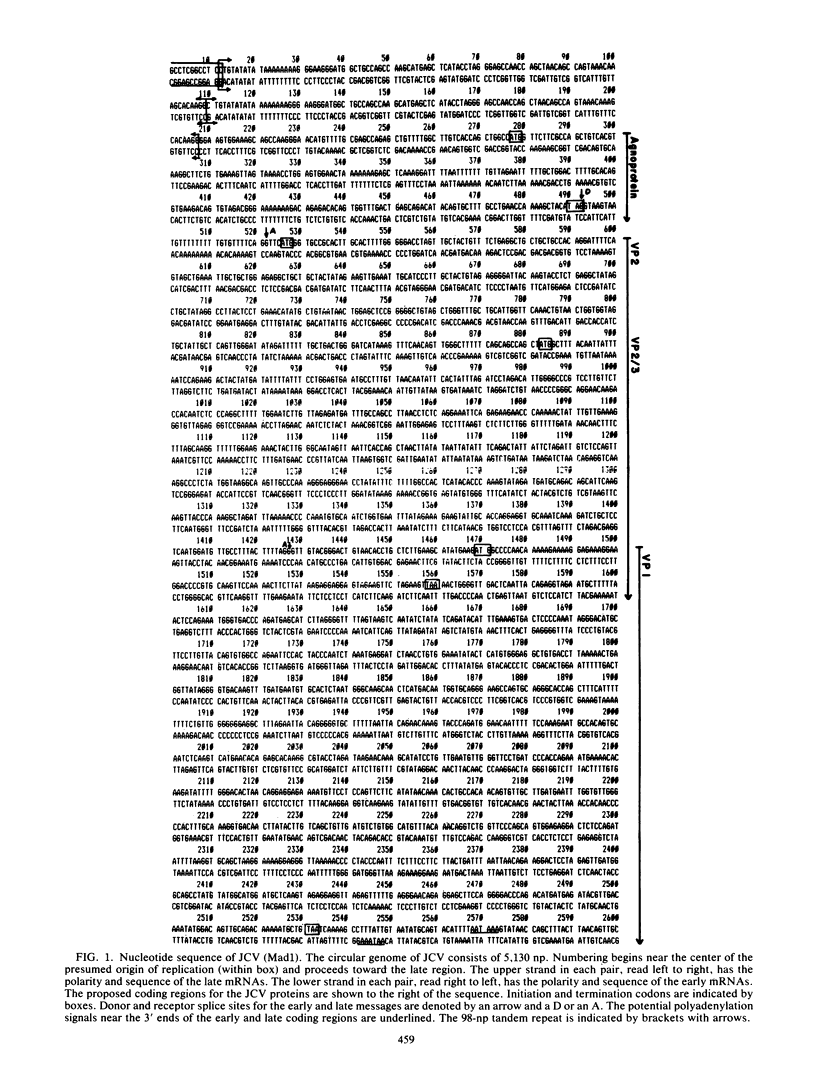
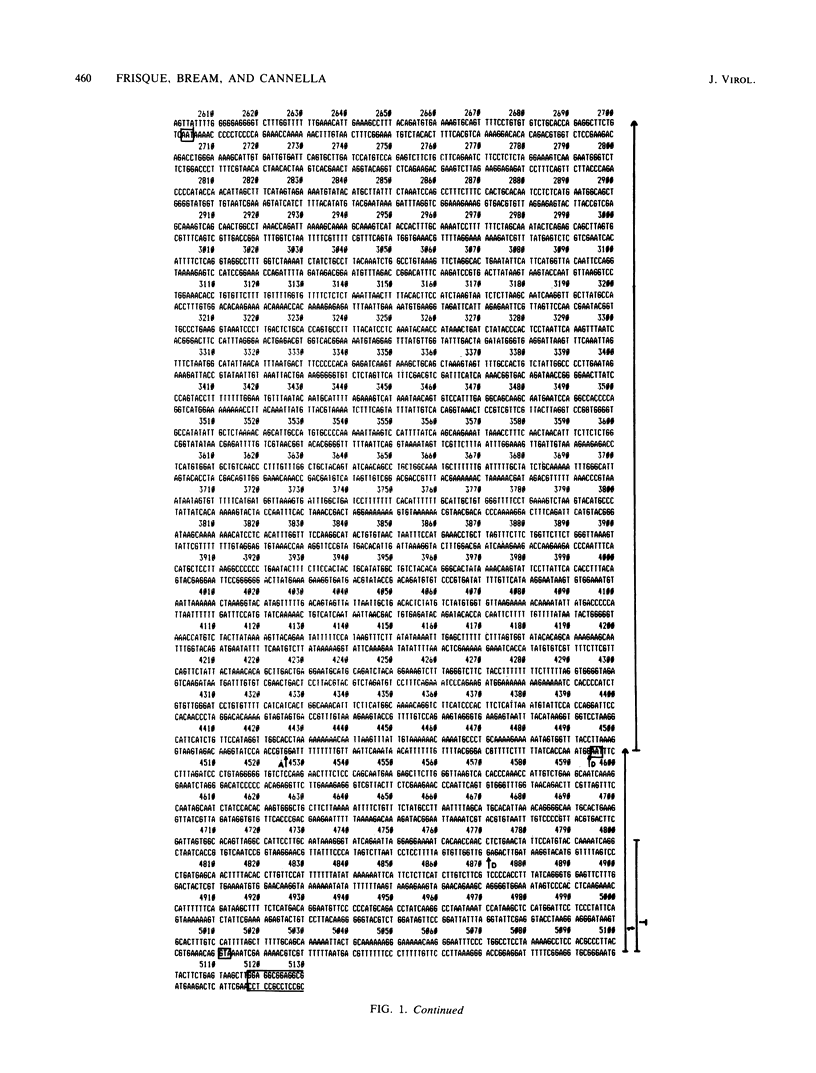
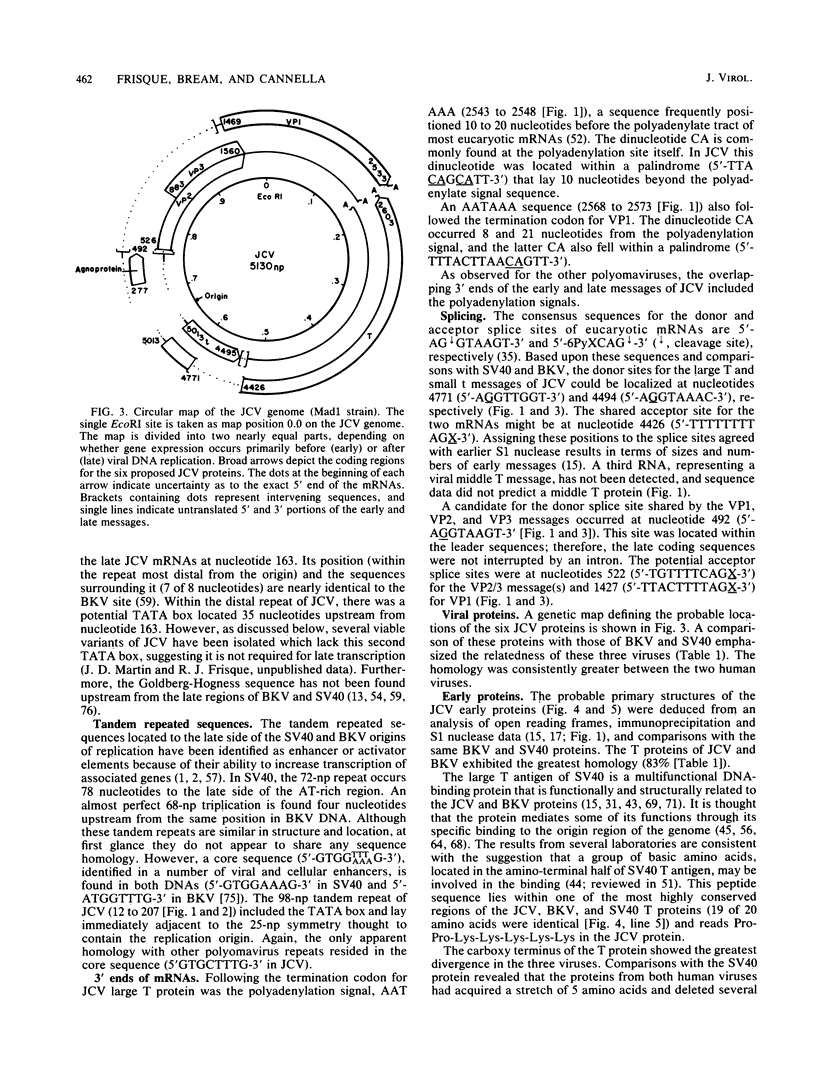
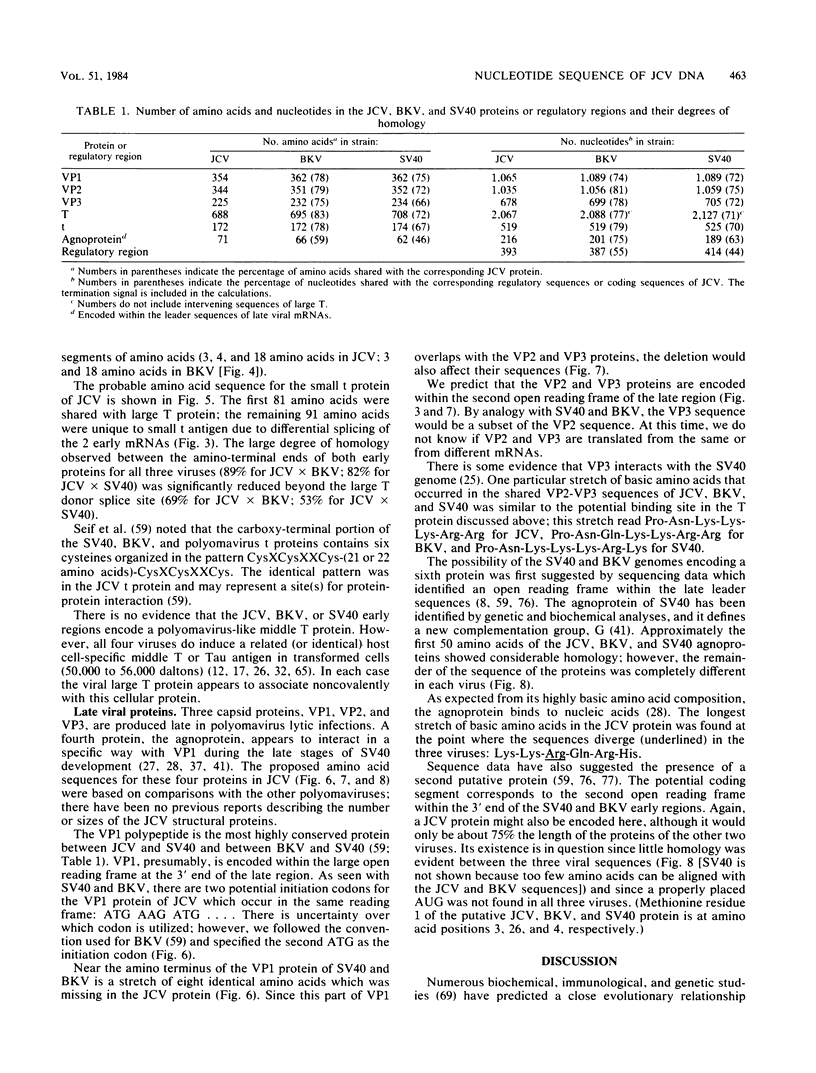
The complete DNA sequence of the human JC virus, which was found to consist of 5,130 nucleotide pairs, is presented. The amino acid sequence of six proteins could be deduced: the early, nonstructural proteins, large T and small t antigens; the late capsid proteins, VP1, VP2, and VP3; and the agnogene product encoded within the late leader sequence, called the agnoprotein in simian virus 40. The extent of homology between JC virus DNA and the genomes of simian virus 40 (69%) and BK virus (75%) confirmed the close evolutionary relationship of these three polyomaviruses. The sequences showing the greatest divergence in these viral DNAs occurred within the tandem repeats located to the late side of the replication origins.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma D. J., Olive D. M., Hartzell S. W., Subramanian K. N. Territorial limits and functional anatomy of the simian virus 40 replication origin. Proc Natl Acad Sci U S A. 1982 Jan;79(2):381–385. doi: 10.1073/pnas.79.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B. J., Davis M. S., Yamaguchi J., Bergsma D. J., Subramanian K. N. Definition of the simian virus 40 early promoter region and demonstration of a host range bias in the enhancement effect of the simian virus 40 72-base-pair repeat. Proc Natl Acad Sci U S A. 1983 Feb;80(3):721–725. doi: 10.1073/pnas.80.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia A. L., Lewton B. A., Tjian R., Tegtmeyer P. Topography of simian virus 40 A protein-DNA complexes: arrangement of pentanucleotide interaction sites at the origin of replication. J Virol. 1983 Apr;46(1):143–150. doi: 10.1128/jvi.46.1.143-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Subramanian K. N., Pan J., Weissman S. M. Structure of a large segment of the genome of simian virus 40 that does not encode known proteins. Proc Natl Acad Sci U S A. 1977 Mar;74(3):827–831. doi: 10.1073/pnas.74.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Mantei N., Weissmann C. DNA sequences preceding the rabbit beta-globin gene are required for formation in mouse L cells of beta-globin RNA with the correct 5' terminus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1411–1415. doi: 10.1073/pnas.78.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Farrell M. P., Mäntyjärvi R. A., Pagano J. S. T antigen of BK papovavirus in infected and transformed cells. J Virol. 1978 Mar;25(3):871–877. doi: 10.1128/jvi.25.3.871-877.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Martin J. D., Padgett B. L., Walker D. L. Infectivity of the DNA from four isolates of JC virus. J Virol. 1979 Nov;32(2):476–482. doi: 10.1128/jvi.32.2.476-482.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J. Nucleotide sequence of the region encompassing the JC virus origin of DNA replication. J Virol. 1983 Apr;46(1):170–176. doi: 10.1128/jvi.46.1.170-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J. Regulatory sequences and virus-cell interactions of JC virus. Prog Clin Biol Res. 1983;105:41–59. [PubMed] [Google Scholar]
- Frisque R. J., Rifkin D. B., Walker D. L. Transformation of primary hamster brain cells with JC virus and its DNA. J Virol. 1980 Jul;35(1):265–269. doi: 10.1128/jvi.35.1.265-269.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura F. K., Deininger P. L., Friedmann T., Linney E. Mutation near the polyoma DNA replication origin permits productive infection of F9 embryonal carcinoma cells. Cell. 1981 Mar;23(3):809–814. doi: 10.1016/0092-8674(81)90445-1. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Lebowitz P., Frisque R. J., Gluzman Y. Identification of a promoter component involved in positioning the 5' termini of simian virus 40 early mRNAs. Proc Natl Acad Sci U S A. 1981 Jan;78(1):100–104. doi: 10.1073/pnas.78.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y., Sambrook J. F., Frisque R. J. Expression of early genes of origin-defective mutants of simian virus 40. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3898–3902. doi: 10.1073/pnas.77.7.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Padgett B. L., Walker D. L. Comparison of infectious JC virus DNAs cloned from human brain. J Virol. 1983 Jan;45(1):299–308. doi: 10.1128/jvi.45.1.299-308.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld G. C., de Boer E., Shewmaker C. K., Flavell R. A. DNA sequences necessary for transcription of the rabbit beta-globin gene in vivo. Nature. 1982 Jan 14;295(5845):120–126. doi: 10.1038/295120a0. [DOI] [PubMed] [Google Scholar]
- Gruss P., Dhar R., Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. doi: 10.1073/pnas.78.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Estes M. K., Pagano J. S. Structure and function of the polypeptides in simian virus 40. I. Existence of subviral deoxynucleoprotein complexes. J Virol. 1972 Jun;9(6):923–929. doi: 10.1128/jvi.9.6.923-929.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Hutchinson M. A., Eckhart W. Translation of polyoma virus T antigens in vitro. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5917–5921. doi: 10.1073/pnas.75.12.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson V., Chalkley R. Use of whole-cell fixation to visualize replicating and maturing simian virus 40: identification of new viral gene product. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6081–6085. doi: 10.1073/pnas.78.10.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Nomura S., Anderson C. W., Khoury G. Identification of the SV40 agnogene product: a DNA binding protein. Nature. 1981 May 28;291(5813):346–349. doi: 10.1038/291346a0. [DOI] [PubMed] [Google Scholar]
- Katinka M., Vasseur M., Montreau N., Yaniv M., Blangy D. Polyoma DNA sequences involved in control of viral gene expression in murine embryonal carcinoma cells. Nature. 1981 Apr 23;290(5808):720–722. doi: 10.1038/290720a0. [DOI] [PubMed] [Google Scholar]
- Katinka M., Yaniv M., Vasseur M., Blangy D. Expression of polyoma early functions in mouse embryonal carcinoma cells depends on sequence rearrangements in the beginning of the late region. Cell. 1980 Jun;20(2):393–399. doi: 10.1016/0092-8674(80)90625-x. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Goldman N. D., Khoury G. Functional similarity between the early antigens of simian virus 40 and human papovavirus BK. J Virol. 1979 Apr;30(1):141–147. doi: 10.1128/jvi.30.1.141-147.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Law M. F., Martin J. D., Takemoto K. K., Howley P. M. The colinear alignment of the genomes of papovaviruses JC, BK, and SV40. Virology. 1979 Jul 30;96(2):576–587. doi: 10.1016/0042-6822(79)90113-2. [DOI] [PubMed] [Google Scholar]
- Levinson B., Khoury G., Vande Woude G., Gruss P. Activation of SV40 genome by 72-base pair tandem repeats of Moloney sarcoma virus. Nature. 1982 Feb 18;295(5850):568–572. doi: 10.1038/295568a0. [DOI] [PubMed] [Google Scholar]
- London W. T., Houff S. A., Madden D. L., Fuccillo D. A., Gravell M., Wallen W. C., Palmer A. E., Sever J. L., Padgett B. L., Walker D. L. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus). Science. 1978 Sep 29;201(4362):1246–1249. doi: 10.1126/science.211583. [DOI] [PubMed] [Google Scholar]
- Margolskee R. F., Nathans D. Suppression of a VP1 mutant of simian virus 40 by missense mutations in serine codons of the viral agnogene. J Virol. 1983 Nov;48(2):405–409. doi: 10.1128/jvi.48.2.405-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Mellon P., Parker V., Gluzman Y., Maniatis T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell. 1981 Dec;27(2 Pt 1):279–288. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Murphy A., Barkan A. Mutants deleted in the agnogene of simian virus 40 define a new complementation group. J Virol. 1983 Jan;45(1):36–46. doi: 10.1128/jvi.45.1.36-46.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura T., Jikuya H., Soeda E., Yoshiike K. Genomic structure of human polyoma virus JC: nucleotide sequence of the region containing replication origin and small-T-antigen gene. J Virol. 1983 Jan;45(1):73–79. doi: 10.1128/jvi.45.1.73-79.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura T., Takemoto K. K. Helper function for adenovirus replication in monkey cells by BK human papovavirus. Virology. 1979 Oct 15;98(1):279–282. doi: 10.1016/0042-6822(79)90549-x. [DOI] [PubMed] [Google Scholar]
- Morrison B., Kress M., Khoury G., Jay G. Simian virus 40 tumor antigen: isolation of the origin-specific DNA-binding domain. J Virol. 1983 Jul;47(1):106–114. doi: 10.1128/jvi.47.1.106-114.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Tjian R. Construction and analysis of simian virus 40 origins defective in tumor antigen binding and DNA replication. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6491–6495. doi: 10.1073/pnas.77.11.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Rogers C. M., Walker D. L. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect Immun. 1977 Feb;15(2):656–662. doi: 10.1128/iai.15.2.656-662.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973 Apr;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Hodach A. E., Chou S. M. JC Papovavirus in progressive multifocal leukoencephalopathy. J Infect Dis. 1976 Jun;133(6):686–690. doi: 10.1093/infdis/133.6.686. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Varakis J. N. Differential neurooncogenicity of strains of JC virus, a human polyoma virus, in newborn Syrian hamsters. Cancer Res. 1977 Mar;37(3):718–720. [PubMed] [Google Scholar]
- Prives C., Barnet B., Scheller A., Khoury G., Jay G. Discrete regions of simian virus 40 large T antigen are required for nonspecific and viral origin-specific DNA binding. J Virol. 1982 Jul;43(1):73–82. doi: 10.1128/jvi.43.1.73-82.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. Sequence at the 3' end of globin mRNA shows homology with immunoglobulin light chain mRNA. Nature. 1974 Nov 29;252(5482):359–362. doi: 10.1038/252359a0. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Piatak M., Weissman S. M. Simian virus 40 early mRNA's. I. Genomic localization of 3' and 5' termini and two major splices in mRNA from transformed and lytically infected cells. J Virol. 1979 Apr;30(1):279–296. doi: 10.1128/jvi.30.1.279-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rieth K. G., Di Chiro G., London W. T., Sever J. L., Houff S. A., Kornblith P. L., McKeever P. E., Buonomo C., Padgett B. L., Walker D. L. Experimental glioma in primates: a computed tomography model. J Comput Assist Tomogr. 1980 Jun;4(3):285–290. doi: 10.1097/00004728-198006000-00001. [DOI] [PubMed] [Google Scholar]
- Rio D., Robbins A., Myers R., Tjian R. Regulation of simian virus 40 early transcription in vitro by a purified tumor antigen. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5706–5710. doi: 10.1073/pnas.77.10.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N., Kress M., Gruss P., Khoury G. BK viral enhancer element and a human cellular homolog. Science. 1983 Nov 18;222(4625):749–755. doi: 10.1126/science.6314501. [DOI] [PubMed] [Google Scholar]
- Ruley H. E., Fried M. Sequence repeats in a polyoma virus DNA region important for gene expression. J Virol. 1983 Jul;47(1):233–237. doi: 10.1128/jvi.47.1.233-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Ozer H. L., Ghazey H. N., Kelly T. J., Jr Common structural antigen of papovaviruses of the simian virus 40-polyoma subgroup. J Virol. 1977 Jan;21(1):179–186. doi: 10.1128/jvi.21.1.179-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Kleinberger T., Livingston D. M. Mapping of SV40 DNA replication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell. 1980 Jun;20(2):411–422. doi: 10.1016/0092-8674(80)90627-3. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D. R., Margolskee R. F., Nathans D. Mutational analysis of the simian virus 40 replicon: pseudorevertants of mutants with a defective replication origin. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6128–6131. doi: 10.1073/pnas.76.12.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T. Characterization of tau antigens isolated from uninfected and simian virus 40-infected monkey cells and papovavirus-transformed cells. J Virol. 1980 Nov;36(2):519–525. doi: 10.1128/jvi.36.2.519-525.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K. N., Dhar R., Weissman S. M. Nucleotide sequence of a fragment of SV40 DNA that contains the origin of DNA replication and specifies the 5' ends of "early" and "late" viral RNA. III. Construction of the total sequence of EcoRII-G fragment of SV40 DNA. J Biol Chem. 1977 Jan 10;252(1):355–367. [PubMed] [Google Scholar]
- Takemoto K. K., Rabson A. S., Mullarkey M. F., Blaese R. M., Garon C. F., Nelson D. Isolation of papovavirus from brain tumor and urine of a patient with Wiskott-Aldrich syndrome. J Natl Cancer Inst. 1974 Nov;53(5):1205–1207. doi: 10.1093/jnci/53.5.1205. [DOI] [PubMed] [Google Scholar]
- Tjian R. Protein-DNA interactions at the origin of simian virus 40 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):655–661. doi: 10.1101/sqb.1979.043.01.073. [DOI] [PubMed] [Google Scholar]
- Tyndall C., La Mantia G., Thacker C. M., Favaloro J., Kamen R. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 1981 Dec 11;9(23):6231–6250. doi: 10.1093/nar/9.23.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. L., Padgett B. L., ZuRhein G. M., Albert A. E., Marsh R. F. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973 Aug 17;181(4100):674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Augereau P., Chambon P. The SV40 72 bp repeat preferentially potentiates transcription starting from proximal natural or substitute promoter elements. Cell. 1983 Feb;32(2):503–514. doi: 10.1016/0092-8674(83)90470-1. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K. Change of DNA near the origin of replication enhances the transforming capacity of human papovavirus BK. J Virol. 1982 Jun;42(3):978–985. doi: 10.1128/jvi.42.3.978-985.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K., Nozawa A., Yuasa Y., Uchida S. Viable deletion mutant of human papovavirus BK that induces insulinomas in hamsters. J Virol. 1979 Dec;32(3):934–942. doi: 10.1128/jvi.32.3.934-942.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA: complete nucleotide sequence of a human tumor virus. Science. 1979 Oct 26;206(4417):456–462. doi: 10.1126/science.228391. [DOI] [PubMed] [Google Scholar]
- Zain B. S., Thimmappaya B., Dhar R., Weissman S. M. Nucleotide sequences of DNA encoding the 3' ends of SV40 mRNA. I. The sequence of the DNA fragment Hi-DII,III-G. J Biol Chem. 1978 Mar 10;253(5):1606–1612. [PubMed] [Google Scholar]
- de Villiers J., Schaffner W. A small segment of polyoma virus DNA enhances the expression of a cloned beta-globin gene over a distance of 1400 base pairs. Nucleic Acids Res. 1981 Dec 11;9(23):6251–6264. doi: 10.1093/nar/9.23.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]


