Abstract
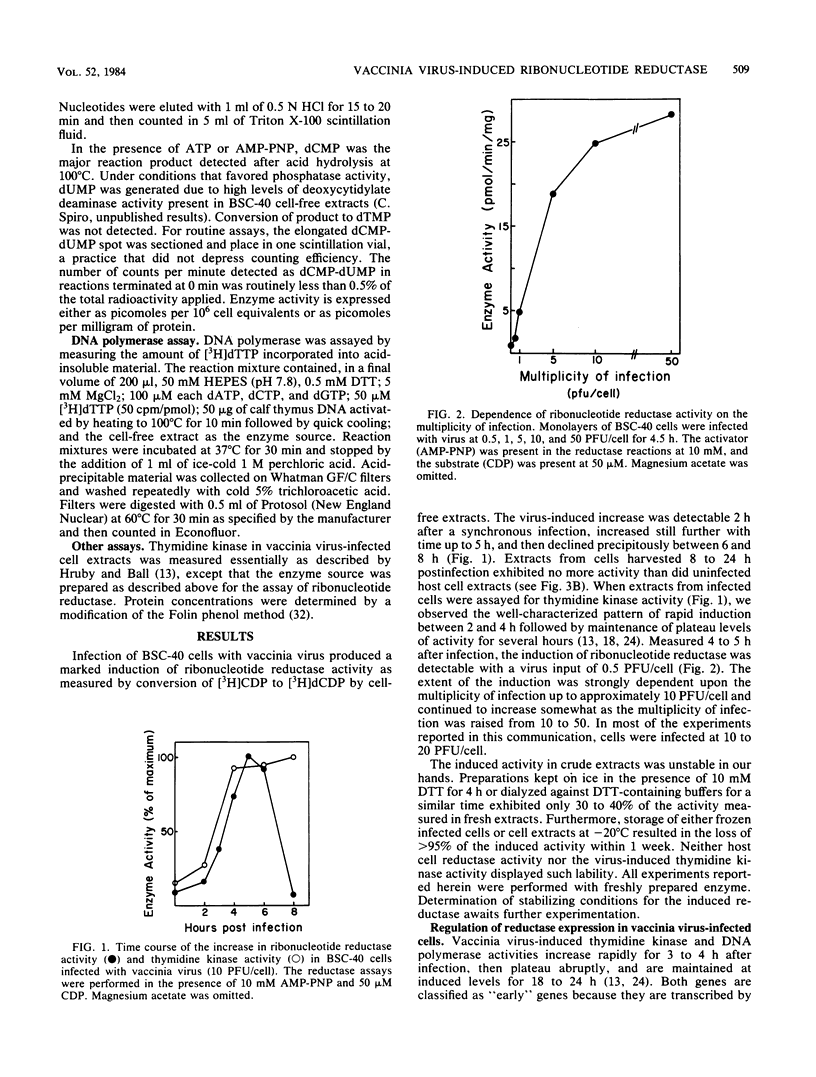
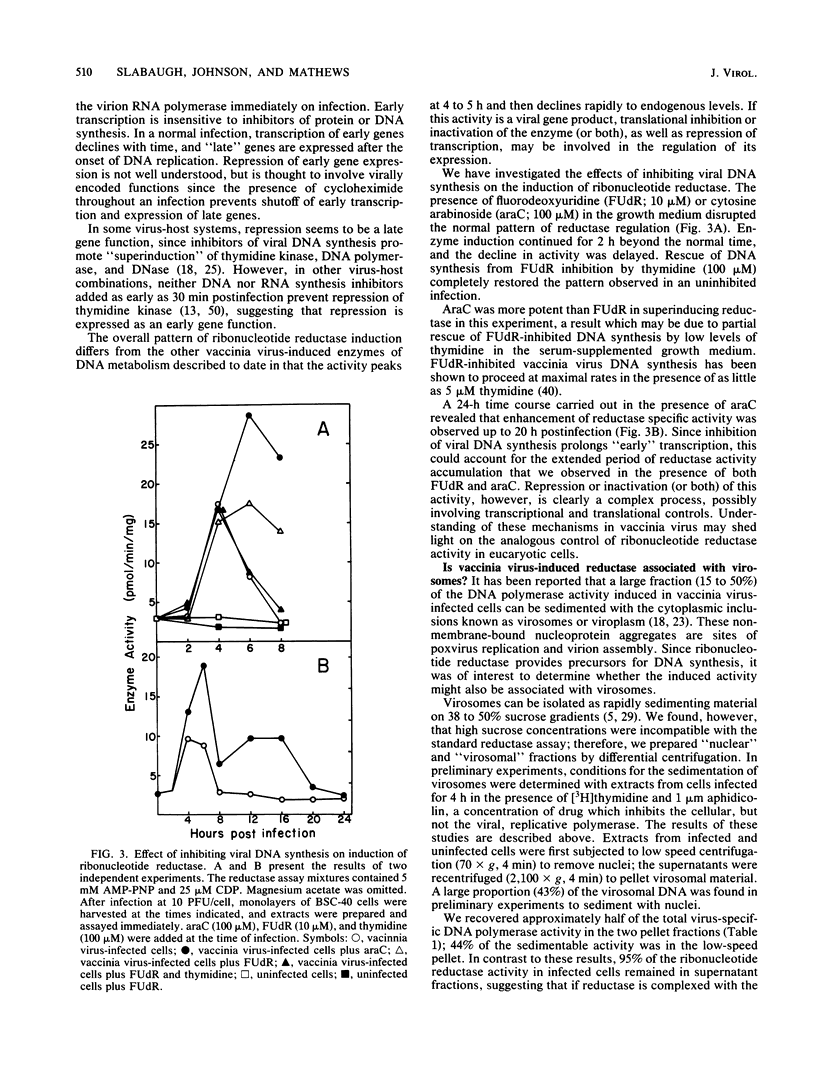
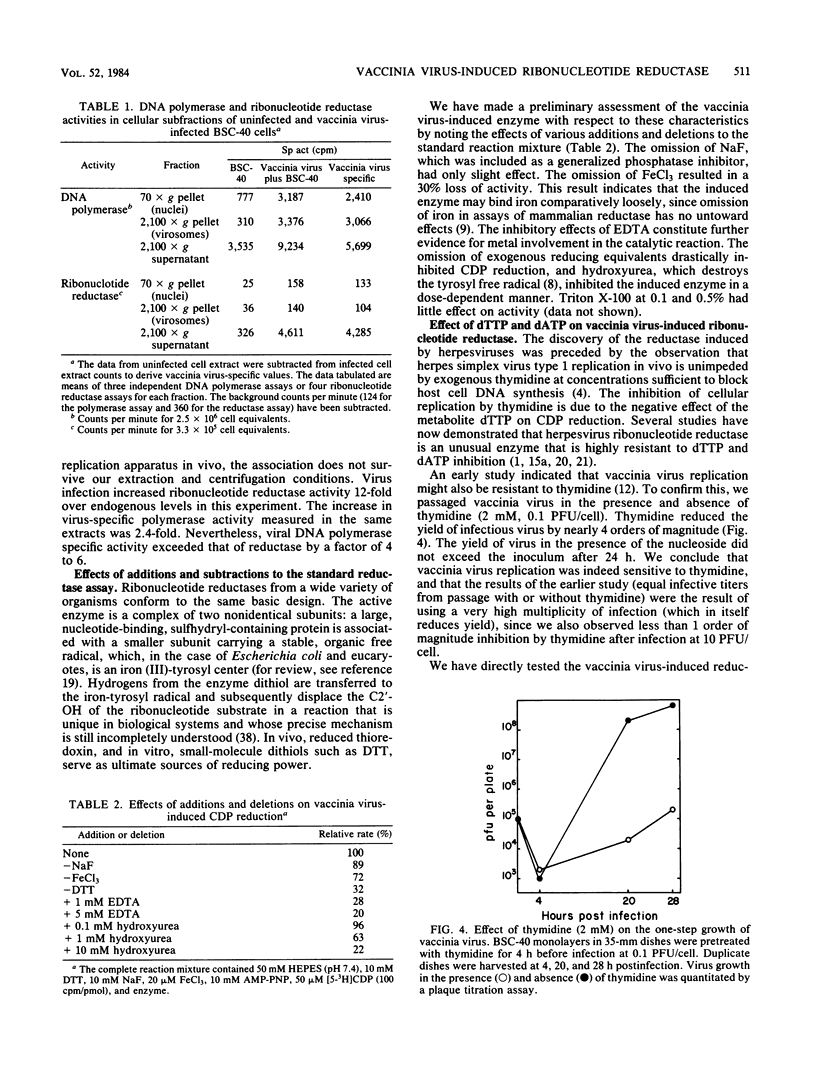
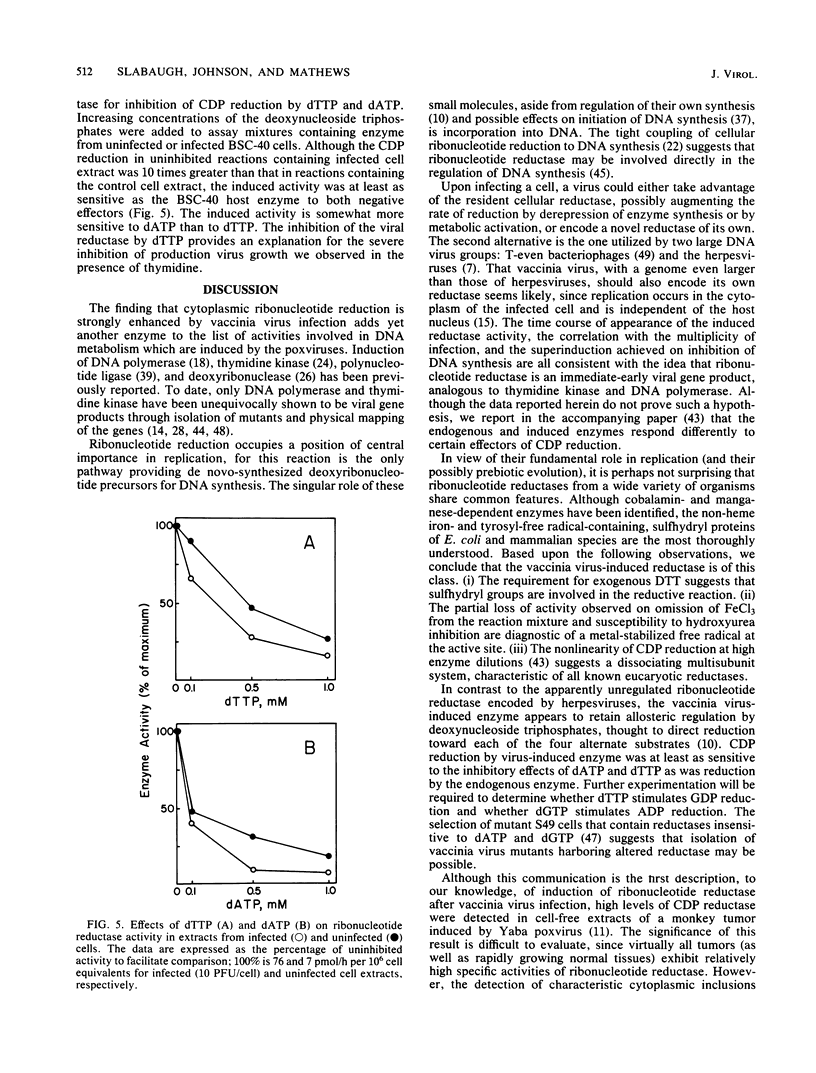
Infection of monkey kidney (BSC-40) cells with vaccinia virus strain WR resulted in a marked increase in ribonucleoside diphosphate reductase (EC 1.17.4.1) activity as measured by CDP reduction in cell-free extracts. After a synchronous infection, increased activity was detected at 2 h, peaked at 4 to 5 h, and then declined between 6 and 8 h to the endogenous cellular level. The induction, detectable at 0.5 PFU/cell, correlated strongly with multiplicity of infection to 10 PFU/cell and continued to increase to 50 PFU/cell. It paralleled the previously described induction of viral DNA polymerase and thymidine kinase, suggesting that the reductase may also be a product of early transcription of the viral genome. The inhibition of DNA synthesis throughout infection resulted in prolonged accumulation of reductase activity and delayed and incomplete down-regulation at 8 h, suggesting that repression involves late functions. Rescue of fluorodeoxyuridine-inhibited DNA synthesis with exogenous thymidine restored the normal pattern. Preferential association of the induced reductase with the cytoplasmic sites of vaccinia virus DNA replication (virosomes) was not detected. The induced enzyme is similar in several respects to other eucaryotic ribonucleotide reductases, but is distinct from host cell reductase in response to certain modulators of reductase activity (M. B. Slabaugh and Christopher K. Mathews, J. Virol. 52:501-506, 1984). Full activity required an activator, exogenous reducing equivalents, and iron. Hydroxyurea, EDTA, dATP, and dTTP inhibited CDP reduction, setting this reductase apart from T4 reductase, which is not inhibited by dATP, and from herpesvirus reductase, which requires no activation and is insensitive to deoxyribonucleoside triphosphate inhibition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averett D. R., Lubbers C., Elion G. B., Spector T. Ribonucleotide reductase induced by herpes simplex type 1 virus. Characterization of a distinct enzyme. J Biol Chem. 1983 Aug 25;258(16):9831–9838. [PubMed] [Google Scholar]
- Brakel C., Kates J. R. Poly(A) polymerase from vaccinia virus-infected cells. I. Partial purification and characterization. J Virol. 1974 Oct;14(4):715–723. doi: 10.1128/jvi.14.4.715-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Cohen G. H. Ribonucleotide reductase activity of synchronized KB cells infected with herpes simplex virus. J Virol. 1972 Mar;9(3):408–418. doi: 10.1128/jvi.9.3.408-418.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. ISOLATION AND PROPERTIES OF VACCINIA MUTANTS DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Feb;22:214–225. doi: 10.1016/0042-6822(64)90006-6. [DOI] [PubMed] [Google Scholar]
- Dahl R., Kates J. R. Intracellular structures containing vaccinia DNA: isolation and characterization. Virology. 1970 Oct;42(2):453–462. doi: 10.1016/0042-6822(70)90288-6. [DOI] [PubMed] [Google Scholar]
- Dutia B. M. Ribonucleotide reductase induced by herpes simplex virus has a virus-specified constituent. J Gen Virol. 1983 Mar;64(Pt 3):513–521. doi: 10.1099/0022-1317-64-3-513. [DOI] [PubMed] [Google Scholar]
- Ehrenberg A., Reichard P. Electron spin resonance of the iron-containing protein B2 from ribonucleotide reductase. J Biol Chem. 1972 Jun 10;247(11):3485–3488. [PubMed] [Google Scholar]
- Engström Y., Eriksson S., Thelander L., Akerman M. Ribonucleotide reductase from calf thymus. Purification and properties. Biochemistry. 1979 Jul 10;18(14):2941–2948. doi: 10.1021/bi00581a004. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Thelander L., Akerman M. Allosteric regulation of calf thymus ribonucleoside diphosphate reductase. Biochemistry. 1979 Jul 10;18(14):2948–2952. doi: 10.1021/bi00581a005. [DOI] [PubMed] [Google Scholar]
- Gordon H. L., Fiel R. J. Ribonucleotide reductase activity in cell-free extracts of Yaba poxvirus tumor and normal monkey tissues. Cancer Res. 1969 Jul;29(7):1350–1355. [PubMed] [Google Scholar]
- Groyon R. M., Kniazeff A. J. Vaccinia virus infection of synchronized pig kidney cells. J Virol. 1967 Dec;1(6):1255–1264. doi: 10.1128/jvi.1.6.1255-1264.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Ball L. A. Control of expression of the vaccinia virus thymidine kinase gene. J Virol. 1981 Nov;40(2):456–464. doi: 10.1128/jvi.40.2.456-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Ball L. A. Mapping and identification of the vaccinia virus thymidine kinase gene. J Virol. 1982 Aug;43(2):403–409. doi: 10.1128/jvi.43.2.403-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Guarino L. A., Kates J. R. Vaccinia virus replication. I. Requirement for the host-cell nucleus. J Virol. 1979 Feb;29(2):705–715. doi: 10.1128/jvi.29.2.705-715.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D., Bacchetti S. Is ribonucleotide reductase the transforming function of herpes simplex virus 2? Nature. 1983 Mar 3;302(5903):76–79. doi: 10.1038/302076a0. [DOI] [PubMed] [Google Scholar]
- Huszar D., Bacchetti S. Partial purification and characterization of the ribonucleotide reductase induced by herpes simplex virus infection of mammalian cells. J Virol. 1981 Feb;37(2):580–588. doi: 10.1128/jvi.37.2.580-588.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- Jungwirth C., Joklik W. K. Studies on "early" enzymes in HeLa cells infected with vaccinia virus. Virology. 1965 Sep;27(1):80–93. doi: 10.1016/0042-6822(65)90145-5. [DOI] [PubMed] [Google Scholar]
- Langelier Y., Buttin G. Characterization of ribonucleotide reductase induction in BHK-21/C13 Syrian hamster cell line upon infection by herpes simplex virus (HSV). J Gen Virol. 1981 Nov;57(Pt 1):21–31. doi: 10.1099/0022-1317-57-1-21. [DOI] [PubMed] [Google Scholar]
- Lankinen H., Gräslund A., Thelander L. Induction of a new ribonucleotide reductase after infection of mouse L cells with pseudorabies virus. J Virol. 1982 Mar;41(3):893–900. doi: 10.1128/jvi.41.3.893-900.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A. Ribonucleotide reductase rom regenerating rat liver. Eur J Biochem. 1969 Nov;11(1):113–121. doi: 10.1111/j.1432-1033.1969.tb00747.x. [DOI] [PubMed] [Google Scholar]
- MCAUSLAN B. R. THE INDUCTION AND REPRESSION OF THYMIDINE KINASE IN THE POXVIRUS-INFECTED HELA CELL. Virology. 1963 Nov;21:383–389. doi: 10.1016/0042-6822(63)90199-5. [DOI] [PubMed] [Google Scholar]
- Magee W. E., Miller O. V. Immunological evidence for the appearance of a new DNA-polymerase in cells infected with vaccinia virus. Virology. 1967 Jan;31(1):64–69. doi: 10.1016/0042-6822(67)90008-6. [DOI] [PubMed] [Google Scholar]
- Moss B., Cooper N. Genetic evidence for vaccinia virus-encoded DNA polymerase: isolation of phosphonoacetate-resistant enzyme from the cytoplasm of cells infected with mutant virus. J Virol. 1982 Aug;43(2):673–678. doi: 10.1128/jvi.43.2.673-678.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer R. W., Graves R. L. The mechanism of cytoplasmic orthopoxvirus DNA replication. Cell. 1981 Dec;27(2 Pt 1):391–401. doi: 10.1016/0092-8674(81)90422-0. [DOI] [PubMed] [Google Scholar]
- NOYES W. F. OBSERVATIONS ON TWO POX-TUMOR VIRUSES. Virology. 1965 Apr;25:666–669. doi: 10.1016/0042-6822(65)90097-8. [DOI] [PubMed] [Google Scholar]
- Parkhurst J. R., Peterson A. R., Heidelberger C. Breakdown of HeLa cell DNA mediated by vaccinia virus. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3200–3204. doi: 10.1073/pnas.70.11.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Freimuth P., Stein A. Shope fibroma virus. I. Biological and molecular properties of a cytocidal and a noncytocidal strain. J Virol. 1982 Jan;41(1):97–103. doi: 10.1128/jvi.41.1.97-103.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., Kates J. Viral-specific polypeptides associated with newly replicated vaccinia DNA. Virology. 1975 Jul;66(1):128–139. doi: 10.1016/0042-6822(75)90184-1. [DOI] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P., Ehrenberg A. Ribonucleotide reductase--a radical enzyme. Science. 1983 Aug 5;221(4610):514–519. doi: 10.1126/science.6306767. [DOI] [PubMed] [Google Scholar]
- Reichard P. From deoxynucleotides to DNA synthesis. Fed Proc. 1978 Jan;37(1):9–14. [PubMed] [Google Scholar]
- SHATKIN A. J., SALZMAN N. P. Deoxyribonucleic acid synthesis in vaccinia virus-infected HeLa cells. Virology. 1963 Apr;19:551–560. doi: 10.1016/0042-6822(63)90050-3. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Shatkin A. J. Polynucleotide ligase activity in cells infected with simian virus 40, polyoma virus, or vaccinia virus. J Virol. 1969 Nov;4(5):719–726. doi: 10.1128/jvi.4.5.719-726.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabaugh M. B., Mathews C. K. Vaccinia virus-induced ribonucleotide reductase can be distinguished from host cell activity. J Virol. 1984 Nov;52(2):501–506. doi: 10.1128/jvi.52.2.501-506.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar P., Condit R. C. Selection for temperature-sensitive mutations in specific vaccinia virus genes: isolation and characterization of a virus mutant which encodes a phosphonoacetic acid-resistant, temperature-sensitive DNA polymerase. Virology. 1983 Jul 30;128(2):444–457. doi: 10.1016/0042-6822(83)90269-6. [DOI] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Tompkins W. A., Walker D. L., Hinze H. C. Cellular deoxyribonucleic acid synthesis and loss of contact inhibition in irradiated and contact-inhibited cell cultures infected with fibroma virus. J Virol. 1969 Nov;4(5):603–609. doi: 10.1128/jvi.4.5.603-609.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Caras I. W., Eriksson S., Weinberg G. L., Wormsted M. A., Martin D. W., Jr Demonstration of normal and mutant protein M1 subunits of deoxyGTP-resistant ribonucleotide reductase from mutant mouse lymphoma cells. J Biol Chem. 1981 Oct 10;256(19):10189–10192. [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y. C., Dubovi E. J., Tessman I. Control of pyrimidine biosynthesis by phage T4: mutants unable to catalyze the reduction of cytidine diphosphate. Virology. 1969 Apr;37(4):615–623. doi: 10.1016/0042-6822(69)90279-7. [DOI] [PubMed] [Google Scholar]
- Zaslavsky V., Yakobson E. Control of thymidine kinase synthesis in IHD vaccinia virus-infected thymidine kinase-deficient LM cells. J Virol. 1975 Jul;16(1):210–213. doi: 10.1128/jvi.16.1.210-213.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


