Abstract
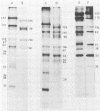
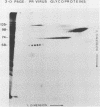
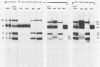
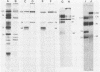
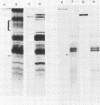
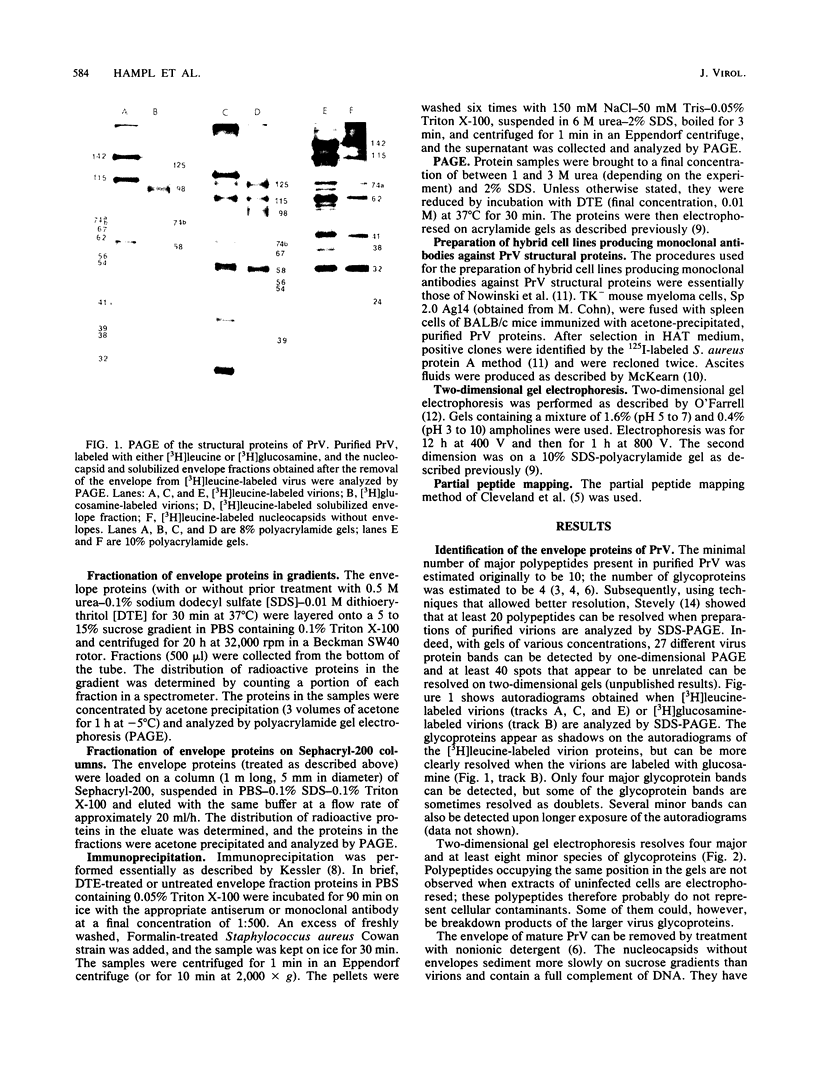
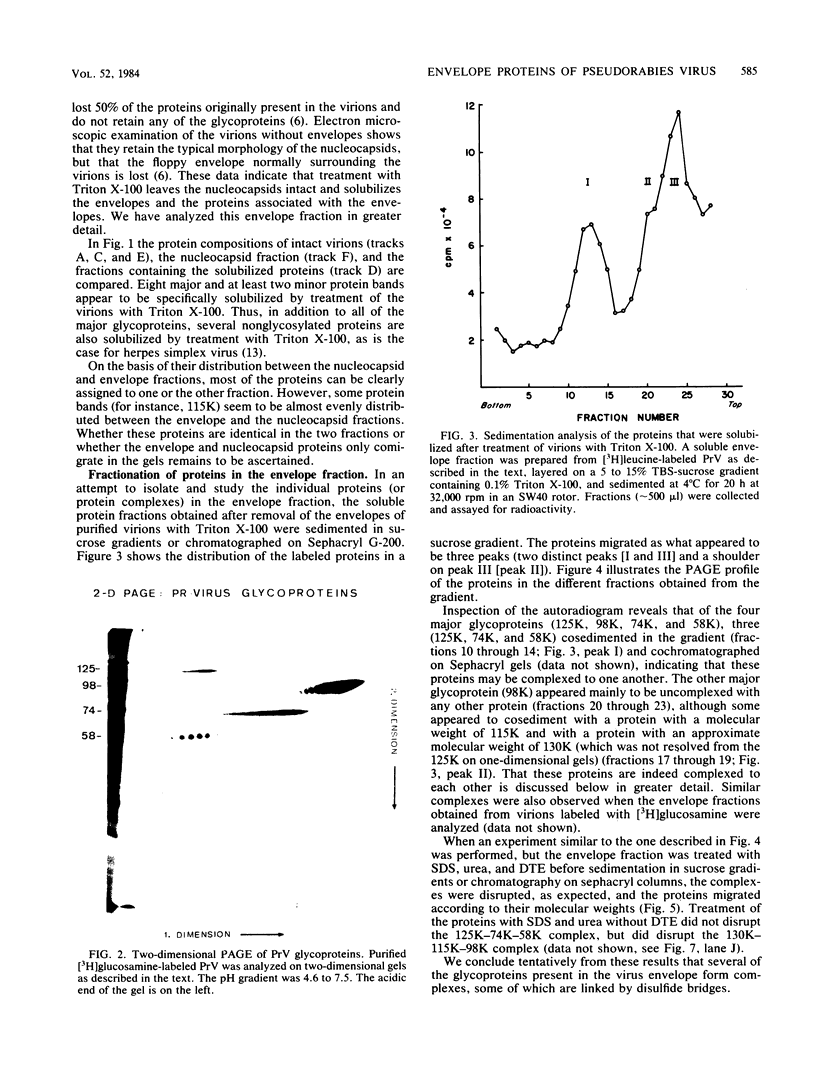
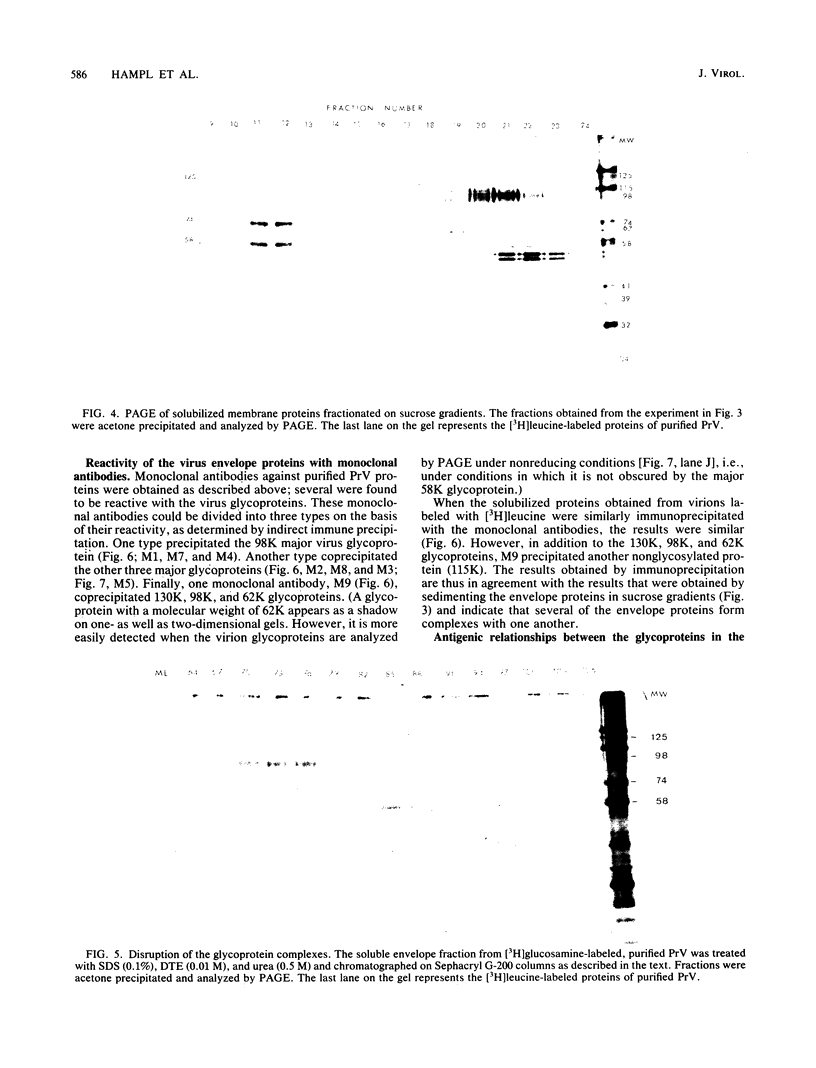
Previously we have reported that among the proteins of purified pseudorabies virions there are four major glycoproteins (T. Ben-Porat and A. S. Kaplan, Virology 41:265-273, 1970). Several minor glycoproteins can also be identified by two-dimensional gel electrophoresis. Removal of the viral envelope with Triton X-100 selectively removes from the virions all of the glycoproteins as well as several non-glycosylated proteins. Sedimentation analysis or chromatography of these proteins reveals that several are complexed with one another, some being covalently linked via disulfide bridges. Analysis of the proteins by immunoprecipitation with monoclonal antibodies reactive with the membrane proteins showed also that three of the four major virus glycoproteins (125K, 74K, and 58K; gIIa, gIIb, and gIIc, respectively) are linked covalently by disulfide bridges. Furthermore, all three share extensive sequence homology as indicated by the identity of their antigenic determinants and by partial peptide mapping; they probably originate from a single protein precursor. The fourth major glycoprotein (98K; gIII) is not complexed to any other protein. Three minor glycoproteins (130K [gI], 98K [gIV], and 62K [gV]), which form a noncovalently linked complex with a 115K nonglycosylated protein, have also been identified. Of the monoclonal antibodies used in this study, only those reactive with the major 98K glycoprotein (gIII) inhibit virus adsorption and neutralize virus infectivity in the absence of complement. However, all react with surface components of the virion, indicating that the proteins with which they react are exposed on the surface of the virions. A nomenclature for the pseudorabies virus glycoproteins is proposed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Demarchi J. M., Kaplan A. S. Characterization of defective interfering viral particles present in a population of pseudorabies virions. Virology. 1974 Sep;61(1):29–37. doi: 10.1016/0042-6822(74)90239-6. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. V. Viral glycoproteins. Virology. 1970 Jun;41(2):265–273. doi: 10.1016/0042-6822(70)90078-4. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Shimono H., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. IV. Analysis of the proteins in viral particles isolated from the cytoplasm and the nucleus. Virology. 1970 Jun;41(2):256–264. doi: 10.1016/0042-6822(70)90077-2. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- KAPLAN A. S., VATTER A. E. A comparison of herpes simplex and pseudorabies viruses. Virology. 1959 Apr;7(4):394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- Kaplan A. S., Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus, VI. Characterization of the proteins of the viral membrane. Proc Natl Acad Sci U S A. 1970 Jul;66(3):799–806. doi: 10.1073/pnas.66.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Ladin B. F., Ihara S., Hampl H., Ben-Porat T. Pathway of assembly of herpesvirus capsids: an analysis using DNA+ temperature-sensitive mutants of pseudorabies virus. Virology. 1982 Jan 30;116(2):544–561. doi: 10.1016/0042-6822(82)90147-7. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Lostrom M. E., Tam M. R., Stone M. R., Burnette W. N. The isolation of hybrid cell lines producing monoclonal antibodies against the p15(E) protein of ecotropic murine leukemia viruses. Virology. 1979 Feb;93(1):111–126. doi: 10.1016/0042-6822(79)90280-0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevely W. S. Virus-induced proteins in pseudorabies-infected cells. II. Proteins of the virion and nucleocapsid. J Virol. 1975 Oct;16(4):944–950. doi: 10.1128/jvi.16.4.944-950.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]