Abstract
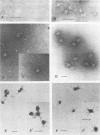
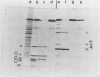
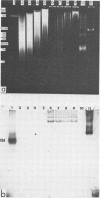
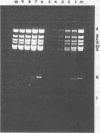
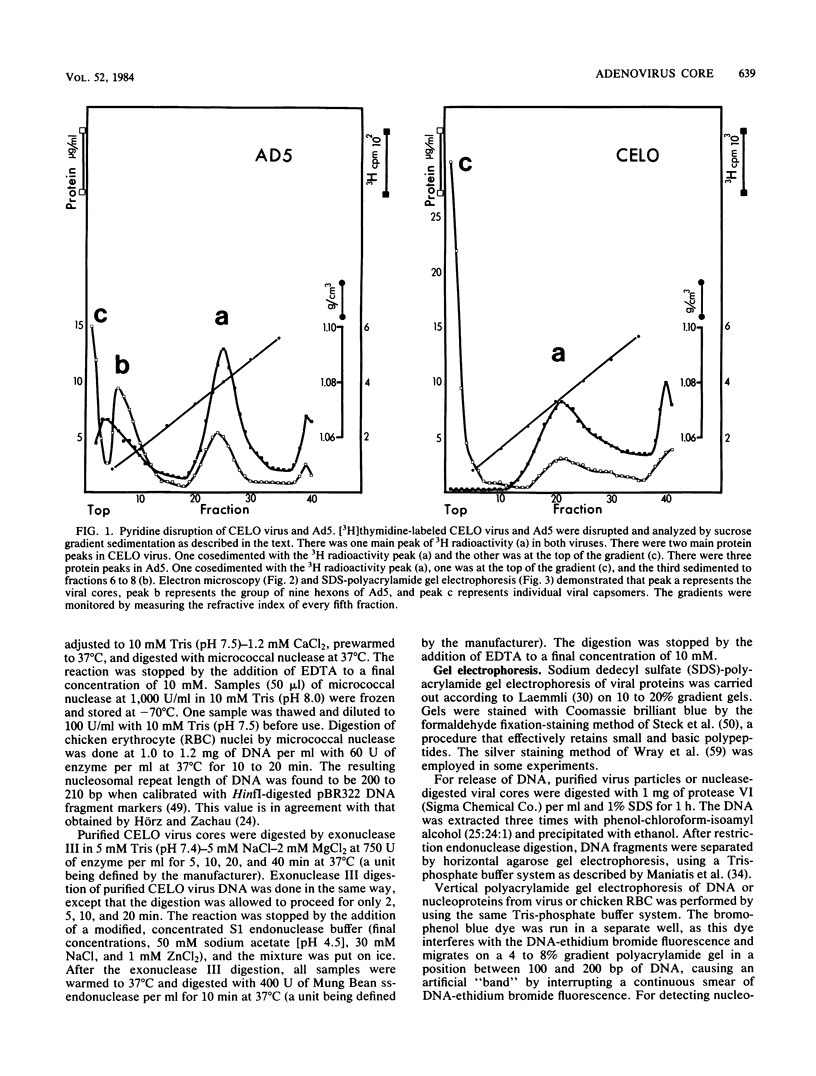
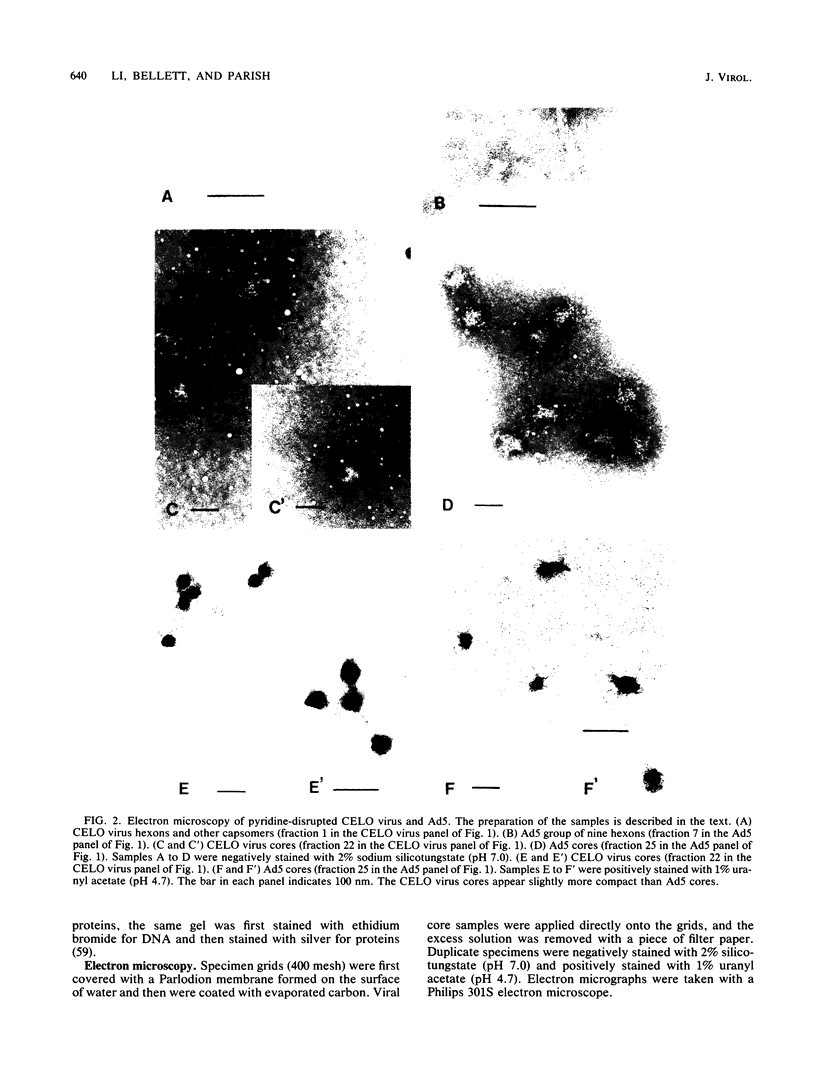
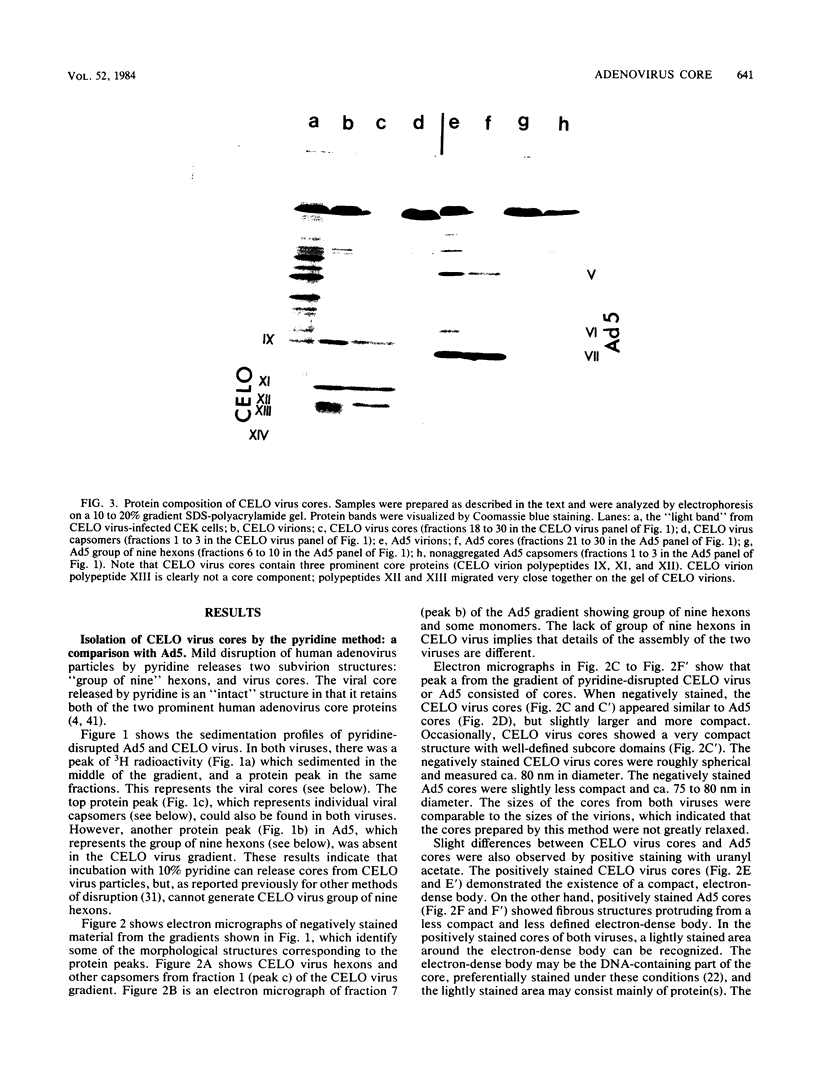
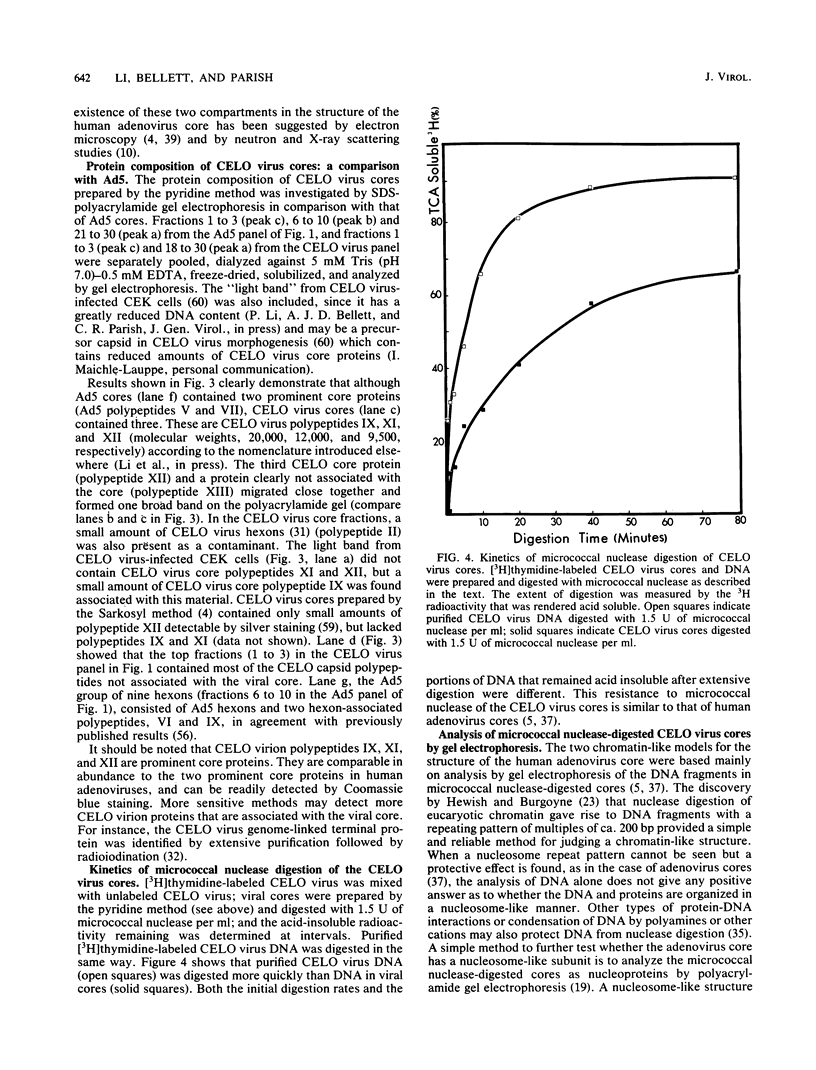
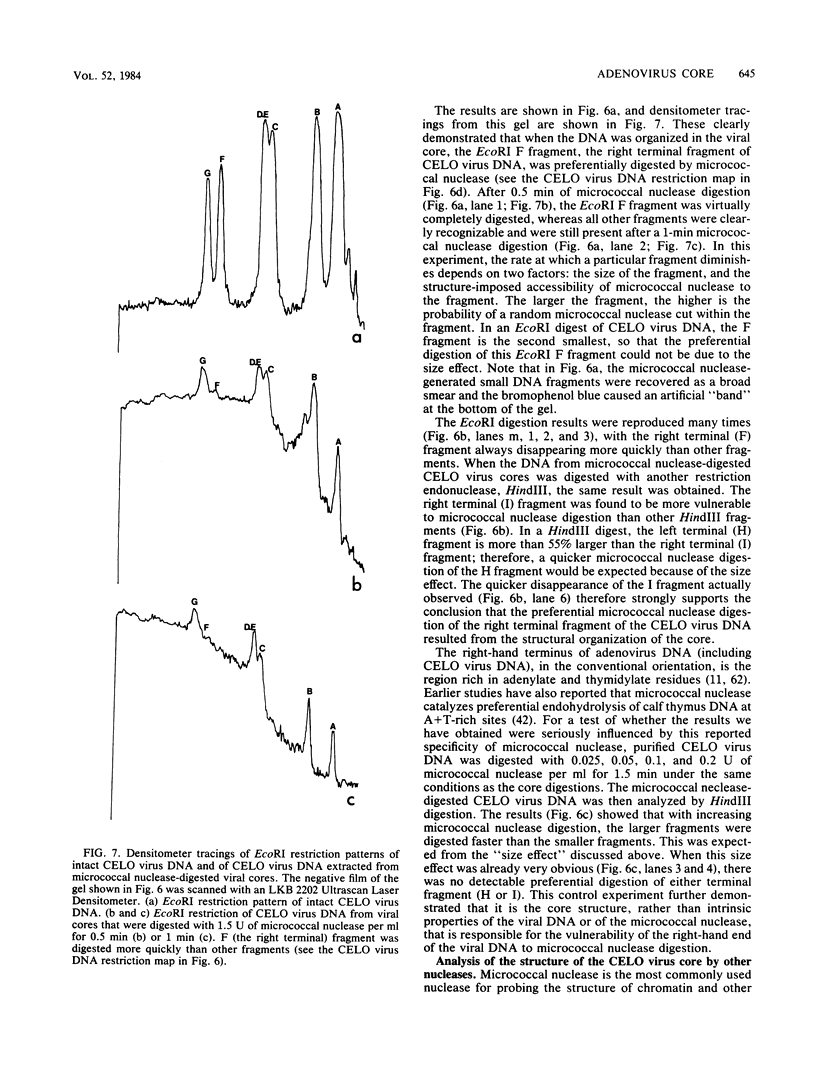
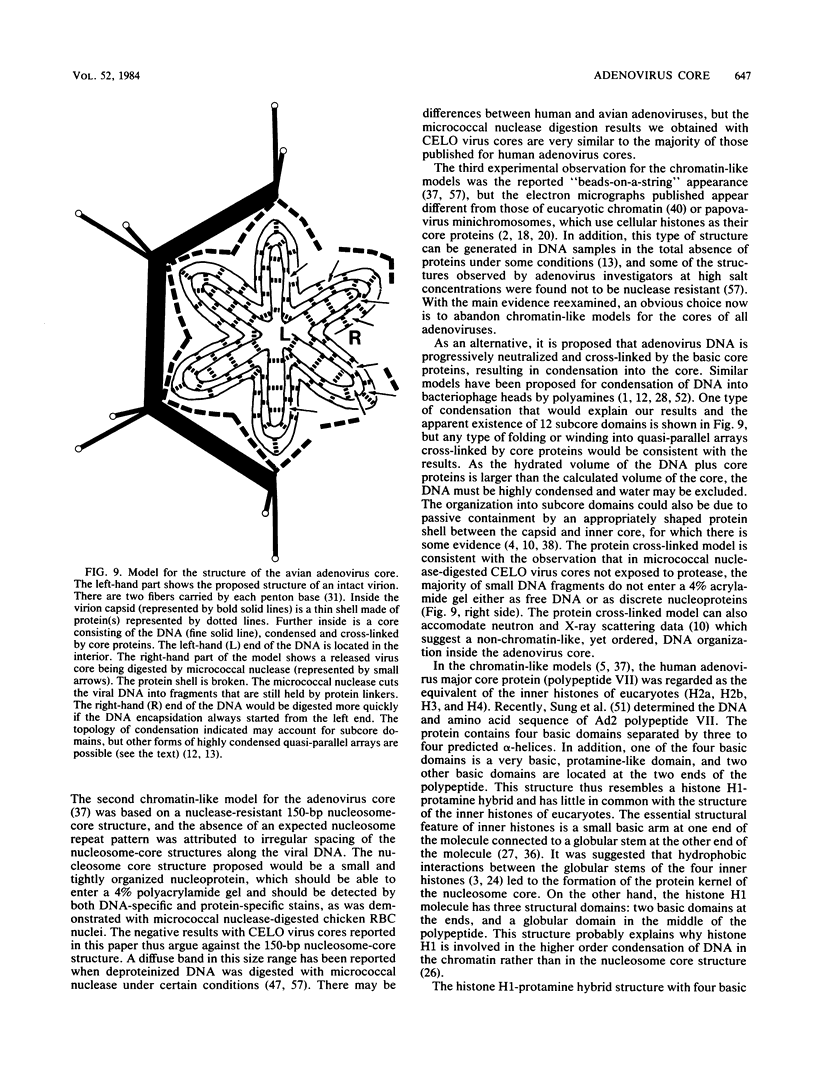
CELO virus (fowl adenovirus 1) contained three core polypeptides of molecular weights 20,000, 12,000, and 9,500. The core was similar to that of human adenoviruses, with some evidence of compact subcore domains. Micrococcal nuclease digestion of CELO virus cores produced a smear of DNA fragments of gradually decreasing size, with no nucleosome subunit or repeat pattern. Moreover, when digested cores were analyzed without protease treatment, there was again no evidence of a nucleosome substructure; neither DNA fragments nor core proteins entered a 4% polyacrylamide gel. The organization of the core is thus quite unlike that of chromatin. Restriction endonuclease analysis of the DNA from digested cores showed that the right end was on the outside of the core. We suggest that adenovirus DNA is condensed into the core by cross-linking and neutralization by the core proteins, beginning with the packaging sequence at the center of the core and ending with the right end of the DNA on the outside.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker A., Marko M., Gold M. Early events in the in vitro packaging of bacteriophage lambda DNA. Virology. 1977 May 1;78(1):291–305. doi: 10.1016/0042-6822(77)90100-3. [DOI] [PubMed] [Google Scholar]
- Bellard M., Oudet P., Germond J. E., Chambon P. Subunit structure of simian-virus-40 minichromosome. Eur J Biochem. 1976 Nov 15;70(2):543–553. doi: 10.1111/j.1432-1033.1976.tb11046.x. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Moss T., Hayashi H., Hjelm R. P., Suau P., Stephens R. M., Baldwin J. P., Crane-Robinson C. Nucleosomes, histone interactions, and the role of histones H3 and H4. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):277–286. doi: 10.1101/sqb.1978.042.01.030. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Westphal M., Burlingham B. T., Winterhoff U., Doerfler W. Structure and composition of the adenovirus type 2 core. J Virol. 1975 Aug;16(2):366–387. doi: 10.1128/jvi.16.2.366-387.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Engelking H. M., Pearson G. D. Chromatin-like organization of the adenovirus chromosome. Proc Natl Acad Sci U S A. 1976 Feb;73(2):401–404. doi: 10.1073/pnas.73.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E. Genome structure of incomplete particles of adenovirus. J Virol. 1976 Aug;19(2):685–708. doi: 10.1128/jvi.19.2.685-708.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E., Groff D. E., Fedor M. J. Adenovirus chromatin structure at different stages of infection. Mol Cell Biol. 1981 Dec;1(12):1094–1105. doi: 10.1128/mcb.1.12.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E., Mullenbach T. Synthesis of defective viral DNA in HeLa cells infected with adenovirus type 3. J Virol. 1978 Apr;26(1):61–70. doi: 10.1128/jvi.26.1.61-70.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisova T. S., Sitnikov B. S., Gibadulin R. A. Izuchenie fragmentatsii DNK ptich'ego adenovirusa CELO s pomoshch'iu spetsificheskikh endonukleaz R. HpaI, R. EcoRI, R. HindIII. Mol Biol (Mosk) 1979 Sep-Oct;13(5):1021–1034. [PubMed] [Google Scholar]
- Devaux C., Timmins P. A., Berthet-Colominas C. Structural studies of adenovirus type 2 by neutron and X-ray scattering. J Mol Biol. 1983 Jun 15;167(1):119–132. doi: 10.1016/s0022-2836(83)80037-0. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Kleinschmidt A. K. Denaturation pattern of the DNA of adenovirus type 2 as determined by electron microscopy. J Mol Biol. 1970 Jun 28;50(3):579–593. doi: 10.1016/0022-2836(70)90086-0. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Harrison S. C. DNA arrangement in isometric phage heads. Nature. 1977 Aug 18;268(5621):598–602. doi: 10.1038/268598a0. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The compaction of DNA helices into either continuous supercoils or folded-fiber rods and toroids. Cell. 1978 Feb;13(2):295–306. doi: 10.1016/0092-8674(78)90198-8. [DOI] [PubMed] [Google Scholar]
- Everitt E., Lutter L., Philipson L. Structural proteins of adenoviruses. XII. Location and neighbor relationship among proteins of adenovirion type 2 as revealed by enzymatic iodination, immunoprecipitation and chemical cross-linking. Virology. 1975 Sep;67(1):197–208. doi: 10.1016/0042-6822(75)90417-1. [DOI] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Pettersson U., Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973 Mar;52(1):130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- Fedor M. J., Daniell E. DNase I cleavage of adenoviral nucleoprotein. Nucleic Acids Res. 1983 Jul 11;11(13):4417–4434. doi: 10.1093/nar/11.13.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R. B., Berk A. J. Cis-acting induction of adenovirus transcription. Cell. 1983 Jul;33(3):683–693. doi: 10.1016/0092-8674(83)90011-9. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Winberg G. Encapsidation of adenovirus 16 DNA is directed by a small DNA sequence at the left end of the genome. Cell. 1980 Jul;20(3):787–795. doi: 10.1016/0092-8674(80)90325-6. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Hosokawa K., Sung M. T. Isolation and characterization of an extremely basic protein from adenovirus type 5. J Virol. 1976 Mar;17(3):924–934. doi: 10.1128/jvi.17.3.924-934.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörz W., Zachau H. G. Deoxyribonuclease II as a probe for chromatin structure. I. Location of cleavage sites. J Mol Biol. 1980 Dec 15;144(3):305–327. doi: 10.1016/0022-2836(80)90093-5. [DOI] [PubMed] [Google Scholar]
- Igo-Kemenes T., Hörz W., Zachau H. G. Chromatin. Annu Rev Biochem. 1982;51:89–121. doi: 10.1146/annurev.bi.51.070182.000513. [DOI] [PubMed] [Google Scholar]
- Isenberg I. Histones. Annu Rev Biochem. 1979;48:159–191. doi: 10.1146/annurev.bi.48.070179.001111. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Syvanen M., Masuda T. DNA packaging steps in bacteriophage lambda head assembly. J Mol Biol. 1975 Jan 15;91(2):175–186. doi: 10.1016/0022-2836(75)90158-8. [DOI] [PubMed] [Google Scholar]
- Kosturko L. D., Sharnick S. V., Tibbetts C. Polar encapsidation of adenovirus DNA: cloning and DNA sequence of the left end of adenovirus type 3. J Virol. 1982 Sep;43(3):1132–1137. doi: 10.1128/jvi.43.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Younghusband H. B., Wrigley N. G. Purification and properties of chick embryo lethal orphan virus (an avian adenovirus). Virology. 1971 Sep;45(3):598–614. doi: 10.1016/0042-6822(71)90175-9. [DOI] [PubMed] [Google Scholar]
- Li P., Bellett A. J., Parish C. R. A comparison of the terminal protein and hexon polypeptides of avian and human adenoviruses. J Gen Virol. 1983 Jun;64(Pt 6):1375–1379. doi: 10.1099/0022-1317-64-6-1375. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx K. A., Reynolds T. C. Spermidine-condensed phi X174 DNA cleavage by micrococcal nuclease: torus cleavage model and evidence for unidirectional circumferential DNA wrapping. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6484–6488. doi: 10.1073/pnas.79.21.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Mirza M. A., Weber J. Structure of adenovirus chromatin. Biochim Biophys Acta. 1982 Jan 26;696(1):76–86. doi: 10.1016/0167-4781(82)90012-4. [DOI] [PubMed] [Google Scholar]
- Nermut M. V. Structural elements in adenovirus cores. Evidence for a "core shell" and linear structures in "relaxed" cores. Arch Virol. 1979;62(2):101–116. doi: 10.1007/BF01318063. [DOI] [PubMed] [Google Scholar]
- Nermut M. V. The architecture of adenoviruses: recent views and problems: Brief review. Arch Virol. 1980;64(3):175–196. doi: 10.1007/BF01322699. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Boring J. W., Brown J. C. Ion etching of human adenovirus 2: structure of the core. J Virol. 1984 Jul;51(1):52–56. doi: 10.1128/jvi.51.1.52-56.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E. Spheroid chromatin units (v bodies). Science. 1974 Jan 25;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Höglund S., Lonberg-Holm K., Philipson L. Structural proteins of adenoviruses. IV. Sequential degradation of the adenovirus type 2 virion. Virology. 1970 Oct;42(2):341–358. doi: 10.1016/0042-6822(70)90278-3. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Bellett J. D. A circular DNA-protein complex adenoviruses and its possible role in DNA replication. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):523–531. doi: 10.1101/sqb.1974.039.01.064. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Rylatt D. B., Parish C. R. Protein determination on an automatic spectrophotometer. Anal Biochem. 1982 Mar 15;121(1):213–214. doi: 10.1016/0003-2697(82)90578-4. [DOI] [PubMed] [Google Scholar]
- Schaller J. P., Yohn D. S. Transformation potentials of the noninfectious (defective) component in pools of adenoviruses type 12 and simian adenovirus 7. J Virol. 1974 Aug;14(2):392–401. doi: 10.1128/jvi.14.2.392-401.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant A., Tigges M. A., Raskas H. J. Nucleosome-like structural subunits of intranuclear parental adenovirus type 2 DNA. J Virol. 1979 Mar;29(3):888–898. doi: 10.1128/jvi.29.3.888-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Steck G., Leuthard P., Bürk R. R. Detection of basic proteins and low molecular weight peptides in polyacrylamide gels by formaldehyde fixation. Anal Biochem. 1980 Sep 1;107(1):21–24. doi: 10.1016/0003-2697(80)90486-8. [DOI] [PubMed] [Google Scholar]
- Sung M. T., Cao T. M., Coleman R. T., Budelier K. A. Gene and protein sequences of adenovirus protein VII, a hybrid basic chromosomal protein. Proc Natl Acad Sci U S A. 1983 May;80(10):2902–2906. doi: 10.1073/pnas.80.10.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvanen M., Yin J. Studies of DNA packaging into the heads of bacteriophage lambda. J Mol Biol. 1978 Dec 15;126(3):333–346. doi: 10.1016/0022-2836(78)90044-x. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- Tate V. E., Philipson L. Parental adenovirus DNA accumulates in nucleosome-like structures in infected cells. Nucleic Acids Res. 1979 Jun 25;6(8):2769–2785. doi: 10.1093/nar/6.8.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C. Viral DNA sequences from incomplete particles of human adenovirus type 7. Cell. 1977 Sep;12(1):243–249. doi: 10.1016/0092-8674(77)90202-1. [DOI] [PubMed] [Google Scholar]
- Vayda M. E., Rogers A. E., Flint S. J. The structure of nucleoprotein cores released from adenovirions. Nucleic Acids Res. 1983 Jan 25;11(2):441–460. doi: 10.1093/nar/11.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrant R. W., Kim S. H. alpha-Helix-double helix interaction shown in the structure of a protamine-transfer RNA complex and a nucleoprotamine model. Nature. 1978 Jan 12;271(5641):130–135. doi: 10.1038/271130a0. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yasue H., Ishibashi M. Chick embryo lethal orphan (CELO) virus-induced early and late polypeptides. Virology. 1977 May 1;78(1):216–233. doi: 10.1016/0042-6822(77)90093-9. [DOI] [PubMed] [Google Scholar]
- Younghusband H. B., Bellett A. J. Denaturation pattern of the deoxyribonucleic acid from chicken embryo lethal orphan virus. J Virol. 1972 Oct;10(4):855–857. doi: 10.1128/jvi.10.4.855-857.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younghusband H. B., Bellett A. J. Mature form of the deoxyribonucleic acid from chick embryo lethal orphan virus. J Virol. 1971 Sep;8(3):265–274. doi: 10.1128/jvi.8.3.265-274.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]